Abstract
Portal vein glucose sensors detect variations in glycemia to induce a nervous signal that influences food intake and glucose homeostasis. Previous experiments using high infusions of glucose suggested a metabolic sensing involving glucose transporter 2 (GLUT2). Here we evaluated the afferent route for the signal and candidate molecules for detecting low glucose fluxes. Common hepatic branch vagotomy did not abolish the anorectic effect of portal glucose, indicating dorsal transmission. GLUT2-null mice reduced their food intake in response to portal glucose signal initiated by protein-enriched diet. A similar response of Trpm5-null mice and portal infusions of sweeteners also excluded sugar taste receptors. Conversely, infusions of alpha-methylglucose, but not 3-O-methylglucose, decreased food intake, while phlorizin prevented the effect of glucose. This suggested sensing through SGLT3, which was expressed in the portal area.
From these results we propose a finely tuned dual mechanism for portal glucose sensing that responds to different physiological conditions.
Abbreviations: (3-O-MDG), 3-O-methyl-d-glucopyranose; (5-HT), 5-hydroxytryptamin/serotonin; (αMDG), α-methylglucopyranoside; (EGP), endogenous glucose production; (GFAP), glial fibriallary acidic protein; (GLP1), glucagon-like peptide 1; (G6PC), glucose-6-phosphatase catalytic subunit; (GLUT), glucose transporter; (GAPDH), glyceraldehyde-3-phosphate dehydrogenase; (PGP9.5), protein gene product 9.5; (PED), protein-enriched diet; (SED), starch-enriched diet; (SGLT), sodium glucose co-transporter; (Trpm5), transient receptor potential melastin 5
Keywords: Glucose sensing, Portal vein, SGLTs, Peripheral nervous signal, Glucose metabolism, Food intake
1. Introduction
Glucose-sensing units in the portal vein area are implicated in the control of feeding and glucose homeostasis. This hepatoportal glucose sensor is activated by a nonnegative portoarterial gradient [1], which induces a nervous afferent signal through vagal and spinal nerves [2,3] that results in decreased food intake [4,5], appearance of a food preference [6], increased insulin secretion [7] and glucose utilization by various tissues [8,9], depending on experimental conditions. Hepatoportal sensing also contributes to detection of slowly-induced hypoglycemia [10], in which studies suggested that the sensor is localized upstream of the liver, in the portal area and perhaps extending to the mesenteric vein [10,11]. The hepatoportal area is innervated by spinal (dorsal root) and vagal afferents (ventrally through the common hepatic branch and dorsally through the celiac branch, the former being the more abundant [12]). The exact route for the nervous signal initiated by glucose sensing is still unknown, though the ventral vagal pathway has received much interest (e.g. [3]). Interestingly, the multiple effects of portal glucose sensing on food intake and glucose metabolism are suppressed by capsaicin, a neurotoxic agent that destroys all primary afferent nerves (vagal and spinal) [10,13,14] which are small unmyelinated fibers similar to those affected by neuropathy in diabetic or pre-diabetic patients [12,15].
It is known that protein-enriched diets improve overall glucose control, postprandial blood glucose and glycated hemoglobin in people with type 2 diabetes [16]. In recent studies in rats, we linked portal appearance of neosynthetized glucose to the beneficial effects of high-protein diets on glycemia control. Such diets increase satiety [17] and insulin sensitivity of glucose production [18], and induce intestinal gluconeogenesis in the postabsorptive state in animals [14]. We have recently demonstrated that this small postabsorptive flux of glucose into the portal blood (∼15–20% of endogenous glucose production-EGP) and its detection are sufficient to decrease food intake similarly to dietary proteins [14], and are necessary for high-protein diets to induce satiety [14,19].
How small concentrations of portal glucose initiate the nervous signal is still unclear. Early 2000s, Burcelin et al. showed that in fasted mice, the response to a flux equivalent to EGP requires the presence of GLUT2 [20] and an active glucagon-like peptide 1 (GLP1) receptor [21]. These studies suggested a catabolic mechanism for portal glucose detection similar to the paradigm of the pancreatic beta-cell response, i.e. initiated by cellular entry through GLUT2 [22]. To our knowledge, even though GLUT2 is a potential candidate in accordance with its low affinity for glucose [23], no study explored its role in the hepatoportal detection of low glucose. More recently, several studies on intestinal and hypothalamic glucose-detecting cells proposed extracellular sites for detection of even small concentrations of glucose, in addition to metabolic mechanisms. Sodium–glucose co-transporters such as SGLT3 have been implicated in the activation of hypothalamic glucose-excited neurons [24], the enteric secretion of GLP1 by L-cells [25] or serotonin by enterochromaffin cells [26]. Although more controversial [27,28], a role in the glucose-induced secretion of GLP1 was also suggested for the dimeric sweet taste receptor T1R2+T1R3 [29]. In these models, glucose acts as a signal and binds to an extracellular receptor, and initiates depolarization with Na+ entry (for SGLT3 [30]) or through transient receptor potential melastin 5 (Trpm5) channels (for T1Rs [31]).
In this study, we tested whether GLUT2 or these extracellular mechanisms are implicated in the hepatoportal detection of glucose in the conditions induced by high-protein diets (low flux of glucose in the postabsorptive state). As readout for this detection, we used the decrease in food intake observed both in rats and mice [14,19].
2. Materials and methods
Animals were housed on 12 h light/dark cycle, had free access to water, and, unless indicated otherwise, to a standard rodent starch-enriched diet (SED, Harlan, Lyon, France). All procedures were in accordance with the principles and guidelines of the European Convention for the Protection of Laboratory Animals, and approved by the regional animal care committee (CREEA, CNRS Rhône-Alpes-Auvergne, France).
