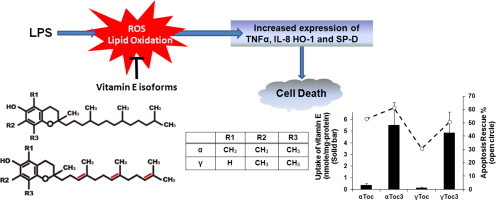
Abstract
Lipopolysaccharide (LPS) induces host inflammatory responses and tissue injury and has been implicated in the pathogenesis of various age-related diseases such as acute respiratory distress syndrome, vascular diseases, and periodontal disease. Antioxidants, particularly vitamin E, have been shown to suppress oxidative stress induced by LPS, but the previous studies with different vitamin E isoforms gave inconsistent results. In the present study, the protective effects of α- and γ-tocopherols and α- and γ-tocotrienols on the oxidative stress induced by LPS against human lung carcinoma A549 cells were studied. They suppressed intracellular reactive oxygen formation, lipid peroxidation, induction of inflammatory mediator cytokines, and cell death. Tocopherols were incorporated into cultured cells much slower than tocotrienols but could suppress LPS-induced oxidative stress at much lower intracellular concentration than tocotrienols. Considering the bioavailability, it was concluded that α-tocopherol may exhibit the highest protective capacity among the vitamin E isoforms against LPS-induced oxidative stress.
Abbreviations: DCFH, Dichlorofluorescein; DPPP, Diphenyl-1-pyrenylphosphine; LPS, Lipopolysaccharide; MTT, 3-[4,5-dimethylthiazol-2-yl]2,5-dipheyltetrazolium bromide; NF-κB, Nuclear factor-kappaB; ROS, Reactive oxygen species; SP-D, Pulmonary surfactant protein D; Toc, Tocopherol; Toc3, Tocotrienol; TNF-α, Tumor necrosis factor α
Keywords: Lipid peroxidation, Lipopolysaccharide (LPS), Oxidative stress, Tocopherol, Tocotrienol, Vitamin E
Graphical abstract
Highlights
► Lipopolysaccharide (LPS) induces oxidative stress in cultured cells. ► Protective effect of four vitamin E isoforms against LPS cytotoxicity was studied. ► α- and γ-tocopherols and tocotrienols suppressed oxidative damage and cell death. ► Tocotorienols were incorporated into cells much faster than tocopherols. ► α-Tocopherol may exert the highest capacity to inhibit LPS-induced cytotoxicity.
Introduction
Lipopolysaccharide (LPS), a highly conserved cell wall component of gram-negative bacteria, is known to initiate signaling cascade for inflammatory mediator expression including cytokines such as tumor necrosis factor α (TNF-α) and interleukin (IL)-6, inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX2), and nuclear factor-kappaB (NF-κB) [1]. It is accepted that dysregulated inflammatory responses result in various age-related diseases including acute respiratory distress syndrome (ARDS), neurodegenerative and vascular diseases [2] and also periodontal disease [3,4]. Oxidative stress mediated by reactive oxygen/nitrogen species (ROS/RNS) has been implicated in the pathogenesis induced by LPS which accelerates the formation of nitric oxide (NO) [5] and prostaglandin E2 (PGE2) [6].
Consequently, the effects of antioxidants against LPS-induced oxidative stress have been studied extensively. Notably, the role of vitamin E against deleterious effects of LPS has been the subject of many in vitro and in vivo studies. In general, it has been observed that vitamin E suppresses inflammatory responses and oxidative damage induced by LPS in both cell culture systems and animal experiments [7–11]. Vitamin E is composed of eight isoforms: α, β, γ, and δ-tocopherols and α, β, γ, and δ-tocotrienols. Tocopherols contain a saturated phytyl side chain, while tocotrienols contain three double bonds in the side chain, and α, β, γ, and δ-forms differ in the number and position of methyl groups on the chromanol ring [12]. It was reported that α-tocopherol (αToc) effectively prevented interferon-gamma/LPS-induced dopaminergic neuron degeneration [13] and that αToc decreased LPS-induced lipid peroxidation and IL-6 in murine microglia and brain [14]. In a rat model of acute lung inflammation caused by intratracheally administered LPS, aerosol-administered αToc attenuated lung inflammation [15]. Furthermore, αToc was found to promote recovery from LPS-induced infection in aged mice [16], and a low embryo implantation rate due to systemic administration of LPS was partially reversed or prevented by αToc [17].
The relative preventive capacity of different vitamin E isoforms against LPS-induced inflammation and oxidative stress has been the subjects of many studies. It was found that γToc inhibited proinflammatory PGE2 and leukotriene B4 (LTB4) production, decreased TNF-α, and attenuated inflammation-mediated damage more effectively than αToc [18,19]. Further, a combination of aspirin and γToc, but not a combination of aspirin and αToc, exerted some advantage over aspirin alone in terms of anti-inflammatory effects and attenuation of aspirin-induced adverse effects [20,21]. In a study on the effects of αToc or γToc against LPS-triggered non-alcoholic steatohepatitis (NASH) in an obese (ob/ob) mouse model, the intraperitonial injection of LPS increased serum alanine aminotransferase (ALT) and both αToc and γToc decreased this response and also hepatic malondialdehyde (MDA) and TNF-α production [22].
