Abstract
Disruption of the gene BSCL2 causes a severe, generalised lipodystrophy, demonstrating the critical role of its protein product, seipin, in human adipose tissue development. Seipin is essential for adipocyte differentiation, whilst the study of seipin in non-adipose cells has suggested a role in lipid droplet formation. However, its precise molecular function remains poorly understood. Here we demonstrate that seipin can inducibly bind lipin 1, a phosphatidic acid (PA) phosphatase important for lipid synthesis and adipogenesis. Knockdown of seipin during early adipogenesis decreases the association of lipin 1 with membranes and increases the accumulation of its substrate PA. Conversely, PA levels are reduced in differentiating cells by overexpression of wild-type seipin but not by expression of a mutated seipin that is unable to bind lipin 1. Together our data identify lipin as the first example of a seipin-interacting protein and reveals a novel molecular function for seipin in developing adipocytes.
Keywords: Seipin, Adipogenesis, Lipodystrophy, Lipin, Endoplasmic reticulum
1. Introduction
The severe metabolic disease seen in individuals with congenital generalised lipodystrophy (CGL) provides the most compelling illustration of the importance of functional adipose tissue for human health [1–3]. Mutations in BSCL2, encoding the protein seipin, cause Berardinelli-Seip Congenital Lipodystrophy Type 2 (BSCL2), the most severe human lipodystrophy described with affected individuals almost entirely lacking adipose tissue [1,4]. Seipin plays an essential cell-autonomous role in adipogenesis [5,6], however its precise molecular function remains poorly understood. Given its importance, the delineation of the role of seipin in adipogenesis is likely to give novel insights into this process. Seipin resides in the endoplasmic reticulum (ER) membrane [7,8] but lacks homology to any proteins of known function. Yeast lacking the orthologue of seipin, YLR404W/FLD1, and cells including dermal fibroblasts or lymphoblastoid cells from BSCL2 patients, make aberrant lipid droplets when loaded with fatty acids suggesting a role in lipid synthesis [9–12]. Moreover, yeast lacking Fld1 exhibit altered lipid droplet dynamics, inheritance and lipolysis [13].
Recently, detailed studies of MEFs isolated from seipin null mice showed dramatically increased cAMP signalling and lipolysis causing failure of adipogenesis in culture during the first few days [14]. This has provided the first insight into how seipin null preadipocytes may fail during adipogenesis, however, the molecular details regarding how seipin loss alters cAMP levels and lipolysis, and whether this is a direct or indirect effect, requires further clarification.
Disruption of the gene encoding 1-acylglycerol-3-phosphate O-acyltransferase 2 (AGPAT2) causes a similar, severe generalised lipodystrophy to that seen in BSCL2. Moreover, disruption of either seipin or AGPAT2 expression also similarly inhibits the differentiation of preadipocytes in culture [6,15] suggesting that seipin and AGPAT2 might function in a common pathway. We noted that the phosphatidic acid (PA) phosphatase lipin 1 which acts immediately downstream of AGPAT2 lacks a transmembrane domain to target it to the ER membrane where AGPAT2 resides. Lipin 1 is essential for adipocyte differentiation and lipid biogenesis in culture [16–18]. Moreover, disruption of the gene encoding lipin 1 results in the lipodystrophic fld mouse model, whilst overexpression of lipin 1 in adipose tissue increases adipocyte TG storage and adipose mass [19,20]. Here we show that seipin acts as a lipin binding protein and that, consistent with this, altering seipin expression can modulate PA levels in differentiating preadipocytes.
2. Materials and methods
2.1. Cell culture and PA assays
HEK293 cells were grown in DMEM containing 10% FBS and transiently transfected using Fugene 6 transfection reagent (Roche). 3T3-L1 preadipocytes were cultured and differentiated as previously described [6,16]. Preadipocytes were transiently transfected using lipofectamine LTX (Invitrogen) for DNA or lipofactamine RNAimax (Invitrogen) for siRNA (ABI) according to the manufacturer's instructions. Cells stably expressing myc-seipin, AGPAT2 or lipin 1-GFP were generated as described in [6]. Constructs to express HA-tagged lipin 1α or lipin 1β, Flag-seipin, myc-seipin, or mutant forms of myc-seipin or Flag-seipin were as previously described [6,7,16] or generated by site directed mutagenesis. Lipids were extracted and PA levels determined using a total phosphatidic assay kit (CAYMAN, cat. Nr. 700240) according to the manufacturer's instructions. PA values were calculated from a standard curve in each experiment and normalised to protein content in cell lysates. For BiFC experiments HEK or COS7 cells were transfected with seipin–Yn and lipin 1–Yc plasmids using Fugene6 (Roche). Cells were incubated at 37 °C for 4 h, at 32 °C for 20 h then at 30 °C followed by a 24 h incubation. Cells were then fixed or harvested.
2.2. Gene expression analysis
RNA was purified using RNeasy kits (Qiagen) according to manufacturer's instructions. cDNA was synthesised and real-time PCR was performed by Taqman or Sybr Green reagents (ABI) as previously described [6]. Primers were as described in [6] or for lipin 1: forward 5′CGAGGGAGTTCTCTCTAGCTCTTG3′, reverse 5′GCAGACTTACTGACCAGCTCAGAGT3′.
2.3. Immunoprecipitations and western blotting
Cells were lysed in 50 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, 50 mM n-octyl-β-d-glucopyranoside plus protease inhibitors (Complete EDTA free, Roche). Samples were sonicated, incubated on ice for 20 min, then centrifuged at 16,000×g at 4 °C for 10 min. Supernatants were collected and protein concentration determined using DC Protein Assay (BioRad). 1.5 mg of lysates were incubated with anti-c-Myc (9E10) or anti-Flag antibody conjugated to agarose beads (Santa Cruz) for 2 h at 4 °C then beads were collected by centrifugation at 3000×g. Beads were then washed 4 times in lysis buffer. Flag immunoprecipitates were eluted with Flag peptide, Myc immunoprecipitates were eluted by addition of reducing laemmli buffer with 5% β-mercaptoethanol. Samples were separated by SDS-PAGE and transferred to PVDF membrane (Amersham). Membranes were blocked in TBS containing 0.1% Tween 20 (TBST) supplemented with 3% BSA (anti-myc) or 5% milk (HA, lipin 1, derlin 1 and calnexin) then probed with antibodies to myc (clone 4A6 Millipore), HA tag (Y-11, Santa Cruz), lipin 1 [16], AKT (Cell signalling), derlin 1 (generously provided by Dr. Stephen High, University of Manchester, UK) [21] or calnexin (abcam). Following incubation with HRP linked secondary antibodies, blots were visualised by ECL. All western blots are representative of at least 3 independent experiments.