2.1. Food intake on standard and protein-enriched diets
Food intake of male adult control (C57BL/6J) and transgenic (ripglut1;glut2−/− [20] and Trpm5 knock-out [31]) mice (n=4–8 per group) was monitored every day for 2–3 weeks using an 8-chamber Oxymax system (Columbus Instruments). After 5–6 days on SED, mice were switched to a protein-enriched diet (PED), deriving from SED in its starch-glucose/protein ratio (50%/17% vs. 13%/53%, w/w). Both diets were isocaloric (3.3 kcal/g). Proteins in PED were soya protein and casein (50/50). Global and group comparisons were carried out using repeated measures analysis of variance. In each group, daily food intake after diet change was compared to the mean intake for the first days on SED using Student's t-test for paired values. Significance levels were set at 0.05.
2.2. Gene and protein expression analysis
Animals were killed in the postabsorptive state (6 h after food removal). Total RNAs and protein were extracted from frozen tissues with TRIzol reagent (Invitrogen), using glycogen to improve mRNA yield in small samples (mice portal area). Reverse transcription, real-time PCR and Western blots were performed as described previously [18], using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA for normalization, and anti-G6PC, anti-GLUT2 (Chemicon, Temecula, CA), anti-SGLT3 (Interchim, Montluçon, France) antibodies at 1:5000, 1:2500, 1:1000 dilutions, respectively. Table 1 contains primer sequences.
Table 1.
Primer sequences.
| Gene | Forward primer (5′−3′) | Reverse primer (5′−3′) |
|---|---|---|
| GLUT2 | GCTGGAAGAAGCGTATCAGG | AATCCTGATTGCCCAGAATG |
| SGLT3a | TGCTGAAGACGAACCGAAGCAC | ACCAGCAGCAAGGCAAACGA |
| SGLT3b | TCGTACAGCGCTGCTTATGTGGT | ACCGCAGTGCCACACTGTTTCT |
| GAPDH | TTCCAGTATGACTCCACTCACG | AGACTCCACGACATACTCAGCA |
2.3. Portal vein infusions
Adult male Sprague-Dawley rats (Harlan, Lyon, France, n=3–9 per group) were equipped with an indwelling catheter, positioned at the junction of the mesenteric and the pancreaticoduodenal veins, as previously described [14]. To inactivate afferents, during surgery the portal vein area was isolated from surrounding tissues using paraffin films and cotton compresses. A gauze compress moistened with 80 μl of capsaicin (10 mg/ml in water:ethanol:tween20, 8:1:1, vol/vol), was applied around the portal vein for 15 min. For ventral vagotomy, the left and caudal hepatic lobes were gently deflected, and the common hepatic branch, along with the fascia surrounding the nerve, was completely sectioned after mid-level between the liver and the esophagus (see [32] for schematics), with the aid of a binocular microscope. Successful ablation was verified at the end of the experiment for each animal. In preliminary experiments, we were unsuccessful in specifically inactivating afferents of this common hepatic branch using capsaicin, because of diffusion on the nearby portal vein (thereby destroying both vagal and spinal fibers). Rats were allowed to recover from surgery for one week with free access to SED and water.
Infusion experiments were performed as described previously [14]. Briefly, 4 h before light onset, rats were isolated without food (free access to water) and infused with saline or test solutions. Upon light onset, rats were given SED while infusion was maintained. Food intake was measured 3 h later. Each rat was studied at least three times with each solution, infused in a random order. Mean values for each solution were first calculated in each rat. These values were then used to calculate mean values for each solution in each group (saline vs. test) and compared using Student's t-test for paired values. When saline was compared to two or more solutions, global comparison was performed using analysis of variance, followed by multiple paired comparisons with Holm–Bonferroni correction (all significance level at 0.05).
2.4. Immunofluorescence
These procedures were performed as previously described [14,33]. Tissues removed comprised an extended portal vein area (containing mesenteric vein and all hepatic lobes). Before freezing, tissues were embedded and vessels were injected with Tissue-Tek® (Sakura-Finetek, Villeneuve-d'Ascq, France) using custom-made syringes. Coronal sections were analyzed using antibodies indicated in Supplementary Table 1. Staining was absent when primary antibodies were omitted. For double labeling, no cross-reactivity occurred, as assessed by inverting staining order or omitting the second primary antibody. The relative extent of innervation was assessed by counting all visible reactive fibers within randomly selected sample field views (200 μm×200 μm) on 5 different sections of intact or capsaicin-treated portal veins, and values were compared using Student's t-test (significance level at 0.05).
3. Results
3.1. Ventral vagal innervation is not necessary for portal glucose effects on food intake
To evaluate the supposed role of vagal afferents as route for the glucose signal, we performed a surgical ablation of the common hepatic branch of the vagus in rats. This is the major branch of hepatoportal vagal innervation [12,34], and it also innervates parts of the stomach, pyloric sphincter, pancreas and proximal duodenum. We measured food intake in response to either saline (thus allowing control for potential baseline physiological changes) or glucose perfusion in our experimental paradigm (25 μmol/kg/min, in the postabsorptive state). Despite this local vagotomy, portal glucose elicited a similar decrease in food intake (−52%) compared to saline infusions (P=0.01), as observed in intact, non-vagotomized, animals (Figure 1).
Figure 1.
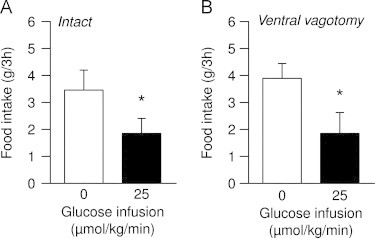
Ventral vagal innervation is not necessary for portal glucose effects on food intake. Effect of infusion of saline (0, white bars) or glucose (25 μmol/kg/min, black bars) on the food intake of SED-fed rats, either (A) non-denervated or (B) vagotomized at the level of the common hepatic branch (n=3–6 per group); data are expressed as means±SEM; *P<0.05 vs. saline.