Tocotrienols (Toc3) have received much attention recently because of a potential biological function distinct from tocopherols [23]. The tocotrienol-rich fraction of palm oil was found to inhibit induction of iNOS and COX2 expression and protect human monocytic THP-1 cells from LPS-induced cell death [24]. α, γ, or δToc3 inhibited TNF-α secretion in LPS-stimulated RAW 264.7 cells and reduced serum levels of TNF-α after LPS treatment in BALB/c mice [11]. It was reported that low concentrations of δToc3 (<20 μM) blocked LPS-induced gene expression of TNF-α, IL-1β, IL-6 and iNOS, while higher concentrations (40 μM) increased gene expression of the latter in peritoneal macrophages as compared to control group [11]. Further, αToc3, γToc3 and δToc3 suppressed LPS-induced NO production in microglia, δToc3 being the most potent [25].
Thus, previous studies indicate contradictory outcomes for the anti-inflammatory effects of vitamin E isoforms [26]. One of the reasons may be ascribed to a marked difference in the rates of incorporation into cultured cells [27] and bioavailability of vitamin E isoforms [28]. Tocotrienols are incorporated into cultured cells much faster than the corresponding tocopherols [27,29,30], which makes the apparent efficacy of tocotrienols larger than tocopherols. This also suggests that the capacity of antioxidants should be assessed based upon the actual intracellular concentrations rather than those added into the cell culture medium. In most previous studies, intracellular concentrations of antioxidants added into the culture medium were not measured. The present study was performed aiming at elucidating the preventive effects of four kinds of vitamin E isoforms, αToc, γToc, αToc3, and γToc3, against the oxidative stress and cytotoxicity induced by LPS using human lung carcinoma A549 cultured cells. The intracellular concentrations of four vitamin E isoforms were measured and considered in assessment of their preventive effects.
Materials and methods
Materials
Dulbecco's modified eagle medium (DMEM) was obtained from Gibco Invitrogen Corporation, (Grand Island, NY), fetal bovine serum from JRH Biosciences (Lenexa, KS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) from Nacalai Tesque (Kyoto, Japan), LPS (serotype Escherichia coli O55: B5; phenol extracted) and dichlorofluorescein diacetate (DCFH-DA) from Sigma-Aldrich (St. Louis, MO), and diphenyl-1-pyrenylphosphine (DPPP) from Dojindo (Kumamoto, Japan). The natural isoforms of vitamin E were kindly supplied by Eisai Co. Ltd. (Tokyo, Japan). Other chemicals were of the highest quality commercially available.
Cell culture and determination of cell viability
Human lung carcinoma A549 cells, used as a model of lung tissue cells considering the effect of LPS in ARDS, were purchased from the Riken BioResource Center (Tsukuba, Japan). Cells were routinely cultured in DMEM containing 10% heat-inactivated fetal bovine serum (FBS; CELLect GOLD; MP Biomedicals Incorporated, Solon, OH), 100 units/mL of penicillin, 100 μg/mL of streptomycin, and 250 ng/mL of amphotericin B (Nacalai Tesque Inc., Kyoto, Japan).
Cells were incubated in humidified atmosphere of 5% carbon dioxide and 95% air at 37 °C. In order to assess the effect of vitamin E isoforms, cells were treated with Toc and Toc3 at different concentrations for 24 h, and then LPS was added to the medium.
The cell viability was measured by three assays, lactate dehydrogenase (LDH) release assay, MTT assay, and Annexin V/PI double staining assay. LDH release assay was conducted along the protocol outlined by the manufacture for the Cytotoxic Detection Kit (Roche Diagnostics, Penzberg, Germany). Maximum LDH release was determined by the incubation of cells with 1% Triton X-100. Data are expressed as a percentage of maximum LDH release, after subtraction of background determined from serum medium alone [27].
To determine mitochondrial function, which is another indicator of cell viability, the MTT assay was conducted as described previously [27]. Briefly, cells were incubated with 0.5 mg/ml MTT solution in fresh medium at 37 °C for 2 h. Isopropyl alcohol containing 0.04 N HCl was added to the culture medium (3:2, v/v), and they were mixed by pipette until the formazan was completely dissolved. The optical density of formazan was measured at 570 nm using a Multiskan Ascent plate reader (Thermo Labsystems, Helsinki, Finland).
The apoptotic cell death was measured from the extent of phosphatidylserine (PS) exposure [31], which was measured by the binding of annexin V-FITC according to the protocol outlined by the manufacture in the Apoptosis Detection-kit (Medical & Biological Laboratories). The treated cells were stained with annexin V-FITC and propidium iodide (PI), followed by analysis with a Cytomics FC500 Flow Cytometry System (Beckman Coulter, Inc., Maiami, FL) with a 488 nm argon laser. Data were collected from 10,000 events.
Determination of vitamin E uptake into cultured cells
Intracellular vitamin E was detected using HPLC systems with electrochemical detection as described previously [27]. Cell samples in PBS were mixed with chloroform/methanol (2:1, v/v), and then cellular vitamin E in the lower chloroform layer was analyzed with an HPLC using a post-column amperometric electrochemical detector (NANOSPACE SI-1, Shiseido, Japan) set at 800 mV combined with an octadecyl-bounded silica column (LC18, 5 μm, 250×4.6 mm2, Sigma-Aldrich, Japan, Co.); methanol-tert-butyl alcohol (95:5, v/v) containing 50 mM sodium perchlorate was used as an eluent at a rate of 0.7 ml/min.