2.4. Constructs
Fusion constructs for BiFC experiments were generated using a similar strategy to that described in [22]. Briefly, the YFPn (1–158) fragment was inserted downstream of seipin in pcDNA 3.1 to generate the SC–Yn fusion. The C-terminal YFPc (155–239) fragment was amplified and inserted upstream or downstream of lipin 1 in pcDNA 3.1 to make LN–Yc and LC–Yc constructs respectively. The lipin 1 catalytic domain (amino acids 581–890 of lipin 1α, or the same fragment in which the DXDXTV motif was mutated to inactivate its catalytic activity [23]), were amplified and inserted downstream of human Seipin 1–275 (SΔC) in pEGFP to generate SΔC–Lcat and SΔC–Lpm, respectively.
2.5. Immunofluorescence
Cells were grown on glass coverslip, treated then fixed permeablised, blocked and probed as previously described [24]. Antibodies used were as described for western blotting. Highly cross adsorbed Alexafluor anti-rabbit 594 or anti-mouse 488 secondary antibodies were used for detection (Invitrogen). Cover slips were mounted in ProLong Gold+DAPI medium (Invitrogen) and analyzed on a Zeiss 510 Meta confocal microscope. Fluorescence from 405 nm excitation of DAPI was collected through a 420–480 bandpass filter, 488 excitation emission was collected through a bandpass 505–575 filter and emission from 594 excitation through a longpass 615 filter. Images were recorded and analysed using Zeiss ZEN software and 63× objective. BiFc images were acquired using a 63× objective. In each of three independent experiments 8 randomly chosen fields were imaged and the sum of YFP fluorescence intensity above background was computed using Volocity 5 (Perkin Elmer).
2.6. Cellular fractionation
Lysates, microsomal and soluble fractions were isolated from 3T3-L1 cells differentiated for 2 days essentially as previously described by Harris et al. [23] except that microsomal fractions were resuspended in 20% of the original sample volume.
2.7. Statistical analysis
Quantitative data are represented as mean±SEM. For statistical analysis the differences between two groups were examined with Student's paired t test, and between groups with ANOVA followed by a Tukey's post-hoc test. P≤0.05 was considered statistically significant.
3. Results
3.1. Lipin isoforms can be co-imunoprecipitated with seipin
To examine whether lipin 1 and seipin might interact we co-transfected HEK293 cells with HA-tagged lipin 1α, or lipin 1β, in the presence, or absence, of flag-tagged human seipin (Figure 1A). Two translations of the human seipin protein have been described, differing by an N-terminal extension of 64 amino acids [4,25]. As it is not currently clear which of these may be the most relevant in adipogenesis each was co-transfected with the lipin 1 isoforms. Both lipin 1α and lipin 1β were detected in precipitates only when co-transfected with Flag-seipin (Figure 1A) demonstrating that both lipin 1 isoforms are able to bind each of the predicted translations of seipin.
Figure 1.
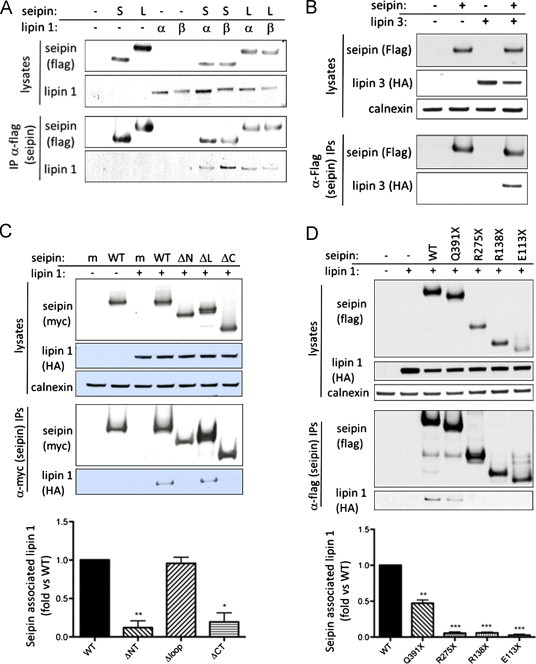
Seipin binds to lipin 1 via its C terminus. (A) HEK293 cells were transfected with empty vector (–) or Flag-tagged short form (S) or long form (L) of seipin in the presence or absence of HA-tagged lipin 1α (α) or lipin 1β (β). Lysates or anti-Flag immunoprecipitates were separated by SDS PAGE and blotted for Flag or Lipin 1. (B) HEK293 cells were transfected with empty vector (–) or Flag-tagged long form of seipin in the presence or absence of HA-tagged lipin 3 as indicated. Lysates or anti-Flag immunoprecipitates were separated by SDS PAGE and blotted for Flag or HA. (C) HEK293 cells were transfected with wild-type myc-seipin (WT) or mutants lacking the N-terminus (ΔNT), the ER lumenal loop region (ΔLP) or the C-terminus (ΔCT) in the absence or presence of Lipin 1β as indicated. Lysates or anti-myc immunoprecipitates were separated by SDS PAGE and western blotted for myc, HA and calnexin. (D) HEK293 cells were co-transfected with wild-type Flag-seipin or Flag-seipin where pathogenic mutations had been introduced to generate Q391X, R275X, R138X or E113X premature stop mutants of the protein. Lysates or anti-flag immunoprecipitates were separated by SDS PAGE and western blotted for flag, HA and calnexin. In both (C) and (D) quantification of Lipin 1β co-immunoprecipitating with wild-type and mutant forms of myc-seipin as in (C). Data are means±SEM, n=4 (C) or n=3 (D). * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001 versus co-immunoprecipitation with wild-type seipin.
As with lipin 1, lipin 3 co-precipitated with seipin when both proteins were co-expressed showing that seipin is capable of binding other lipin isoforms (Figure 1B). As previously reported, we found that lipin 2 was poorly expressed when transfected [26,27] preventing us from specifically examining its interaction with seipin.
3.2. The interaction with lipin 1 requires the N- and C-terminal cytoplasmic domains of seipin.
To investigate the regions of seipin required for lipin binding we employed seipin mutants in which the cytosolic N terminus (ΔNT), the ER lumenal loop (ΔLP) or the C terminus (ΔCT) had been deleted [7] (schematically shown in Figure S1A). These mutants have been demonstrated previously to all insert into the ER membrane with appropriate topology [7]. Wild-type or mutant forms of seipin were then co-transfected with lipin 1β. Lipin 1β was used as it appears predominantly cytosolic and to be more involved in lipogenesis than lipin 1α [28]. Seipin proteins were immunoprecipitated from lysates of transfected cells and association with lipin 1β analysed by western blotting. This revealed that ΔNT–seipin and ΔCT–seipin [7] were both unable to co-immunoprecipitate lipin1 β (Figure 1C). In contrast, ΔLP–seipin retained the ability to interact strongly with lipin1β. Consistent with these observations, the pathogenic premature stop mutations of seipin identified in BSCL2 patients that disrupt the C-terminus of seipin E113X, R138X and R275X (numbered for the short form of seipin) all essentially failed to co-immunoprecipitate lipin 1 (Figure 1D). In addition the most C-terminal reported pathogenic premature stop mutant Q391X, which lacks only the last 8 amino acids co-immunoprecipitated less than 50% of the lipin 1 associated with wild-type seipin. Together these data suggest that both the N- and C-terminal cytoplasmic domains of seipin are involved in the association with lipin 1.