This strongly suggests that ventral vagal innervation is not essential to convey the nervous signal elicited by glucose detection in the portal vein.
3.2. GLUT2 is present in the portal area but not necessary for the effect of glucose signal on satiety
In order to evaluate implication of GLUT2 in the detection of a low portal flux of glucose that leads to satiety, we first examined its presence in the portal area (comprising the portal vein, the hepatic artery and the common bile duct) from rats and mice. In both species, GLUT2 mRNA and protein were expressed (Figure 2A, B). We used liver-expressed glucose-6-phosphatase (catalytic subunit, G6PC) to control the absence of hepatocytes in the portal samples (Figure. 2B). We then tried to visualize GLUT2 in the portal area using immunohistochemistry, especially in relation to nervous structures, that convey the portal glucose signal [3]. Using glial fibriallary acidic protein (GFAP) and protein gene product 9.5 (PGP9.5) as markers we could observe a dense network of entangled glial and neuronal cells around the portal vein, hepatic artery and bile duct. In the portal (and mesenteric) vein, and contrary to the hepatic artery, glial and neuronal cells were seen ending near the lumen, suggesting potential detection of circulating metabolites (Supplementary Figure 1). However we failed to visualize GLUT2 in the area, despite strong immunodetection in the liver or pancreas. Moreover, using portal injections of 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-d-glucose (2-NBDG), a fluorescent 2-deoxyglucose analog transported by GLUT2 that accumulates in the cell without catabolism, we could not detect candidate cells transporting glucose through GLUT2 in the portal area (despite strong 2-NBDG visualization in the liver).
Figure 2.
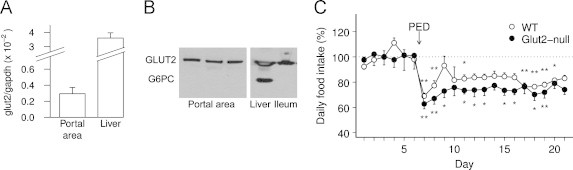
GLUT2 is present in the portal area but not necessary for glucose sensing. A: relative mRNA expression of GLUT2 in the portal area and liver of rats (n=3); data are expressed as means±SEM. B: protein expression of GLUT2 and glucose-6-phosphatase (catalytic unit, G6PC) in the portal area, liver and ileum of mice (representative image; three different animals are represented for portal vein). C: daily food intake of wild-type (n=8, white circles) and GLUT2-null mice (n=6, black circles) fed successively on a standard chow diet (SED, day 1–6) then on a protein-enriched diet (PED, starting day 7); for each animal data are expressed in percentage of its mean daily food intake during the 6 days under SED (reference), and represented as means±SEM for each group; *P<0.05 and **P<0.01 compared to this reference.
Then we functionally investigated implication of GLUT2 in low glucose detection using a transgenic approach and recent results demonstrating that the satiety elicited by protein-enriched diets is caused by intestinal production of glucose in the postabsorptive state and its portal detection [14,19]. After 6 days on standard chow diet (SED, 20% energy from protein), wild-type and GLUT2-null mice were switched to protein-enriched diet (PED, 64% energy from protein) for 2 weeks. As described previously for standard diet [35], GLUT2-null mice had higher mean daily food intake than wild-type (P<0.0005) under SED (6.3 g/d vs. 4.4 g/d), and PED (4.6 g/d vs. 3.6 g/d), with similar body weight (P=0.6 between genotypes). In agreement with previous results on mice and rats [19,36], both groups ate slightly less the first day of diet change and adjusted their intake for 3 days. Then both wild-type and GLUT2-null mice ate daily around 20% less under PED than under SED (−18±3% and −26±4% for WT and GLUT2-null respectively, P=0.09 between genotypes), and maintained this lower food intake throughout the experiment (Figure 2C).
These data demonstrate that though present in the portal area, GLUT2 is not necessary for the effects of PED on food intake that are mediated by portal glucose signal.
3.3. Trpm5 activation is not responsible for the portal glucose signal
To investigate if the activation of Trpm5, a key component of the sweet taste transduction cascade controlled by T1Rs, could be involved in the portal detection of glucose, we infused one of two structurally different sweeteners, acesulfame K and sucralose, through the portal vein of rats in the postabsorptive state at 13 μmol/kg/min and 6.7 μmol/kg/min respectively. These rates were chosen for comparison with infusions of glucose sufficient to elicit satiety (12–25 μmol/kg/min [14]), and taking into account their relative affinity for sugar taste receptors (around twice as high for sucralose vs. acesulfame K [37]). We measured subsequent food intake on standard chow during 3 h. Both sweeteners did not significantly modify food intake compared to saline infusions (Figure 3A, B, P=0.23 for acesulfame K, P=0.25 for sucralose).
Figure 3.
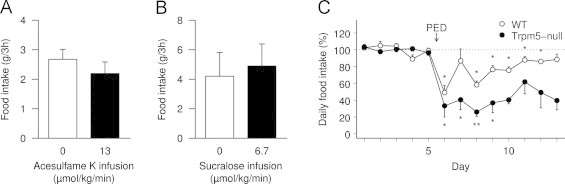
Sugar taste sensing is not responsible for portal glucose sensing. A, B: effect of portal vein infusion of saline (0, white bars), acesulfame K (A, 13 μmol/kg/min, black bar) or sucralose (B, 6.7 μmol/kg/min, black bar) on the food intake of SED-fed rats (n=3–6 per group); data are expressed as means±SEM. C: daily food intake of wild-type (white circles) and Trpm5-null (black circles) mice (n=fed successively on a standard chow diet (SED, day 1–5)) then on a protein-enriched diet (PED, starting day 6); for each animal data are expressed in percentage of its mean daily food intake during 5 days under SED (reference), and represented as means±SEM for each group; *P<0.05 and **P<0.01 compared to this reference.