Measurement of intracellular oxidative stress
Intracellular ROS were detected using 2′,7′-dichlorofluorescein diacetate (DCFH-DA; Sigma-Aldrich, St. Louis, MO). DCFH-DA was dissolved in dimethyl sulfoxide (DMSO) at 5 mM as a stock solution and stored at −20 °C. When used in an experiment, it was diluted 500 times with serum-free medium. After exposure of the cells to LPS for 6 h, the medium was changed to serum-free DMEM containing 10 μM DCFH-DA and incubated for 30 min at 37 °C. Cells were then washed once with PBS, collected by 0.25% trypsinization, washed once with PBS, and resuspended in 500 μl of PBS. Cell samples in PBS were excited with a 488-nm argon ion laser in a Cytomics FC500 flow cytometry system (Beckman Coulter, Inc., Brea, CA), and the DCF emission was measured at 525 nm. Data were collected from at least 5000 gated events.
Intracellular lipid hydroperoxides were measured using DPPP [32]. DPPP was dissolved in DMSO at 5 mM as a stock solution and stored at −20 °C. When used in an experiment, it was diluted 100-times with serum-free medium. After exposure of the cells to LPS for 6 h, the medium was changed to serum-free DMEM containing 50 μM DPPP and incubated for 30 min at 37 °C. Cells were then washed once with PBS, harvested by trypsinization, washed once with PBS, and re-suspended in 3 ml of PBS. Cell samples in PBS were excited with a 351-nm argon ion laser and the fluorescence emission of DPPP oxide was measured at 380 nm using an FR-5300PC spectrophotometer (Shimadzu Corporation, Kyoto, Japan). After measurement, the cells were collected and measured for protein concentration. DPPP oxide level was adjusted by cellular protein concentration.
Protein extraction and Western blot analysis
To obtain total cell extracts, treated cells were collected, washed with ice-cold PBS, and resuspended in lysis buffer (150 mM NaCl, 50 mM Tris–HCl, pH 7.4, 50 mM NaF, 5 mM ethylenediamine tetraacetic acid (EDTA), 0.5% Triton X-100, and 1 mM NasVO4 with a protease inhibitor cocktail tablet (Roche Diagnostics, Penzberg, Germany) at 4 °C for 30 min. Nuclei and unlysated cellular debris were removed by centrifugation at 15,000g for 5 min. Equal amounts of protein (from 10 to 50 μg) were loaded and separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). In all cases, the detection of specific proteins in the total cell extracts was carried out by Western blot analysis, following the previously described procedure [27].
Real time polymerase chain reaction
The expression of target genes was determined using Real Time polymerase chain reaction (PCR) as described previously [33]. Total RNA was isolated from cells using the RNeasy mini kit (Qiagen GmbH, Hilden, Germany). cDNA synthesis was carried out with a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA). Real Time-PCR system was conducted using a Step One Real Time-PCR system (Applied Biosystems), and PCR amplification was detected with power SYBR Green PCR master mix (Applied Biosystems). Human ribosomal protein L32 (RiboL32) was used as an endogenous control. The primers for amplification were: RiboL32, 5′-GTA ACT GGC GGA AAC CCA-3′ (forward), 5′-AGA TCT GGC CCT TGA ATC TTC-3′ (reverse); TNF-α, 5′-TGC TCC TCA CCC ACA CCA T-3′ (forward), 5′-GGA GGT TGA CCT TGG TCT GGT A-3′ (reverse); IL-8, 5′-AAA CCA CCG GAA GGA ACC A-3′ (forward), 5′-CAC GGC CAG CTT GGA AGT-3′ (reverse); HO-1, 5′-GGG TGA TAG AAG AGG CCA AGA CT-3′ (forward), 5′-AGC TCC TGC AAC TCC TCA AAG A -3′ (reverse).
Statistical analysis
All results were expressed as mean±SD of at least three independent experiments. The statistical significance of difference between determinations was calculated by Student's t-test and an analysis of variance (ANOVA) using Dunnett test for multiple comparisons.
Results
Firstly, the LPS-induced oxidative stress and cytotoxicity against A549 cells and the preventive effects of vitamin E were studied. The addition of LPS into the cell culture medium increased intracellular ROS and lipid hydroperoxides six times and twofold as measured by DCFH and DPPP assay respectively (Figs. 1 and 2). The ROS level increased and plateaued at 4 h after addition of LPS. αToc, αToc3, γToc, and γToc3 all reduced the level of ROS significantly. DPPP is a useful probe for detection of lipid hydroperoxides, but not for hydrogen peroxide [32,34]. The results shown in Fig. 2 indicate that lipid hydroperoxides increased at the initial stage and then decreased, probably because of the reduction by peroxidases. αToc, αToc3, γToc, and γToc3 all suppressed the formation of lipid hydroperoixdes significantly.
Fig. 1.