3.3. Bimolecular fluorescence complementation analysis confirms the interaction of seipin and lipin 1 can occur in intact cells
To further validate the interaction between seipin and lipin we employed a bimolecular fluorescence complementation (BiFC) assay in which the N-terminal fragment of yellow fluorescent protein (YFP) was fused to the C-terminus of seipin (SC–Yn) and the C-terminal fragment of YFP fused to the C-terminus of lipin 1β (LC–Yc). When these constructs were co-expressed in HEK293 cells YFP signal could be clearly detected demonstrating an interaction between the two proteins (Figure 2A, left panels, quantified in Figure 2B). In contrast, no signal could be detected if the C-terminal fragment of YFP was fused to the N-terminus of lipin (LN–Yc) and co-expressed with the SC–Yn seipin (Figure 2A right panels) despite abundant expression of LN–Yc lipin (Figure 2B lower panels). This control strongly implies that the signal obtained with the LC–Yc lipin construct reports a specific and direct interaction with SC–Yn seipin and not mere co-localisation in a common subcellular location. As detailed subcellular localisation is difficult to visualise in HEK cells we also examined the interaction of SC–Yn seipin and LC–Yc lipin in COS7 cells. Using an antibody to a Flag sequence inserted in the seipin SC–Yn seipin construct the reticular pattern consistent with localisation to the ER was observed and a subset of this staining overlaid with YFP signal indicating that a population of SC–Yn seipin was complexed with LC–Yc lipin (Figure 2C upper panels). Similarly a subpopulation of the LC–Yc lipin, visualised with anti-lipin antibody also coincided with YFP fluorescence (Figure 2C lower panels). These data, using a robust and independent method, confirms that the specific interaction of lipin and seipin implied by co-immunoprecipitation can occur in intact cells.
Figure. 2.
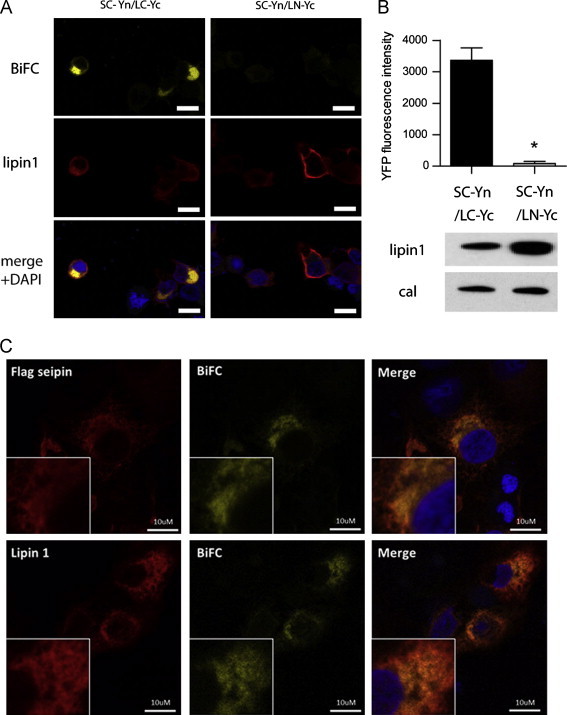
Bimolecular fluorescence complementation (BiFC) analysis of the seipin/lipin interaction. (A) Plasmids encoding the N-terminal fragment of YFP fused to the C terminus of seipin (SC–Yn) and the C terminal fragment of YFP fused either to the C (LC–Yc) or N terminus (LN–Yc) of lipin 1 were co-transfected in HEK cells and stained with anti-lipin1 antibodies (red) and DAPI to label nuclei. The direct interaction between seipin and lipin 1 is indicated by the presence of YFP signal (yellow) due to apposition of the two fragments of the YFP protein. White bars indicate 10 μm. (B) Quantified BiFc signal intensity. n=3±SEM, * indicates difference from SC–Yn/LC–Yc p<0.05. A representative western blot using anti-lipin1 antibody to detect LC–Yc and LN–Yc expression and showing calnexin as a loading control is shown below. (C) COS7 cells were transfected with plasmids encoding the seipin SC–Yn and lipin 1 LC–Yc constructs and stained with anti-Flag antibodies (upper panels) or anti-Lipin 1 antibodies (lower panels) and DAPI to label nuclei. As in (A) YFP signal (yellow) indicates tight apposition of the two fragments of the YFP protein. White bars indicate 10 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Seipin can inducibly interact with lipin in maturing adipocytes
Despite its important role in early adipogenesis, endogenous lipin 1 protein is difficult to detect until later in adipocyte development. For this reason we turned to maturing adipocytes to examine the interaction of seipin with endogenous lipin 1. At present we have been unable to convincingly detect endogenous seipin in 3T3-L1 cells by western blotting using any of the antibodies currently available. Hence, we utilised 3T3-L1 cells stably expressing myc-seipin. We hypothesised that seipin may tether lipin 1 at the ER membrane to facilitate its involvement in lipid biosynthesis in developing adipocytes. Exogenous fatty acids can induce the translocation of lipin activity to intracellular membranes [29] whilst insulin is known to increase lipogenesis in adipocytes and activates proadipogenic pathways in differentiating preadipocytes [30–32]. We therefore investigated whether the proposed interaction between seipin and lipin may be influenced by insulin and fatty acids. Myc-seipin was immunoprecipitated from serum starved adipocytes incubated in the presence or absence of fatty acids and/or insulin. In these experiments immunoprecipitates were heated to elute the myc-seipin and ensure maximum recovery of any co-immunoprecipitated lipin 1. Consistent with previous reports by ourselves and others [5–7] seipin can form high molecular weight complexes when separated by SDS-PAGE, a phenomenon specifically resulting from the heating of samples prior to loading as recently demonstrated by Fei et al. [10] (Figure S1B). No endogenous lipin 1 was detected in anti-myc immunoprecipitates from control cells or in immunoprecipitates from serum starved or insulin treated myc-seipin expressing cells (Figure 3A). However, endogenous lipin 1 was detectable in immunoprecipitates from myc-seipin expressing cells treated with fatty acids, an effect significantly potentiated by the addition of insulin. Consistent with the co-immunopreciptation data, confocal microscopy of serum starved cells revealed that peak areas of seipin expression (marked with white arrows) did not correspond strongly with the peak areas of endogenous lipin 1 expression in the same cells suggesting poor co-localisation under basal conditions (Figure 3B upper panels). In contrast, when cells were treated for 30 min with insulin and fatty acids almost all the regions of strongest seipin staining corresponded with areas of peak staining for endogenous lipin 1 (Figure 3B lower panels). Quantitative analysis of seipin and lipin fluorescence revealed a significant increase in overlay of the two signals in fatty acid and insulin treated cells compared with untreated cells (Figure 3C). Importantly, as the binding of seipin to endogenous lipin 1 and their co-localisation is acutely inducible, this indicates that the interaction is both specific and regulated.