Then we used Trpm5-null mice in the same paradigm as GLUT2-null mice. Trpm5-null mice are indeed deprived of the cation channel required for a response to sweet taste [31], without significant effect on food intake under SED (4.16 g/d vs. 4.14 g/d for wild-type, P=0.99). As in the experiment with GLUT2-null mice, when adapted to protein-enriched diets, both wild-type and Trpm5-null mice ate less under PED (3.13 g/d and 1.52 g/d, i.e. −17±4% and −54±12% vs. SED for WT and Trpm5-null respectively, P<0.01 between genotypes, Figure 3C).
These data demonstrate that Trpm5 activation, an essential step in the sweet taste transduction cascade, is not responsible for the satiety response to portal glucose.
3.4. Portal glucose sensing exhibits the characteristics of detection through SGLT3
We then explored the hypothesis that portal glucose leads to a nervous signal through binding to SGLT3, as was proposed for hypothalamic neurons [24] and enterochromaffin cells [26].
We first measured food intake in response to portal infusions in the postabsorptive state of the non-metabolizable glucose analog α-methylglucopyranoside (αMDG), a specific substrate of all SGLTs but not of GLUTs. As shown in Figure 4A, portal infusions of αMDG (25 μmol/kg/min) induced a significant 37% decrease in the food intake of rats compared to saline infusions (2.44±0.31 g vs. 3.89±0.41 g, P<0.05).
Figure 4.
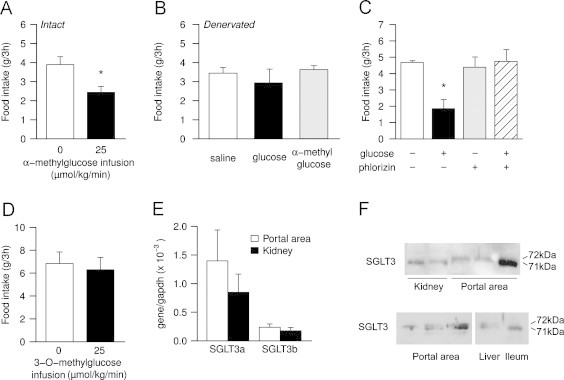
Portal glucose sensor exhibits the characteristics of SGLTs, particularly SGLT3. A: effect of infusion of saline (0, white bar) or α-methylglucose (25 μmol/kg/min, black bar) on the food intake of SED-fed rats (n=9 per group); data are expressed as means±SEM; *P<0.05 vs. saline. B: effect of infusions of saline (white bar), glucose (25 μmol/kg/min, black bar), or α-methylglucose (25 μmol/kg/min, gray bar) in rats with capsaicin-treated portal vein (n=5 per group); data are expressed as means±SEM. C: effect of infusion of saline (white bar), glucose (25 μmol/kg/min, black bar), phlorizin (0.2 μmol/kg/min, gray bar), or phlorizin and glucose (0.2 μmol/kg/min and 25 μmol/kg/min respectively, hatched bar) on the food intake of SED-fed rats (n=4); data are expressed as means±SEM; *P<0.05 vs. saline. D: effect of infusion of saline (0, white bar) or 3-O-methylglucose (25 μmol/kg/min, black bar) on the food intake of SED-fed rats (n=5); data are expressed as means±SEM. E: relative mRNA expression of SGLT3a and SGLT3b in the portal area (white bars) and kidney (black bars) of rats (n=3); data are expressed as means±SEM. F: protein expression of SGLT3 in the portal area, kidney, liver and ileum of mice (representative image; six different animals are represented for portal vein, two different animals for kidney). In each lane, the two spots correspond in weight to the two isoforms SGLT3a and SGLT3b.
To verify that the effect is due to portal detection, since SGLTs are present in different organs throughout the body [38], we treated portal veins of rats with capsaicin, a neurotoxic agent which inactivates weakly myelinated or unmyelinated afferent fibers, and therefore prevents portal glucose signal [14]. To confirm the efficacy of denervation, we observed that the number of neuronal and glial cells in the portal area was substantially decreased (Supplementary Figure 2) in line with the relative abundance of afferents vs. efferents in vagal hepatoportal innervation [34], and that portal infusions of glucose (25 μmol/kg/min) had no effect on food intake (Figure 4B, 2.93±0.73 g vs. 3.44±0.29 g, P=0.82 for global comparison). When receiving portal infusions of αMDG (25 μmol/kg/min), denervated animals did not decrease their food intake (Figure 4B, 3.63±0.20 g), indicating that the satiety elicited by portal infusions of this SGLTs substrate is mediated by a portal nervous signal.
To further evaluate the role of SGLTs in portal glucose detection, we used phlorizin, a competitive SGLT inhibitor. In order to limit adverse effects (e.g. on renal glucose reabsorption), we infused phlorizin at the very low rate of 0.2 μmol/kg/min. When infused alone, we did not observe any significant effect of this inhibitor neither on glycemia (4.65±0.21 mM vs. 5.00±0.1 mM for saline, P=0.33) nor on food intake (Figure 4C, 4.39±0.63 g vs. 4.69±0.10 g for saline, P=0.63 vs. saline). However, when co-infused with glucose (25 μmol/kg/min), phlorizin abolished the decrease in food intake elicited by portal glucose (Figure. 4C, 4.75±0.72 g, P=1 vs. saline).
To specify which SGLT could be responsible for this detection, we used 3-O-methyl-d-glucopyranose (3-O-MDG), which is transported by SGLT1 but does not bind to SGLT3 (nor SGLT4) [39]. In response to portal 3-O-MDG infusions, rats did not significantly change their food intake compared to saline (Figure 4D, 6.30±1.07 g vs. 6.84±1.01 g, P=0.63), suggesting that SGLT1 is not involved in portal glucose signal.