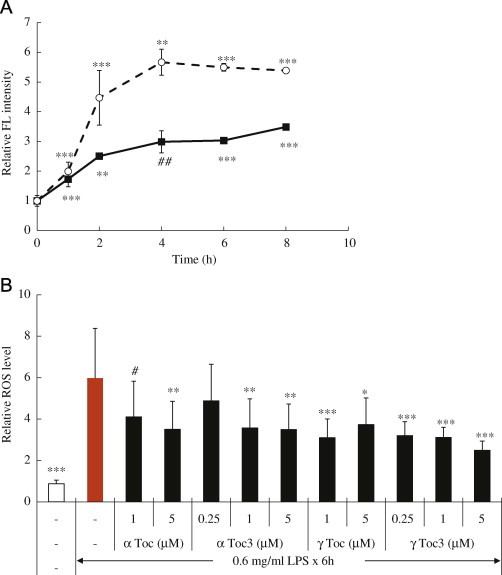
Production of reactive oxygen species (ROS) as measured by DCFH induced by LPS added into the A549 cell culture medium. (A) Fluorescence (FL) intensity from DCFH as described in Materials and methods. Closed square: 0.6 mg/ml LPS; open circle: 0.75 mg/ml LPS. (B) Effects of vitamin E isoforms preincubated for 24 h on the intracellular ROS levels. ⁎⁎⁎p<0.001, ⁎⁎p<0.005, ⁎p<0.01, ♯♯p<0.025, ♯p<0.05 compared with controls without LPS.
Fig. 2.
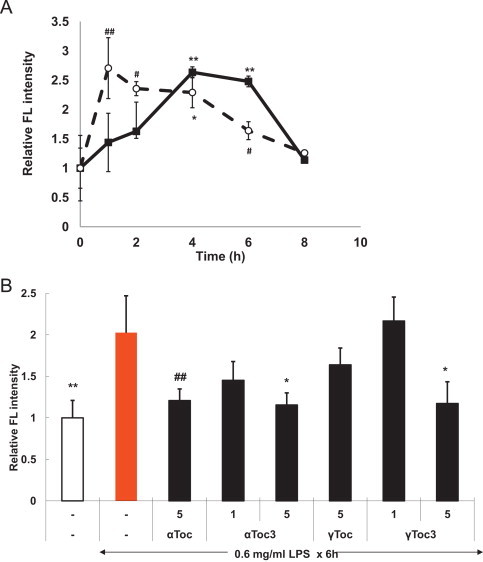
(A) Production of lipid hydroperoxides measured with DPPP induced by LPS in A549 cells as described in Materials and methods. Closed square: 0.6 mg/ml LPS; open circle: 0.75 mg/ml LPS. (B) Effects of vitamin E isoforms preincubated for 24 h. ⁎⁎⁎p<0.001, ⁎⁎p<0.005, ⁎p<0.01, ♯♯p<0.025, ♯p<0.05 compared with controls without LPS.
LPS-induced cell death was assessed by the three methods described in the Methods section. The cellular viability decreased with increasing LPS concentrations (Fig. 3). αToc, αToc3, γToc, and γToc3 suppressed LPS cytotoxicity, but the effects were not significant except for the apoptotic cell death. The difference between the effects of vitamin E isoforms was not significant.
Fig. 3.
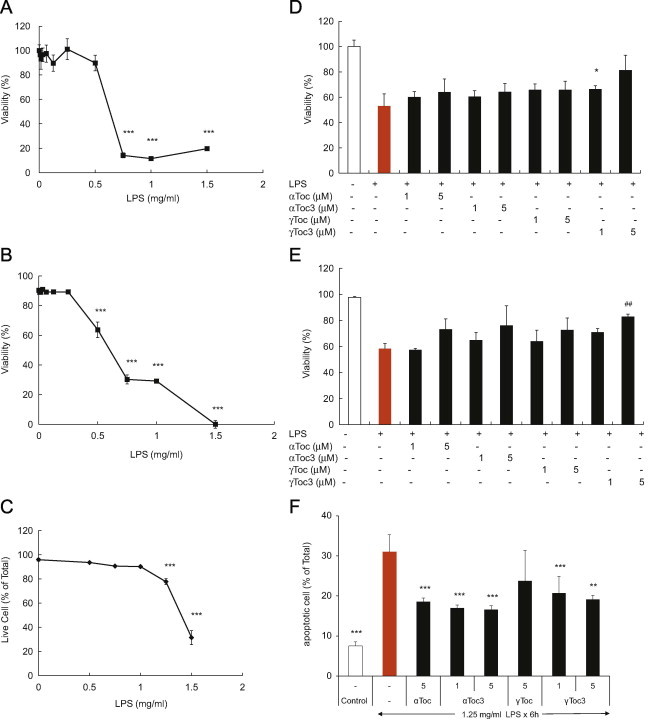
Cytotoxicity of LPS on A549 cells and preventive effects of vitamin E isoforms as measured by (A), (D) MTT, (B), (E) LDH, and (C), (F) Annexin V-FITC and propidium iodide assay as described in Materials and methods. ⁎⁎⁎p<0.001, ⁎⁎p<0.005, ⁎p<0.01, ♯♯p<0.025, ♯p<0.05 compared with controls without LPS.