Figure 3.
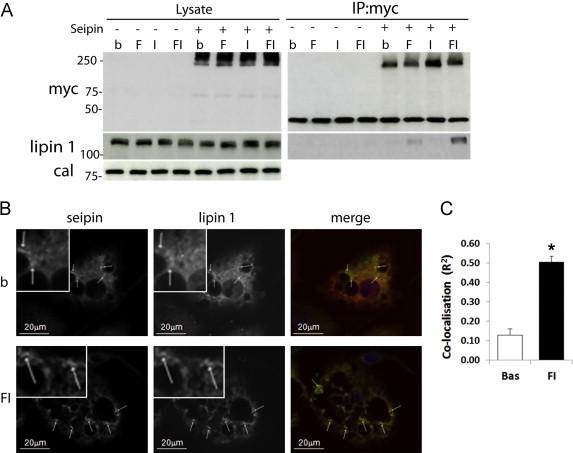
Endogenous lipin 1 inducibly binds to seipin in maturing adipocytes. (A) Following differentiation for 7 days control 3T3-L1 adipocytes or adipocytes stably expressing myc-sepin were serum starved for 2 h. Adipocytes were left untreated (b) or incubated with 330 μM each of oleate and linoleate (F), 100 nM insulin (I) or a combination of both (FI) for 30 min. Thirty micro grams of cell lysates or anti-myc immunoprecipitates were western blotted for myc-seipin, endogenous lipin 1 or calnexin. (B) Myc-seipin expressing adipocytes were incubated for 30 min in the absence (b) or presence (FI) of 330 μM each of oleate and linoleate and 100 nM insulin, fixed and immunostained for myc-seipin and endogenous lipin 1. Individual images are shown in grayscale and an ROI containing peak myc-seipin staining of each cell shown at higher magnification (inset). Merged image shows overlay of myc-seipin (green) and lipin 1 (red). White arrows reference regions of high seipin intensity. (C) Quantification of endogenous lipin and myc-seipin co-localisation in adipocytes under basal (Bas) conditions or following treatment with 330 μM each of oleate and linoleate and 100 nM insulin for 30 min (FI) n=12. Data shown are ±SEM, * indicates difference from basal p<0.005. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Seipin knockdown or overexpression increases or decreases, respectively, the levels of the lipin 1 substrate phosphatidic acid in differentiating adipocytes
Next we investigated whether the interaction with seipin may be important for lipin 1 function during early adipogenesis. Post-confluent 3T3-L1 preadipocytes were transfected with control siRNA or siRNA targeting seipin and induced to differentiate for 2 days. Analysis of mRNA expression demonstrated that siRNA targeting seipin significantly reduced seipin expression in these cells (Figure 4A). Seipin siRNA did not significantly affect lipin 1 mRNA levels at this early time point (Figure S2A). However, prolonged siRNA knockdown of seipin subsequently impaired adipogenesis consistent with the known requirement for seipin in this process (Figure S2B) [5,6]. Seipin knockdown modestly increased PA levels in 3T3-L1 preadipocytes differentiated for 2 days, however these changes were only significant with one siRNA tested (Figure 4B). To increase the flux through this pathway we repeated these experiments with 3T3-L1 preadipocytes stably expressing AGPAT2. In these cells, knockdown of seipin led to more robust and significant increase in PA levels (Figure 4C). Conversely, when 3T3-L1 preadipocytes were transfected with wild-type seipin and differentiated for 2 days, PA levels were significantly reduced compared to those in mock transfected cells (Figure 4D). In contrast, equivalent expression of the mutant ΔCT–seipin protein, which cannot bind lipin 1, did not affect levels of PA in these cells.
Figure 4.
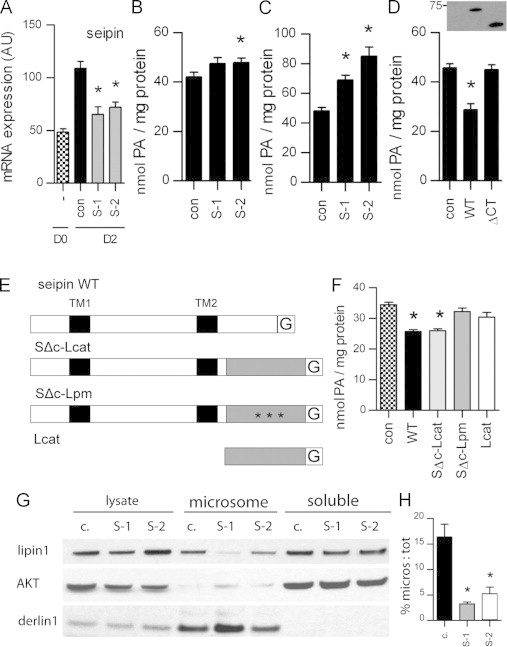
Inhibiting or increasing seipin expression in 3T3-L1 adipocytes raises or lowers cellular phosphatidic acid (PA) levels, respectively. (A) 3T3-L1 preadipocytes were transfected with control siRNA (con) or siRNAs targeting seipin (S-1, S-2) and induced to differentiate for 2 days. Expression of mRNA encoding seipin was determined by real time PCR on day 0 (D0) and day 2 (D2) of differentiation. Data are normalised to cyclophilin A and expressed as mean±SEM, n=4, * indicates significant difference (p<0.05) versus control siRNA. (B) 3T3-L1 cells or (C) 3T3-L1 cells stably expressing AGPAT2, were transfected with control siRNA (con) or siRNAs targeting seipin (S-1, S-2) and differentiated for 2 days. PA content was determined against a standard curve of exogenous PA. Data are means ±SEM, n=4, * indicates a significant difference (p<0.05) from control siRNA transfected cells. (D) 3T3-L1 cells were transfected with wild-type and mutant forms of myc-seipin and differentiated for 2 days. Total PA content was determined as in (B). Data are means±SEM, n=4, * indicates significant difference (p<0.05) from control cells. An anti-myc western blot of transfected cell lysates is shown above. (E) Schematic of GFP (G) tagged wild-type seipin (WT), fusion constructs in which the C-terminus of sepin is replaced either with the catalytic domain of lipin 1 (SΔC–Lcat) or the catalytic domain of lipin 1 mutated to ablate lipin 1 PAP activity as in [23] (SΔC–Lpm), or a GFP-tagged catalytic domain of wild type lipin 1 (Lcat). (F) 3T3-L1 cells were transfected with GFP (con), GFP tagged wild-type seipin (WT), the seipin fusion constructs SΔC–Lcat or SΔC–Lpm, or Lcat. Following differentiation for 2 days PA levels were determined as in (B)–(D). Data are means±SEM, n=4, * indicates a significant difference (p<0.05) compared to control cells. (G) 3T3-L1 preadipocytes stably expressing GFP-tagged lipin 1β were transfected with control siRNA (con) or siRNA targeting seipin (S-1, S-2), differentiated for 2 days, and microsomal and soluble fractions isolated. Microsomal fractions were resuspended in 20% of original volumes. Fractions and total lysates were western blotted for lipin 1 and AKT or derlin 1 as controls for soluble or microsomal fractions, respectively. (H) Levels of lipin 1 protein in the corresponding lysate and microsomal fractions from each cell extract were quantified using Image J and microsomal lipin 1 expressed as a percentage of total cellular lipin 1. Data are means±SEM, n=4. * indicates significant difference (p<0.05) from control cells.