Given the suggested glucosensing role of SGLT3, we assessed its presence in the portal area, using RT-PCR and Western blots on mouse samples (same samples as in Figure. 2B, i.e. controlled for contamination by hepatocytes). Both SGLT3 mRNA (Figure 4E) and protein (Figure 4F) were identified in the portal area of each animal tested. Western blots revealed two spots corresponding to the two isoforms SGLT3a (71 kDa) and SGLT3b (72 kDa).
Altogether these data strongly suggest that portal detection of a small flux of glucose (equivalent to intestinal glucose production in response to protein-enriched diets) could be mediated by SGLTs, particularly by SGLT3.
4. Discussion
In the context of decreased food intake elicited by portal glucose sensing, our data strongly suggest that under the conditions of a small glucose flux, such as that contributed by intestinal gluconeogenesis elicited by protein-enriched diets, portal glucose detection occurs independently of GLUT2, and is mediated by SGLTs, possibly SGLT3, to eventually induce a dorsally transmitted nervous signal.
Our denervation experiment clearly shows that the ventral vagal pathway is not essential for the transmission of the hepatoportal signal under our postabsorptive conditions. Solely based on our present results, we cannot conclude on the respective importance of the two dorsal pathways, either vagal through the celiac branch or spinal through dorsal root afferents. The spinal route seems more plausible, since celiac branch has minor contribution to innervation, and dorsal root afferents were shown to convey the influence of portal glucose on the electrical activity of neurons in the lateral hypothalamus [40]. Although the contribution of the dorsal vagus remains to be fully investigated, the implication of spinal afferents in the transmission of the hepatoportal glucose signal and its effects has been thus far mainly disregarded, and deserves to be given a fresh impetus.
Transgenic mice helped us establish that GLUT2 is not necessary for the satiety elicited by portal glucose sensing in these conditions of a small glucose flux (12–25 μmol/kg/min, around 20% of endogenous glucose production in the postabsorptive state [14]). We then tested other sensing mechanisms, focusing on extracellular detection through which glucose can act rather as a signaling molecule than as a nutrient per se. T1Rs could be involved in glucose sensing in intestinal L-cells [29]. Despite their ability to induce much higher responses from sweet taste receptors than glucose [37], the sweeteners acesulfame K and sucralose did not elicit satiety when infused in the portal vein. We confirmed that portal glucose sensing does not require the activation of the sweet taste transduction cascade using transgenic mice devoid of Trpm5, an essential cation channel for taste signaling pathway. These mice were surprisingly even more sensitive to protein-enriched diet, perhaps suggesting a partially compensating role for Trpm5 in wild-type mice linked to its implication in the gustatory detection of sweet and aminoacid tastes [31].
Our in vivo experiments strongly suggest that portal glucose could be sensed by SGLTs, particularly SGLT3, to elicit satiety. Food intake was decreased by low portal concentrations of both glucose and αMDG, and glucose-induced satiety was abolished by phlorizin, indicating detection of glucose by SGLTs. The absence of response to 3-O-MDG, and its expression in the portal area pointed out SGLT3 as a very likely candidate, even though we cannot exclude a potential role of other members in the SLC5 family with these sugar responses (e.g. SGLT4, also expressed in the intestine). SGLT3 is particularly plausible since previous results showed that in the SGLT family, SGLT3 is expressed in enteric neurons (in humans [30]) and rather acts as sugar sensor responding to small variations in glucose concentration. In vitro experiments showed indeed that both human [30] and mouse [41] proteins display electrochemical characteristics compatible with sensing of physiological concentrations of glucose. Accordingly, physiological studies on rodents suggested that SGLT3 is involved in glucose sensing by hypothalamic neurons and enterochromaffin cells in the intestinal mucosa [24,26]. Despite our observations of an extensive innervation network (neuronal and glial cells) in the portal area, and more specifically in inner layers of the portal vein membrane (Supplementary Figure 1), we could not visualize cells bearing SGLT3, so we cannot conclude whether glucose sensing through SGLTs occurs directly on portal afferents or on intermediate (e.g. glial or neurotransmitter-releasing) cells.
In our present experiments, GLUT2 is not required for the effects of PED on food intake that are conveyed by portal glucose sensing [14,19]. Previous studies had proposed metabolic mechanisms for this sensing (e.g. for hypoglycaemic detection [42]), and showed that GLUT2 is necessary for the effects of a glucose load into the portal vein [20]. The simplest explanation for this discrepancy certainly lies in the very different experimental conditions, and more specifically in the variations of portal glucose concentration. In our study, sugars are perfused at a very low rate while animals are in the postabsorptive state (25 μmol/kg/min in 250–270 g rats, whose mean portal blood flow is around 16–20 mL/min [43,44], i.e. a rate equivalent to a <0.4 mM increase in portal glycemia). These conditions mimic natural intestinal gluconeogenesis (occurring notably under high protein diet), whereby neosynthetized glucose compensates and eventually delays the onset of a small negative gradient between portal vein and hepatic artery glycemia [14]. In studies by Burcelin et al. [20] a much higher load of glucose was infused in animals fasting for 6 h (2 μL/min of a 330 g/L solution in mice whose mean portal blood flow is around 2 mL/min [45], i.e. an approximate 1.8 mM increase in portal glycemia). Under these physiological conditions a larger negative gradient exists between portal and arterial glycemia at the time of infusion, and is much more than compensated by the glucose load. We propose that the sensitive portal glucose sensor may rely on different mechanisms (an extracellular, energy-sparing, detection through SGLT3 and/or catabolism via entry through GLUT2) depending on the variations of glucose gradient in the portal area. Such a multimodal detection was proposed for glucosensing hypothalamic neurons, with different molecular mechanisms implicated in the response to small (from 0.5 to 2.5 mM) or high (from 5 to 20 mM) changes in glucose concentrations [46]. Both catabolic [47] and extracellular detection mechanisms [48] have been proposed within the subcategory of neurons inhibited by small changes in glucose. Based on our results, we propose that, to induce satiety, portal glucose sensor acts preferably through SGLT3 for “small” (e.g. <1 mM) variations in portal-arterial glucose gradient, and through GLUT2 for “large” (e.g. >1 mM) gradient changes. For the latter, the small electrogenic response through SGLT3 would indeed be inhibited by background currents caused by glucose metabolism (following entry through GLUT2), as suggested by previous results in neurons [24] and L-cells [49].