It has been known that tocotrienols are incorporated into cultured cells more rapidly than tocopherols [27,29,30], which affects their apparent capacity to prevent oxidative stress. The rates of uptake of αToc, γToc, αToc3, and γToc3 into A549 cells were measured. As observed previously for Jurkat cells [27,29] and primary cortical neuron cells [30], αToc3 and γToc3 were incorporated into A549 cells at a much faster rate than αToc and γToc (Fig. 4).
Fig. 4.
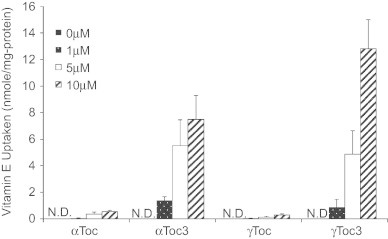
Uptake of vitamin E into A549 cultured cells. Different concentrations of tocopherols and tocotrienols were treated with 2×105 cells/ml A549 cells for 24 h and their intracellular concentrations were measured as described in the Methods section. N.D.: not detected.
LPS treatment increased TNF-α about 10-fold, which was reduced to about 50% by αToc, γToc, αToc3, and γToc3, but the difference in the effect between the four isoforms was small (Fig. 5A). IL-8 was increased about eight times and αToc, γToc, αToc3, and γToc3 reduced the level similarly (Fig. 5B). Heme oxygenase-1 (HO-1) also was increased about 1.5 times, but the effect of vitamin E was small (Fig. 5C). Pulmonary surfactant protein D (SP-D), a member of the collectin protein family which modulates LPS-induced inflammatory cell responses [35], was increased also, but the effect of αToc, γToc, αToc3, and γToc3 was small (Fig. 5D).
Fig. 5.
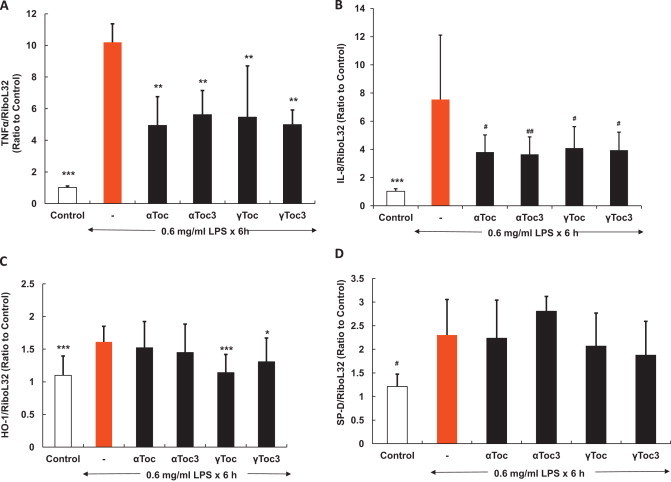
Effects of pre-treatment of vitamin E homologs on cytokine and antioxidant enzyme expression induced by 0.6 mg/ml LPS. Cells pre-treated with vitamin E homologs were harvested for total RNA isolation and real-time PCR analysis. The expression levels of TNF-α, IL-8, HO-1 and SP-D were normalized to that of Ribo-L32 mRNA and are shown relative to those of the control under static conditions. ⁎⁎⁎p<0.001, ⁎⁎p<0.005, ⁎p<0.01, ♯♯p<0.025, ♯p<0.05 in comparison with the LPS-treated cells (Turkey, ANOVA).
Collectively, the above results show that αToc, γToc, αToc3, and γToc3 suppressed the oxidative stress induced by LPS, but the difference in the capacity between the four vitamin E isoforms was not significant. Importantly, αToc and γToc were incorporated into cells much slower than αToc3 and γToc3 yet exerted similar preventive effects at much lower concentrations.
Discussion
LPS plays a pivotal role in the pathogenesis of various age-associated diseases by accelerating the production of ROS/RNS and inflammation-related mediators [1,5,6,36]. In the present study, the accelerated production of ROS/RNS was confirmed from an increase in the fluorescence from DCFH in the LPS-treated A549 cells. DCFH has been used frequently for detection of ROS/RNS. As discussed in recent articles [37,38], care should be taken in the detection, characterization and quantification of ROS/RNS with DCFH, but the results shown in Figs. 1 and 2 suggest that LPS induced the production of active species that could be suppressed by vitamin E and initiate lipid peroxidation. Further, an increase in lipid hydroperoxides was observed as assessed with DPPP, which is a specific probe for lipid hydroperoxides [32,34].
As described above, the protective effects of vitamin E against LPS-induced oxidative stress have been observed in many previous studies, although the relative efficacy of different vitamin E isoforms varied. It was reported that peripherally administered αToc and γToc were equally effective at suppressing the production of F(2)-isoprostanes and F(4)-neuroprostanes, oxidation products from arachidonic and docosahexaenoic acid respectively, caused by kainite, while αToc, but not γToc, was effective at suppressing these lipid peroxidation products induced by the LPS-induced innate immune response [39]. In other studies, γToc was found to be more potent than αToc [18–20]. Further, it was also reported that tocotrienols were superior to tocopherols [11,40].