To further investigate whether the targeting of lipin could be responsible for the PA lowering effects of seipin we generated fusion constructs in which the C-terminus of seipin had been replaced by the catalytic domain of wild-type lipin 1 (SΔC–Lcat) or lipin 1 in which the DXDXTV motif was mutated to inactivate its catalytic activity (SΔC–Lpm) [23] (shown schematically in Figure 4E). In 3T3-L1 cells induced to differentiate for 2 days expression of the SΔC–Lcat fusion construct lowered PA levels to a similar degree to that seen with wild-type seipin (Figure 4F). In contrast, the SΔC–Lpm fusion protein did not decrease PA levels, demonstrating that the catalytic activity of the lipin component was essential for this effect. Importantly, the catalytic domain of lipin alone (Lcat) was unable to alter PA levels demonstrating the key targeting role played by the seipin component of these constructs. These data do not themselves demonstrate that the changes in PA we observe when seipin expression is altered depend on the specific interaction with lipin 1. However, they do show that the direct binding of lipin to seipin is capable of causing the changes in PA levels we observe when seipin is overexpressed and that the magnitude of these changes are consistent with those observed with forced targeting of lipin activity by fusion to ΔCT–seipin.
3.5. Seipin knockdown decreases membrane association of lipin 1 during early adipocyte differentiation
We next examined whether the targeting of lipin to intracellular membranes may depend on seipin expression during early adipogenesis. As the levels of lipin 1 protein are too low to reliably detect by western blotting in these cells we employed 3T3-L1 cells stably expressing Lipin 1β–GFP. These cells were transfected with control siRNA or siRNA targeting seipin and induced to differentiate for 2 days prior to subcellular fractionation. Analysis of seipin mRNA expression confirmed significant knockdown in these cells (Figure S2C). Approximately 15% of the lipin 1β–GFP was recovered in the microsomal fraction in control siRNA treated cells (Figure 4G, quantified in Figure 4H). In contrast, knockdown of seipin significantly reduced the levels of lipin 1 on microsomes. Critically, these data show that seipin is at least partly required for lipin's recruitment to, or retention on, intracellular membranes during early adipogenesis.
4. Discussion
The binding of lipin is the first direct molecular interaction identified for seipin. Moreover, this is the first specific role described for seipin during adipocyte differentiation. This suggests a novel mechanism whereby seipin could act as an important regulator of lipid synthesis and so adipocyte development. Intriguingly, recent studies of seipin orthologues in yeast (Fld1) and drosophila (dSeipin) have also reported increased PA levels in cells where these proteins have been disrupted [33,34]. In yeast lacking Fld1 increased PA levels have been proposed to drive the observed phenotype of supersized lipid droplets [33]. How Fld1 affects PA levels in yeast is not clear. Our data clearly indicate that human seipin requires an intact C-terminus to alter cellular PA levels, however this domain is very short in the yeast Fld1 protein. Moreover a study using the yeast Fld1 protein failed to identify any heterologous binding proteins [35]. Both observations may suggest that PA changes we observe in differentiating adipocytes differ mechanistically from those in yeast lacking Fld1. The drosophila seipin contains a more substantial C-terminus with some homology to the human protein and so might share more functions with the human seipin [34]. Specific experiments comparing yeast, drosophila and human proteins in mammalian adipocytes will be required to determine precisely how the observations made in each model system relates to the role of seipin in adipogenesis.
It remains to be determined whether the binding of lipin 1 at the ER membrane constitutes the major function of seipin in developing adipocytes but it seems likely that seipin also binds other proteins. However, it is plausible that the loss of lipin targeting, and hence activity, contributes significantly the lack of adipose tissue development in BSCL2 patients. Loss of lipin 1 function in the fld mouse drives a complex phenotype including lipodystrophy, demonstrating its importance in adipose tissue development in vivo. However, loss-of-function mutations in lipin 1 have been described in humans with severe rhabdomyolysis without evidence of lipodystrophy [36]. This may reflect functional redundancy amongst lipin isoforms or differences in the lipin isoforms expressed in differentiating human versus mouse adipocytes. Importantly, the interaction we observe between lipin 1 and seipin also occurs with lipin 3 and likely also lipin 2. The role of lipins and their orthologues in lipid biogenesis is very highly conserved through evolution [37]. Thus we propose that the PAP activity of at least 1 lipin isoform will be critical in human adipose tissue development and that seipin could be capable of influencing the function of all lipin isoforms in developing adipocytes. If the lipin targeting function of seipin does play an important role in adipogenesis this may explain the pathogenicity of at least some mutants of seipin found in BSCL2 patients. We observed significantly reduced or no binding of lipin to the pathogenic premature stop mutants of seipin we tested in this study. However, despite robust expression of the R275X mutant of seipin in transiently transfected HEK cells examined here we have consistently found that when stably transfected in preadipocytes the R275X protein is undetectable [6]. Hence we suspect that lack of protein, rather than loss of lipin binding, is more likely to explain the pathogenicity of the R275X mutant and such instability might also affect other pathogenic mutants of seipin. In our preliminary studies we have observed clearly detectable binding of lipin to the pathogenic A212P mutant of seipin, however, we and others have previously reported that this mutant mislocalises in cells [6,10]. Moreover, others have demonstrated that the corresponding mutation of the yeast orthologue of seipin prevents normal oligomerisation of the protein which is also likely to affect its function [35]. Overall it appears that lack of lipin binding may provide a potential pathogenic mechanism for some mutants of seipin found in BSCL2 but that individual point mutations may give rise to lipodystrophy via complex and subtly different mechanisms.