In conclusion, our findings strongly suggest that in conditions of a small flux of glucose in the portal vein (occurring naturally for instance under a high protein diet as a consequence of intestinal gluconeogenesis), satiety elicited by portal glucose sensing is mediated not by GLUT2 but by an extracellular detection through SGLT, possibly SGLT3, that eventually initiates a dorsal nervous signal. We propose that these two mechanisms operate predominantly for large (>∼1 mM) or small (<∼1 mM) variations in porto-arterial glucose gradient, respectively. This enables a fine tuning for the portal glucose signal and its effect on food intake and glucose metabolism. Future studies are now required to fully precise this hypothesis and the nature of the cells and downstream pathways converting these detection mechanisms into a nervous signal.
Conflict of interest
No potential conflict of interest relevant to this article is reported.
Acknowledgments
This work was supported by the INSERM. Our positions were funded by the “Ministère de l′Alimentation, de l′Agriculture et de la Pêche” (F.D.), the CNRS (G.M.), and the “Agence Nationale de Recherche” (grant ANR1.14-NutriSens (A.D.)), the INSERM (C.Z.), the “Ministère de la Recherche et de l′Enseignement Supérieur” (C.D.). B.T. received support from the Swiss National Science Foundation (Grant no. 3100A0-113525).
The authors wish to thank to C.S. Zuker (University of California, San Diego, La Jolla, CA) for providing breeding pairs of Trpm5-null mice, and acknowledge the technical assistance of Hideo Akaoka (U842 INSERM-Université Lyon 1, Lyon, France) for immunofluorescence experiments.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at 10.1016/j.molmet.2012.11.003.
Appendix A. Supplementary materials
Supplementary Material
Figure S1.
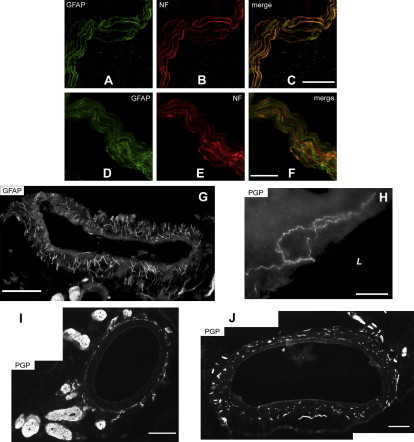
Innervation of rat portal vein (A–H), hepatic artery (I) and common bile duct (J). A–F: confocal microscopy revealed entanglement of neuronal (visualized with 200 kD neurofilament H, NF, in red) and glial fibers (visualized with glial fibriallary acidic protein, GFAP, in green). This entanglement is not detectable at lower magnification, where neuronal and glial proteins appear colocalized. G–J: immunofluorescence of glial fibrillary acidic protein (GFAP) and protein gene product (PGP) 9.5. Panels G, I and J have been composed by juxtaposition of adjacent photographs taken on the same sample. The image on panel H is a magnification of the wall of the portal vein. L: lumen of the portal vein. Scale bars: A–C: 20 μm, D–F: 10 μm, G: 200 μm, H: 50 μm, I,J: 100 μm.
Figure S2.
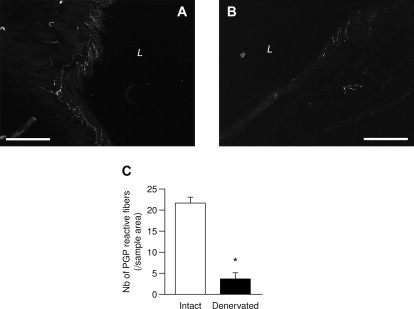
Innervation of portal vein without (A) or after (B) treatment by capsaicin. Immunofluorescence of protein gene product (PGP) 9.5. L: lumen of the portal vein. Scale bar=200 μm. C: number of PGP reactive fibers per samble area (200 μm×200 μm) in intact (white bar) or capsaicin-treated portal vein (black bar) expressed as means±SEM; *P<0.0005 vs. intact
References
- 1.Gardemann A., Strulik H., Jungermann K. A portal-arterial glucose concentration gradient as a signal for an insulin-dependent net glucose uptake in perfused rat liver. FEBS Letters. 1986;202:255–259. doi: 10.1016/0014-5793(86)80697-4. [DOI] [PubMed] [Google Scholar]
- 2.Niijima A. Afferent impulse discharges from glucoreceptors in the liver of the guinea pig. Annals of the New York Academy of Sciences. 1969;157:690–700. doi: 10.1111/j.1749-6632.1969.tb12914.x. [DOI] [PubMed] [Google Scholar]
- 3.Niijima A. Glucose-sensitive afferent nerve fibers in the liver and their role in food intake and blood glucose regulation. Journal of the Autonomic Nervous System. 1983;9:207–220. doi: 10.1016/0165-1838(83)90142-x. [DOI] [PubMed] [Google Scholar]
- 4.Russek M. Demonstration of the influence of a hepatic glucosensitive mechanism on food-intake. Physiology and Behavior. 1970;5:1207–1209. doi: 10.1016/0031-9384(70)90218-0. [DOI] [PubMed] [Google Scholar]
- 5.Yin T.H., Tsai W.H., Barone F.C., Wayner M.J. Effects of continuous intramesenteric infusion of glucose and amino acids on food intake in rats. Physiology and Behavior. 1979;22:1207–1210. doi: 10.1016/0031-9384(79)90278-6. [DOI] [PubMed] [Google Scholar]
- 6.Tordoff M.G., Friedman M.I. Hepatic portal glucose infusions decrease food intake and increase food preference. American Journal of Physiology. 1986;251:R192–R196. doi: 10.1152/ajpregu.1986.251.1.R192. [DOI] [PubMed] [Google Scholar]
- 7.Fukaya M., Mizuno A., Arai H., Muto K., Uebanso T., Matsuo K. Mechanism of rapid-phase insulin response to elevation of portal glucose concentration. American Journal of Physiology—Endocrinology and Metabolism. 2007;293:E515–E522. doi: 10.1152/ajpendo.00536.2006. [DOI] [PubMed] [Google Scholar]