In the present study, αToc, αToc3, γToc, and γToc3 all suppressed the cell death, production of ROS, lipid peroxidation, and expression of TNF-α and IL-8 induced by treatment of LPS to A549 cells, although the difference in relative potency between the four isoforms expressed solely as the added concentration to the media was not significantly different used. As shown above, one of the important and yet often overlooked issue is the marked difference in the rate of incorporation of vitamin E isoforms into cultured cells. Tocotrienols are incorporated into cultured cells much faster than tocopherols, probably due to the shorter side chain length. It may be noted that the rate of incorporation of chromanols into erythrocytes decreases significantly with increasing side chain length [41] and that αToc and αToc3-fortified cells showed similar cellular distribution [29]. The results described above show that αToc and γToc exerted similar preventive effects as αToc3 and γToc3 at much lower intracellular concentrations than those of αToc3 and γToc3.
It should be noted that vitamin E may function as signaling mediator as well as radical-scavenging antioxidant [42,43]. It has been proposed that vitamin E is capable of modulating signal transduction, gene expression, and activity of several enzymes by non-antioxidant function [43,44]. The present study shows that vitamin E is capable of suppressing LPS-induced oxidative stress but does not show explicitly whether non-antioxidant functions may also play a role.
In humans, αToc exhibits the highest affinity toward α-tocopherol transfer protein [45], and, furthermore, tocotrienols are metabolized faster than tocopherols, which make αToc the most abundant vitamin E isoform in humans [28]. The chemical reactivity toward peroxyl radicals of tocopherols and the corresponding tocotrienols are similar and α-forms exhibit higher reactivity than other forms [46].
Taken together we conclude that αToc exerts the most potent protective effect against LPS toxicity despite its slower rate of uptake by cells.
Contribution of authors
KN has carried out most of the experiments. All the authors were involved in the design of the study and interpretation of data. KN and EN edited the manuscript. All the authors have read and approved the final version.
Conflict of interest
The authors report no conflicts of interest.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Keiko Nishio, Email: kei-nishio@aist.go.jp.
Etsuo Niki, Email: etsuo-niki@aist.go.jp.
References
- 1.Rossol M., Heine H., Meusch U., Quandt D., Klein C., Sweet M.J., Hauschildt S. LPS-induced cytokine production in human monocytes and macrophages. Critical Reviews in Immunology. 2011;31:379–446. doi: 10.1615/critrevimmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- 2.Chung H., Cesari M., Anton S., Marzetti E., Giovanini S., Seo A., Carter C., Yu B.P., Leewenburg C. Molecular inflammation: underpinning of ageing and age-related diseases. Ageing Research Reviews. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taubman M.A., Valverde P., Han X., Kawai T. Immune response: the key to bone resorption in periodontal disease. Journal of Periodontology. 2005;76(Suppl.):2033–2041. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- 4.Yoshinaga Y., Ukai T., Kaneko T., Nakatsu S., Shiraishi C., Kuramoto A., Oshino K., Ichimura I., Hara Y. Topical application of lipopolysaccharide into gingival sulcus promotes periodontal destruction in rats immunized with lipopolysaccharide. Journal of Periodontal Research. 2012;7:674–680. doi: 10.1111/j.1600-0765.2012.01486.x. [DOI] [PubMed] [Google Scholar]
- 5.Rada B., Leto T.L. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contributions to Microbiology. 2008;15:164–187. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Díaz-Muñoz M.D., Osma-García I.C., Fresno M., Iñiguez M.A. Involvement of PGE2 and the cAMP signalling pathway in the up-regulation of COX-2 and mPGES-1 expression in LPS-activated macrophages. The Biochemical Journal. 2012;443:451–461. doi: 10.1042/BJ20111052. [DOI] [PubMed] [Google Scholar]
- 7.Suntres Z.E., Shek P.N. Treatment of LPS-induced tissue injury: role of liposomal antioxidants. Shock. 1996;6(1):S57–S64. [PubMed] [Google Scholar]
- 8.Takata J., Ito S., Karube Y., Nagata Y., Matsushima Y. Water-soluble prodrug of vitamin E for parenteral use and its effect on endotoxin-induced liver toxicity. Biological & Pharmaceutical Bulletin. 1997;20:204–209. doi: 10.1248/bpb.20.204. [DOI] [PubMed] [Google Scholar]
- 9.Berg B.M., Godbout J.P., Kelley K.W., Johnson R.W. Alpha-tocopherol attenuates lipopolysaccharide-induced sickness behavior in mice. Brain, Behavior, and Immunity. 2004;18:149–157. doi: 10.1016/S0889-1591(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 10.Rocksén D., Koch B., Sandström T., Bucht A. Lung effects during a generalized Shwartzman reaction and therapeutic intervention with dexamethasone or vitamin E. Shock. 2004;22:482–490. doi: 10.1097/01.shk.0000142254.38630.36. [DOI] [PubMed] [Google Scholar]