Whilst our data demonstrate that seipin may act as a regulator of lipin 1, precisely how this might affect adipogenesis remains to be elucidated. Disruption of either AGPAT2, lipin 1 or seipin inhibits adipogenic gene expression at an early stage in cellular models before the overt appearance of lipid droplets [5,6,15,17,18,28]. Whilst lipin can also directly regulate transcription [17], this is unlikely for AGPAT2 and seipin given their confinement to the ER membrane. In contrast to the ablation of AGPAT2, lipin 1 or seipin, disruption of DGAT enzymes, which are critical for the synthesis of TG from DAG, inhibits TG accumulation but not adipogenesis per se [38]. PA and DAG are key intermediates in the production of phospholipids [39]. We hypothesise that these may provide a key adipogenic signal and that impairment of this, rather than TG synthesis, may represent the critical defect causing lipodystrophy when AGPAT2 or seipin are disrupted. However, given the long-acknowledged role of lipin enzymes in generating PA for the subsequent generation of TG it may be that a significant effect of disrupting the targeting of lipin to the ER by seipin may be to alter TG synthesis in developing adipocytes and that the effect of seipin loss on gene expression may occur via an additional mechanism. Whilst the effect of seipin loss in some non-adipose cells is to increase TG synthesis from exogenous lipid substrates, TG synthesis in lymphoblastoid cells from BSCL2 patients display reduced TG levels [9,10]. This may because seipin plays an important role in TG synthesis from endogenous substrates, or because the effect of seipin loss on TG synthesis specifically differs significantly in different cell types. The precise direct involvement of seipin, if any, in TG synthesis during adipogenesis awaits clarification.
The recent demonstration that MEFs from seipin null mice may undergo relatively normal early adipogenesis with subsequent uncontrolled lipolysis and failure of this process has provided novel and intriguing insights regarding adipogenesis in seipin null cells [14]. The precise molecular mechanisms via which seipin loss may cause this remain to be clarified and might imply a direct role in regulating cAMP signalling, which is increased in seipin-null MEFs. If so the regulation of lipin 1 we have identified and the altered lipolysis observed by Chen et al. may represent independent functions of seipin in developing adipocytes. However, it is equally plausible that the observations of Chen and colleagues are part of a single molecular mechanism which also encompasses our current findings and those reported previously by ourselves and others regarding the requirement for seipin in cultured models of adipogenesis. In differentiating C3H10T1/2 cells lacking seipin and seipin null MEFs early induction of PPARγ expression is relatively normal but the further induction and maintenance of PPARγ levels subsequently fails [6,14]. This might reflect the normal activation of PPARγ by early factors such as C/EBPβ and C/EBPδ which are directly induced by the hormonal cocktail with which adipogenesis is initiated in these cell models. However, sustained PPARγ activation may rely more on signals sensitive to lipin activity, which is known to regulate PPARγ expression in adipogenesis [40]. Inhibition of PPARγ expression in maturing adipocytes has been shown previously to cause de-differentiation and a significant increase in lipolysis and reduced expression of adipogenic markers, somewhat similar to that seen in seipin null MEFs [14,41]. Whilst PPARγ agonists were unable to rescue adipogenesis in seipin null MEFs, this does not exclude a role for reduced expression of PPARγ in causing this phenotype [14]. Therefore, it would be interesting to determine whether ectopic re-expression of PPARγ may rescue the lipolytic phenotype and adipogenesis in seipin null MEFs.
A recent novel mouse model wherein seipin is transgenically overexpressed selectively in adipose tissue has indicated further complexity to the roles of seipin in the regulation of adiposity [42]. Paradoxically these mice display decreased adipose mass, potentially as a result of increased adipocyte lipolysis. Whilst the authors invoke a paradigm of ‘over-differentiation’ the data are also consistent with seipin exhibiting different, stage-dependent functions in developing adipocytes. As such, perhaps via different binding partners or effects on lipid droplet fusion, the molecular means via which seipin is critical for adipogenesis may differ from its role(s) in mature adipocytes, where it is abundantly expressed. Our ongoing studies suggest that seipin may associate with several different proteins some of which are only expressed at specific stages of adipogenesis, consistent with this hypothesis.
5. Conclusions
In summary, our data shows that seipin acts as a novel lipin binding protein and that seipin can target lipin enzymes to the ER in developing adipocytes. This is the first direct molecular function identified for seipin in developing adipocytes and suggests a new mechanism whereby seipin might act as an essential regulator of human adipose tissue development.
Conflict of interest
None declared.
Acknowledgements
This work was supported by the Medical Research Council (MRC) [New Investigator Research Grant number GO800203 (to J.J.R.), Senior Fellowship number G0701446 (to S.S), Program Grant number G09000554 (to S.O.R)], the Swiss National Science Foundation [Grant PBBEP3-123654/PA00P3-129106 (to E.M.A)], the Wellcome Trust [Grant number 078986/Z/06/Z (to S.O.R.)], the Agency for Science, Technology and Research, Singapore(A⁎STAR) (N.R.), the MRC Centre for Obesity and Related Metabolic Disorders (MRC-CORD) [Grant number GO600717] and the National Institute for Health Research Comprehensive Biomedical Research Centre [Grant number CG50826].
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at 10.1016/j.molmet.2012.11.002.
Appendix A. Supplementary materials
Supplementary Figure S1.
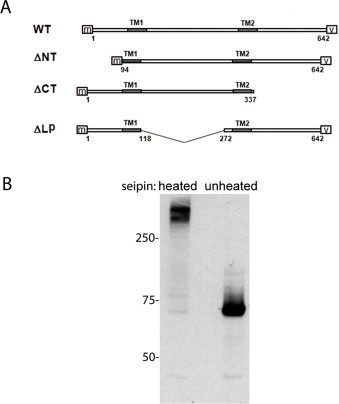
(A) Schematic representation of the wild-type long form of seipin (WT) and mutants lacking the N-terminus(ΔNT), C-terminus (ΔCT) or the ER lumenal loop region (ΔLP). Myc-tag is indicated on the N terminus (m) and V5His tag on the C-terminus (v). (B) HEK293 cells were transfected with myc-tagged wild type seipin. Samples of the same lysates containing 30 μg of protein each were incubated at 95 °C for 5 min prior to loading for separation by SDS-PAGE (heated) or loaded without heating (unheated). Myc-seipin was detected by western blotting with anti-myc antibodies.