- 8.Adkins B.A., Myers S.R., Hendrick G.K., Stevenson R.W., Williams P.E., Cherrington A.D. Importance of the route of intravenous glucose delivery to hepatic glucose balance in the conscious dog. Journal of Clinical Investigation. 1987;79:557–565. doi: 10.1172/JCI112847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore M.C., Hsieh P.S., Neal D.W., Cherrington A.D. Nonhepatic response to portal glucose delivery in conscious dogs. American Journal of Physiology—Endocrinology and Metabolism. 2000;279:E1271–E1277. doi: 10.1152/ajpendo.2000.279.6.E1271. [DOI] [PubMed] [Google Scholar]
- 10.Saberi M., Bohland M., Donovan C.M. The locus for hypoglycemic detection shifts with the rate of fall in glycemia: the role of portal-superior mesenteric vein glucose sensing. Diabetes. 2008;57:1380–1386. doi: 10.2337/db07-1528. [DOI] [PubMed] [Google Scholar]
- 11.Hevener A.L., Bergman R.N., Donovan C.M. Hypoglycemic detection does not occur in the hepatic artery or liver: findings consistent with a portal vein glucosensor locus. Diabetes. 2001;50:399–403. doi: 10.2337/diabetes.50.2.399. [DOI] [PubMed] [Google Scholar]
- 12.Berthoud H.R. Anatomy and function of sensory hepatic nerves. Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology. 2004;280:827–835. doi: 10.1002/ar.a.20088. [DOI] [PubMed] [Google Scholar]
- 13.Dicostanzo C.A., Dardevet D.P., Neal D.W., Lautz M., Allen E., Snead W. Role of the hepatic sympathetic nerves in the regulation of net hepatic glucose uptake and the mediation of the portal glucose signal. American Journal of Physiology—Endocrinology and Metabolism. 2006;290:E9–E16. doi: 10.1152/ajpendo.00184.2005. [DOI] [PubMed] [Google Scholar]
- 14.Mithieux G., Misery P., Magnan C., Pillot B., Gautier-Stein A., Bernard C. Portal sensing of intestinal gluconeogenesis is a mechanistic link in the diminution of food intake induced by diet protein. Cell Metabolism. 2005;2:321–329. doi: 10.1016/j.cmet.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan S.T., Rayman G. The LDIflare: a novel test of C-fiber function demonstrates early neuropathy in type 2 diabetes. Diabetes Care. 2004;27:2930–2935. doi: 10.2337/diacare.27.12.2930. [DOI] [PubMed] [Google Scholar]
- 16.Gannon M.C., Nuttall F.Q., Saeed A., Jordan K., Hoover H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. American Journal of Clinical Nutrition. 2003;78:734–741. doi: 10.1093/ajcn/78.4.734. [DOI] [PubMed] [Google Scholar]
- 17.Barkeling B., Rossner S., Bjorvell H. Effects of a high-protein meal (meat) and a high-carbohydrate meal (vegetarian) on satiety measured by automated computerized monitoring of subsequent food intake, motivation to eat and food preferences. International Journal of Obesity. 1990;14:743–751. [PubMed] [Google Scholar]
- 18.Pillot B., Soty M., Gautier-Stein A., Zitoun C., Mithieux G. Protein feeding promotes redistribution of endogenous glucose production to the kidney and potentiates its suppression by insulin. Endocrinology. 2009;150:616–624. doi: 10.1210/en.2008-0601. [DOI] [PubMed] [Google Scholar]
- 19.Penhoat, A., Mutel, E., Correig, M.A., Pillot, B., Stefanutti, A., Rajas, F., et al. Protein-induced satiety is abolished in the absence of intestinal gluconeogenesis. Physiology and Behavior 2011. [DOI] [PubMed]
- 20.Burcelin R., Dolci W., Thorens B. Glucose sensing by the hepatoportal sensor is GLUT2-dependent: in vivo analysis in GLUT2-null mice. Diabetes. 2000;49:1643–1648. doi: 10.2337/diabetes.49.10.1643. [DOI] [PubMed] [Google Scholar]
- 21.Burcelin R., Da Costa A., Drucker D., Thorens B. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes. 2001;50:1720–1728. doi: 10.2337/diabetes.50.8.1720. [DOI] [PubMed] [Google Scholar]
- 22.Schuit F.C., Huypens P., Heimberg H., Pipeleers D.G. Glucose sensing in pancreatic beta-cells: a model for the study of other glucose-regulated cells in gut, pancreas, and hypothalamus. Diabetes. 2001;50:1–11. doi: 10.2337/diabetes.50.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Thorens B., Charron M.J., Lodish H.F. Molecular physiology of glucose transporters. Diabetes Care. 1990;13:209–218. doi: 10.2337/diacare.13.3.209. [DOI] [PubMed] [Google Scholar]
- 24.O'Malley D., Reimann F., Simpson A.K., Gribble F.M. Sodium-coupled glucose cotransporters contribute to hypothalamic glucose sensing. Diabetes. 2006;55:3381–3386. doi: 10.2337/db06-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gribble F.M., Williams L., Simpson A.K., Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003;52:1147–1154. doi: 10.2337/diabetes.52.5.1147. [DOI] [PubMed] [Google Scholar]
- 26.Freeman S.L., Bohan D., Darcel N., Raybould H.E. Luminal glucose sensing in the rat intestine has characteristics of a sodium–glucose cotransporter. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2006;291:G439–G445. doi: 10.1152/ajpgi.00079.2006. [DOI] [PubMed] [Google Scholar]