- 11.Qureshi A.A., Reis J.C., Papasian C.J., Morrison D.C., Qureshi N. Tocotrienols inhibit lipopolysaccharide-induced pro-inflammatory cytokines in macrophages of female mice. Lipids in Health and Disease. 2010;9:143. doi: 10.1186/1476-511X-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niki E., Traber M.G. Vitamin E history. Annals of Nutrition and Metabolism. 2012;61:207–212. doi: 10.1159/000343106. [DOI] [PubMed] [Google Scholar]
- 13.Shibata H., Katsuki H., Okawara M., Kume T., Akaike A. c-Jun N-terminal kinase inhibition and alpha-tocopherol protect midbrain dopaminergic neurons from interferon-gamma/lipopolysaccharide-induced injury without affecting nitric oxide production. Journal of Neuroscience Research. 2006;83:102–109. doi: 10.1002/jnr.20700. [DOI] [PubMed] [Google Scholar]
- 14.Godbout J.P., Berg B.M., Kelley K.W., Johnson R.W. alpha-Tocopherol reduces lipopolysaccharide-induced peroxide radical formation and interleukin-6 secretion in primary murine microglia and in brain. Journal of Neuroimmunology. 2004;149:101–109. doi: 10.1016/j.jneuroim.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Hybertson B.M., Chung J.H., Fini M.A., Lee Y.M., Allard J.D., Hansen B.N., Cho O.J., Shibao G.N., Repine J.E. Aerosol-administered alpha-tocopherol attenuates lung inflammation in rats given lipopolysaccharide intratracheally. Experimental Lung Research. 2005;31:283–294. doi: 10.1080/01902140590918560. [DOI] [PubMed] [Google Scholar]
- 16.Berg B.M., Godbout J.P., Chen J., Kelley K.W., Johnson R.W. alpha-Tocopherol and selenium facilitate recovery from lipopolysaccharide-induced sickness in aged mice. Journal of Nutrition. 2005;135:1157–1163. doi: 10.1093/jn/135.5.1157. [DOI] [PubMed] [Google Scholar]
- 17.Mayorga M., Iborra A., Estany S., Martínez P. Protective effect of vitamin E in an animal model of LPS-induced inflammation. American Journal of Reproductive Immunology. 2004;52:356–361. doi: 10.1111/j.1600-0897.2004.00233.x. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Q., Elson-Schwab I., Courtemanche C., Ames B.N. gamma-Tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Q., Ames B.N. Gamma-tocopherol but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB Journal. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Q., Moreland M., Ames B.N., Yin X. A combination of aspirin and gamma-tocopherol is superior to that of aspirin and alpha-tocopherol in anti-inflammatory action and attenuation of aspirin-induced adverse effects. The Journal of Nutritional Biochemistry. 2009;20:894–900. doi: 10.1016/j.jnutbio.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S., Lee E.H., Kim S.H., Lee S., Lim S.J. Comparison of three tocopherol analogs as an inhibitor of production of proinflammatory mediators in macrophages. Journal of Pharmacological Sciences. 2012;118:237–244. doi: 10.1254/jphs.11152fp. [DOI] [PubMed] [Google Scholar]
- 22.Chung M.Y., Yeung S.F., Park H.J., Volek J.S., Bruno R.S. Dietary α- and γ-tocopherol supplementation attenuates lipopolysaccharide-induced oxidative stress and inflammatory-related responses in an obese mouse model of nonalcoholic steatohepatitis. The Journal of Nutritional Biochemistry. 2010;21:1200–1206. doi: 10.1016/j.jnutbio.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Tan B., Watson R., Preedy V., editors. 2nd ed. CRC/AOCS Press; Boca Raton: 2012. [Google Scholar]
- 24.Wu S.J., Liu P.L., Ng L.T. Tocotrienol-rich fraction of palm oil exhibits anti-inflammatory property by suppressing the expression of inflammatory mediators in human monocytic cells. Molecular Nutrition & Food Research. 2008;52:921–929. doi: 10.1002/mnfr.200700418. [DOI] [PubMed] [Google Scholar]
- 25.Tan S.W., Ramasamy R., Abdullah M., Vidyadaran S. Inhibitory effects of palm α-, g- and δ-tocotrienol on lipopolysaccharide-induced nitric oxide production in BV2 microglia. Cellular Immunology. 2011;271:205–209. doi: 10.1016/j.cellimm.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 26.McCary C.A., Abdala-Valencia H., Berdnikovs S., Cook-Mills J.M. Supplemental and highly elevated tocopherol doses differentially regulate allergic inflammation: reversibility of α-tocopherol and γ-tocopherol's effects. Journal of Immunology. 2011;186:3674–3685. doi: 10.4049/jimmunol.1003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito Y., Yoshida Y., Akazawa T., Takahashi K., Niki E. Cell death caused by selenium deficiency and protective effect of antioxidants. The Journal of Biological Chemistry. 2003;278:39428–39434. doi: 10.1074/jbc.M305542200. [DOI] [PubMed] [Google Scholar]
- 28.Brigelius-Flohé R., Traber M.G. Vitamin E: function and metabolism. FASEB Journal. 1999;13:1145–1155. [PubMed] [Google Scholar]
- 29.Saito Y., Yoshida Y., Nishio K., Hayakawa M., Niki E. Characterization of cellular uptake and distribution of vitamin E. Annals of the New York Academy of Sciences. 2004;1031:368–375. doi: 10.1196/annals.1331.047. [DOI] [PubMed] [Google Scholar]