Supplementary Figure S2.
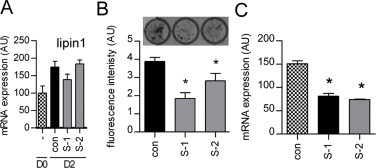
(A) 3T3-L1 adipocytes were transfected with control siRNA (con) or siRNAs targeting seipin (S-1, S-2) on day O of differentiation. Expression of mRNA encoding lipin was determined by real time PCR on day 0 and day 2 of differentiation. Data are normalised to cyclophilin A and expressed as mean±SEM, n=4. (B) Following transfection with control siRNA of siRNA targeting seipin (S-1, S-2) on day 0, day 2 and day 4 of differentiation 3T3-L1 adipocytes were fixed and stained with Bodipy at day 6 of differentiation. Images were acquired using a Storm Scanner (Molecular Dynamics) and fluorescence in each well quantified. Data are mean±SEM, n=5, * indicates significant difference (p<0.05) versus control siRNA treated cells. A representative image is shown above each bar. Knockdown of seipin by siRNA significantly inhibited adipocyte differentiation. (C) 3T3-L1 cells stably overexpressing lipin 1β were transfected with siRNA targeting seipin (S-1 and S-2) at day 0 and differentiated for 2 days and subjected to real time PCR to determine seipin mRNA levels. Data are normalised to cyclophilin A and expressed as mean±SEM, n=4. * indicates significant difference (p<0.05) versus control siRNA treated cells.
References
- 1.Garg A., Agarwal A.K. Lipodystrophies: disorders of adipose tissue biology. Biochimica et Biophysica Acta. 2009;1791:507–513. doi: 10.1016/j.bbalip.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang-Doran I., Sleigh A., Rochford J.J., O'Rahilly S., Savage D.B. Lipodystrophy: metabolic insights from a rare disorder. Journal of Endocrinology. 2010;207:245–255. doi: 10.1677/JOE-10-0272. [DOI] [PubMed] [Google Scholar]
- 3.Rochford J.J. Molecular mechanisms controlling human adipose tissue development: insights from monogenic lipodystrophies. Expert Reviews in Molecular Medicine. 2010;12:e24. doi: 10.1017/S1462399410001547. [DOI] [PubMed] [Google Scholar]
- 4.Magre J., Delepine M., Khallouf E., Gedde-Dahl T., Jr., Van Maldergem L., Sobel E. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nature Genetics. 2001;28:365–370. doi: 10.1038/ng585. [DOI] [PubMed] [Google Scholar]
- 5.Chen W., Yechoor V.K., Chang B.H., Li M.V., March K.L., Chan L. The human lipodystrophy gene product Berardinelli-Seip congenital lipodystrophy 2/seipin plays a key role in adipocyte differentiation. Endocrinology. 2009;150:4552–4561. doi: 10.1210/en.2009-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Payne V.A., Grimsey N., Tuthill A., Virtue S., Gray S.L., Dalla Nora E. The human lipodystrophy gene BSCL2/seipin may be essential for normal adipocyte differentiation. Diabetes. 2008;57:2055–2060. doi: 10.2337/db08-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito D., Fujisawa T., Iida H., Suzuki N. Characterization of seipin/BSCL2, a protein associated with spastic paraplegia 17. Neurobiology of Disease. 2008;31:266–277. doi: 10.1016/j.nbd.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Lundin C., Nordstrom R., Wagner K., Windpassinger C., Andersson H., von Heijne G. Membrane topology of the human seipin protein. FEBS Letters. 2006;580:2281–2284. doi: 10.1016/j.febslet.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 9.Boutet E., El Mourabit H., Prot M., Nemani M., Khallouf E., Colard O. Seipin deficiency alters fatty acid Delta9 desaturation and lipid droplet formation in Berardinelli-Seip congenital lipodystrophy. Biochimie. 2009;91:796–803. doi: 10.1016/j.biochi.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Fei W., Li H., Shui G., Kapterian T.S., Bielby C., Du X. Molecular characterization of seipin and its mutants: implications for seipin in triacylglycerol synthesis. Journal of Lipid Research. 2011;52(12):2136–2147. doi: 10.1194/jlr.M017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fei W., Shui G., Gaeta B., Du X., Kuerschner L., Li P. Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. The Journal of Cell Biology. 2008;180:473–482. doi: 10.1083/jcb.200711136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szymanski K.M., Binns D., Bartz R., Grishin N.V., Li W.P., Agarwal A.K. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20890–20895. doi: 10.1073/pnas.0704154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolinski H., Kolb D., Hermann S., Koning R.I., Kohlwein S.D. A role for seipin in lipid droplet dynamics and inheritance in yeast. Journal of Cell Science. 2011;124(Pt 22):3894–3904. doi: 10.1242/jcs.091454. [DOI] [PubMed] [Google Scholar]
- 14.Chen W., Chang B., Saha P., Hartig S.M., Li L., Reddy V.T. Berardinelli-Seip congenital lipodystrophy-2 (BSCL2)/seipin is a cell autonomous regulator of lipolysis essential for adipocyte differentiation. Molecular and Cellular Biology. 2012;32(6):1099–1111. doi: 10.1128/MCB.06465-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gale S.E., Frolov A., Han X., Bickel P.E., Cao L., Bowcock A. A regulatory role for 1-acylglycerol-3-phosphate-O-acyltransferase 2 in adipocyte differentiation. The Journal of Biological Chemistry. 2006;281:11082–11089. doi: 10.1074/jbc.M509612200. [DOI] [PubMed] [Google Scholar]
- 16.Grimsey N., Han G.S., O'Hara L., Rochford J.J., Carman G.M., Siniossoglou S. Temporal and spatial regulation of the phosphatidate phosphatases lipin 1 and 2. The Journal of Biological Chemistry. 2008;283:29166–29174. doi: 10.1074/jbc.M804278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh Y.K., Lee M.Y., Kim J.W., Kim M., Moon J.S., Lee Y.J. Lipin1 is a key factor for the maturation and maintenance of adipocytes in the regulatory network with CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma 2. The Journal of Biological Chemistry. 2008;283:34896–34906. doi: 10.1074/jbc.M804007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phan J., Peterfy M., Reue K. Lipin expression preceding peroxisome proliferator-activated receptor-gamma is critical for adipogenesis in vivo and in vitro. The Journal of Biological Chemistry. 2004;279:29558–29564. doi: 10.1074/jbc.M403506200. [DOI] [PubMed] [Google Scholar]
- 19.Peterfy M., Phan J., Xu P., Reue K. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nature Genetics. 2001;27:121–124. doi: 10.1038/83685. [DOI] [PubMed] [Google Scholar]