- 27.Reimann F., Habib A.M., Tolhurst G., Parker H.E., Rogers G.J., Gribble F.M. Glucose sensing in L cells: a primary cell study. Cell Metabolism. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujita Y., Wideman R.D., Speck M., Asadi A., King D.S., Webber T.D. Incretin release from gut is acutely enhanced by sugar but not by sweeteners in vivo. American Journal of Physiology—Endocrinology and Metabolism. 2009;296:E473–E479. doi: 10.1152/ajpendo.90636.2008. [DOI] [PubMed] [Google Scholar]
- 29.Jang H.J., Kokrashvili Z., Theodorakis M.J., Carlson O.D., Kim B.J., Zhou J. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. National Academy of Sciences of the United States of America. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diez-Sampedro A., Hirayama B.A., Osswald C., Gorboulev V., Baumgarten K., Volk C. A glucose sensor hiding in a family of transporters. National Academy of Sciences of the United States of America. 2003;100:11753–11758. doi: 10.1073/pnas.1733027100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y., Hoon M.A., Chandrashekar J., Mueller K.L., Cook B., Wu D. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 32.Berthoud H.R., Kressel M., Neuhuber W.L. An anterograde tracing study of the vagal innervation of rat liver, portal vein and biliary system. Anatomy and Embryology (Berlin) 1992;186:431–442. doi: 10.1007/BF00185458. [DOI] [PubMed] [Google Scholar]
- 33.Duraffourd C., De Vadder F., Goncalves D., Delaere F., Penhoat A., Brusset B. Mu-opioid receptors and dietary protein stimulate a gut-brain neural circuitry limiting food intake. Cell. 2012;150:377–388. doi: 10.1016/j.cell.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 34.Berthoud H.R., Neuhuber W.L. Functional and chemical anatomy of the afferent vagal system. Autonomic Neuroscience. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 35.Bady I., Marty N., Dallaporta M., Emery M., Gyger J., Tarussio D. Evidence from glut2-null mice that glucose is a critical physiological regulator of feeding. Diabetes. 2006;55:988–995. doi: 10.2337/diabetes.55.04.06.db05-1386. [DOI] [PubMed] [Google Scholar]
- 36.Jean C., Rome S., Mathe V., Huneau J.F., Aattouri N., Fromentin G. Metabolic evidence for adaptation to a high protein diet in rats. Journal of Nutrition. 2001;131:91–98. doi: 10.1093/jn/131.1.91. [DOI] [PubMed] [Google Scholar]
- 37.Li X., Staszewski L., Xu H., Durick K., Zoller M., Adler E. Human receptors for sweet and umami taste. National Academy of Sciences of the United States of America. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright E.M., Loo D.D., Hirayama B.A. Biology of human sodium glucose transporters. Physiological Reviews. 2011;91:733–794. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 39.Diez-Sampedro A., Lostao M.P., Wright E.M., Hirayama B.A. Glycoside binding and translocation in Na(+)-dependent glucose cotransporters: comparison of SGLT1 and SGLT3. Journal of Membrane Biology. 2000;176:111–117. doi: 10.1007/s00232001081. [DOI] [PubMed] [Google Scholar]
- 40.Schmitt M. Influences of hepatic portal receptors on hypothalamic feeding and satiety centers. American Journal of Physiology. 1973;225:1089–1095. doi: 10.1152/ajplegacy.1973.225.5.1089. [DOI] [PubMed] [Google Scholar]
- 41.Aljure O., Diez-Sampedro A. Functional characterization of mouse sodium/glucose transporter type 3b. American Journal of Physiology—Cell Physiology. 2010;299:C58–C65. doi: 10.1152/ajpcell.00030.2010. [DOI] [PubMed] [Google Scholar]
- 42.Matveyenko A.V., Donovan C.M. Metabolic sensors mediate hypoglycemic detection at the portal vein. Diabetes. 2006;55:1276–1282. doi: 10.2337/db05-1665. [DOI] [PubMed] [Google Scholar]
- 43.Transonic Systems Inc. Portal venous blood flow measurement in the rat, chronic. Rat WB 4th ed.1996.
- 44.Lee H.B., Blaufox M.D. Blood volume in the rat. Journal of Nuclear Medicine. 1985;26:72–76. [PubMed] [Google Scholar]
- 45.Troy S., Soty M., Ribeiro L., Laval L., Migrenne S., Fioramonti X. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metabolism. 2008;8:201–211. doi: 10.1016/j.cmet.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Penicaud L., Leloup C., Fioramonti X., Lorsignol A., Benani A. Brain glucose sensing: a subtle mechanism. Current Opinion in Clinical Nutrition and Metabolic Care. 2006;9:458–462. doi: 10.1097/01.mco.0000232908.84483.e0. [DOI] [PubMed] [Google Scholar]
- 47.Kang L., Dunn-Meynell A.A., Routh V.H., Gaspers L.D., Nagata Y., Nishimura T. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes. 2006;55:412–420. doi: 10.2337/diabetes.55.02.06.db05-1229. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez J.A., Jensen L.T., Fugger L., Burdakov D. Metabolism-independent sugar sensing in central orexin neurons. Diabetes. 2008;57:2569–2576. doi: 10.2337/db08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tolhurst G., Reimann F., Gribble F.M. Nutritional regulation of glucagon-like peptide-1 secretion. Journal of Physiology. 2009;587:27–32. doi: 10.1113/jphysiol.2008.164012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