- 30.Saito Y., Nishio K., Akazawa Y.O., Yamanaka K., Miyama A., Yoshida Y., Noguchi N., Niki E. Cytoprotective effects of vitamin E homologues against glutamate-induced cell death in immature primary cortical neuron cultures: tocopherols and tocotrienols exert similar effects by antioxidant function. Free Radical Biology and Medicine. 2010;49:1542–1549. doi: 10.1016/j.freeradbiomed.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Pigualt C., Follenius-Wund A., Schmutz M., Freyssinet J.M., Brisson A. Formation of two-dimensional arrays of annexin V on phosphatidylserine-containing liposomes. Journal of Molecular Biology. 1994;236:199–208. doi: 10.1006/jmbi.1994.1129. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi M., Shibata M., Niki E. Estimation of lipid peroxidation of live cells using a fluorescent probe, diphenyl-1-pyrenylphosphine. Free Radical Biology and Medicine. 2001;31:164–174. doi: 10.1016/s0891-5849(01)00575-5. [DOI] [PubMed] [Google Scholar]
- 33.Saito Y., Nishio K., Ogawa Y., Kinumi T., Yoshida Y., Masuo Y., Niki E. Molecular mechanisms of 6-hydroxydopamine-induced cytotoxicity in PC12 cells: involvement of hydrogen peroxide-dependent and -independent action. Free Radical Biology and Medicine. 2007;42:675–685. doi: 10.1016/j.freeradbiomed.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Okimoto Y., Warabi E., Wada Y., Niki E., Kodama T., Noguchi N. A novel method of following oxidation of low-density lipoprotein using a sensitive fluorescent probe, diphenyl-1-pyrenylphosphine. Free Radical Biology and Medicine. 2003;35:576–585. doi: 10.1016/s0891-5849(03)00330-7. [DOI] [PubMed] [Google Scholar]
- 35.Yamazoe M., Nishitani C., Takahashi M., Katoh T., Ariki S., Shimizu T., Mitsuzawa H., Sawada K., Voelker D.R., Takahashi H., Kuroki Y. Pulmonary surfactant protein D inhibits lipopolysaccharide (LPS)-induced inflammatory cell responses by altering LPS binding to its receptors. The Journal of Biological Chemistry. 2008;283:35878–35888. doi: 10.1074/jbc.M807268200. [DOI] [PubMed] [Google Scholar]
- 36.Omata Y., Saito Y., Fujita K., Ogawa Y., Nishio K., Yoshida Y., Niki E. Induction of adaptive response and enhancement of PC12 cell tolerance by lipopolysaccharide primarily through the upregulation of glutathione S-transferase A3 via Nrf2 activation. Free Radical Biology and Medicine. 2008;45:1437–1445. doi: 10.1016/j.freeradbiomed.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida Y., Shimakawa S., Itoh N., Niki E. Action of DCFH and BODIPY as a probe for radical oxidation in hydrophilic and lipophilic domain. Free Radical Biology and Medicine. 2003;37:861–872. doi: 10.1080/1071576031000148736. [DOI] [PubMed] [Google Scholar]
- 38.Kalyanaraman B., Darley-Usmar V., Davies K.J., Dennery P.A., Forman H.J., Grisham M.B., Mann G.E., Moore K., Roberts L.J., 2nd., Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radical Biology and Medicine. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milatovic D., VanRollins M., Li K., Montine K.S., Montine T.J. Suppression of murine cerebral F2-isoprostanes and F4-neuroprostanes from excitotoxicity and innate immune response in vivo by alpha- or gamma-tocopherol. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 2005;827:88–93. doi: 10.1016/j.jchromb.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 40.Qureshi A.A., Tan X., Reis J.C., Badr M.Z., Papasian C.J., Morrison D.C., Qureshi N. Inhibition of nitric oxide in LPS-stimulated macrophages of young and senescent mice by δ-tocotrienol and quercetin. Lipids in Health and Disease. 2011;10:239. doi: 10.1186/1476-511X-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niki E., Komuro E., Takahashi M., Urano S., Ito E., Terao K. Oxidative hemolysis of erythrocytes and its inhibition by free radical scavengers. The Journal of Biological Chemistry. 1988;263:19809–19814. [PubMed] [Google Scholar]
- 42.Traber M.G., Atkinson J. Vitamin E, antioxidant and nothing more. Free Radical Biology and Medicine. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azzi A. Molecular mechanism of alpha-tocopherol action. Free Radical Biology and Medicine. 2007;43:16–21. doi: 10.1016/j.freeradbiomed.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Zingg J.M. Molecular and cellular activities of vitamin E analogues. Mini Reviews in Medicinal Chemistry. 2007;7:543–558. doi: 10.2174/138955707780619608. [DOI] [PubMed] [Google Scholar]
- 45.Hosomi A., Arita M., Sato Y., Kiyose C., Ueda T., Igarashi O., Arai H., Inoue K. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Letters. 1997;409:105–108. doi: 10.1016/s0014-5793(97)00499-7. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida Y., Niki E., Noguchi N. Comparative study on the action of tocopherols and tocotrienols as antioxidant: chemical and physical effects. Chemistry and Physics of Lipids. 2003;123:63–75. doi: 10.1016/s0009-3084(02)00164-0. [DOI] [PubMed] [Google Scholar]