- 20.Phan J., Reue K. Lipin, a lipodystrophy and obesity gene. Cell Metabolism. 2005;1:73–83. doi: 10.1016/j.cmet.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Crawshaw S.G., Cross B.C., Wilson C.M., High S. The oligomeric state of Derlin-1 is modulated by endoplasmic reticulum stress. Molecular Membrane Biology. 2007;24:113–120. doi: 10.1080/09687860600988727. [DOI] [PubMed] [Google Scholar]
- 22.Gandotra S., Lim K., Girousse A., Saudek V., O'Rahilly S., Savage D.B. Human frameshift mutations affecting the carboxyl terminus of perilipin increase lipolysis by failing to sequester the adipose triglyceride lipase (ATGL) coactivator, AB-hydrolase containing 5 (ABHD5) The Journal of Biological Chemistry. 2011;286(40):34998–35006. doi: 10.1074/jbc.M111.278853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris T.E., Huffman T.A., Chi A., Shabanowitz J., Hunt D.F., Kumar A. Insulin controls subcellular localization and multisite phosphorylation of the phosphatidic acid phosphatase, lipin 1. The Journal of Biological Chemistry. 2007;282:277–286. doi: 10.1074/jbc.M609537200. [DOI] [PubMed] [Google Scholar]
- 24.Payne V.A., Au W.S., Gray S.L., Nora E.D., Rahman S.M., Sanders R. Sequential regulation of diacylglycerol acyltransferase 2 expression by CAAT/enhancer-binding protein beta (C/EBPbeta) and C/EBPalpha during adipogenesis. The Journal of Biological Chemistry. 2007;282:21005–21014. doi: 10.1074/jbc.M702871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cartwright B.R., Goodman J.M. Seipin—from human disease to molecular mechanism. Journal of Lipid Research. 2012;53(6):1042–1055. doi: 10.1194/jlr.R023754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donkor J., Sariahmetoglu M., Dewald J., Brindley D.N., Reue K. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. The Journal of Biological Chemistry. 2007;282:3450–3457. doi: 10.1074/jbc.M610745200. [DOI] [PubMed] [Google Scholar]
- 27.Liu G.H., Qu J., Carmack A.E., Kim H.B., Chen C., Ren H. Lipin proteins form homo- and hetero-oligomers. The Biochemical Journal. 2010;432:65–76. doi: 10.1042/BJ20100584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterfy M., Phan J., Reue K. Alternatively spliced lipin isoforms exhibit distinct expression pattern, subcellular localization, and role in adipogenesis. The Journal of Biological Chemistry. 2005;280:32883–32889. doi: 10.1074/jbc.M503885200. [DOI] [PubMed] [Google Scholar]
- 29.Martin-Sanz P., Hopewell R., Brindley D.N. Long-chain fatty acids and their acyl-CoA esters cause the translocation of phosphatidate phosphohydrolase from the cytosolic to the microsomal fraction of rat liver. FEBS Letters. 1984;175:284–288. doi: 10.1016/0014-5793(84)80752-8. [DOI] [PubMed] [Google Scholar]
- 30.Assimacopoulos-Jeannet F., Brichard S., Rencurel F., Cusin I., Jeanrenaud B. In vivo effects of hyperinsulinemia on lipogenic enzymes and glucose transporter expression in rat liver and adipose tissues. Metabolism: Clinical and Experimental. 1995;44:228–233. doi: 10.1016/0026-0495(95)90270-8. [DOI] [PubMed] [Google Scholar]
- 31.Gathercole L.L., Morgan S.A., Bujalska I.J., Hauton D., Stewart P.M., Tomlinson J.W. Regulation of lipogenesis by glucocorticoids and insulin in human adipose tissue. PloS One. 2011;6:e26223. doi: 10.1371/journal.pone.0026223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowe C.E., O'Rahilly S., Rochford J.J. Adipogenesis at a glance. Journal of Cell Science. 2011;124:2681–2686. doi: 10.1242/jcs.079699. [DOI] [PubMed] [Google Scholar]
- 33.Fei W., Shui G., Zhang Y., Krahmer N., Ferguson C., Kapterian T.S. A role for phosphatidic acid in the formation of supersized lipid droplets. PLoS Genetics. 2011;7:e1002201. doi: 10.1371/journal.pgen.1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian Y., Bi J., Shui G., Liu Z., Xiang Y., Liu Y. Tissue-autonomous function of Drosophila seipin in preventing ectopic lipid droplet formation. PLoS Genetics. 2011;7:e1001364. doi: 10.1371/journal.pgen.1001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Binns D., Lee S., Hilton C.L., Jiang Q.X., Goodman J.M. Seipin is a discrete homooligomer. Biochemistry. 2010;49:10747–10755. doi: 10.1021/bi1013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michot C., Hubert L., Brivet M., De Meirleir L., Valayannopoulos V., Muller-Felber W. LPIN1 gene mutations: a major cause of severe rhabdomyolysis in early childhood. Human Mutation. 2010;31:E1564–E1573. doi: 10.1002/humu.21282. [DOI] [PubMed] [Google Scholar]
- 37.Carman G.M., Han G.S. Phosphatidic acid phosphatase, a key enzyme in the regulation of lipid synthesis. The Journal of Biological Chemistry. 2009;284:2593–2597. doi: 10.1074/jbc.R800059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris C.A., Haas J.T., Streeper R.S., Stone S.J., Kumari M., Yang K. DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. Journal of Lipid Research. 2011;52:657–667. doi: 10.1194/jlr.M013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nohturfft A., Zhang S.C. Coordination of lipid metabolism in membrane biogenesis. Annual Review of Cell and Developmental Biology. 2009;25:539–566. doi: 10.1146/annurev.cellbio.24.110707.175344. [DOI] [PubMed] [Google Scholar]
- 40.Zhang P., Takeuchi K., Csaki L.S., Reue K. Lipin-1 phosphatidic phosphatase activity modulates phosphatidate levels to promote peroxisome proliferator-activated receptor gamma (PPARgamma) gene expression during adipogenesis. The Journal of Biological Chemistry. 2012;287:3485–3494. doi: 10.1074/jbc.M111.296681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamori Y., Masugi J., Nishino N., Kasuga M. Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes. 2002;51:2045–2055. doi: 10.2337/diabetes.51.7.2045. [DOI] [PubMed] [Google Scholar]
- 42.Cui X., Wang Y., Meng L., Fei W., Deng J., Xu G. Overexpression of a short human seipin/BSCL2 isoform in mouse adipose tissue results in mild lipodystrophy. American Journal of Physiology: Endocrinology and Metabolism. 2012;302(6):E705–E713. doi: 10.1152/ajpendo.00237.2011. [DOI] [PubMed] [Google Scholar]


