Abstract
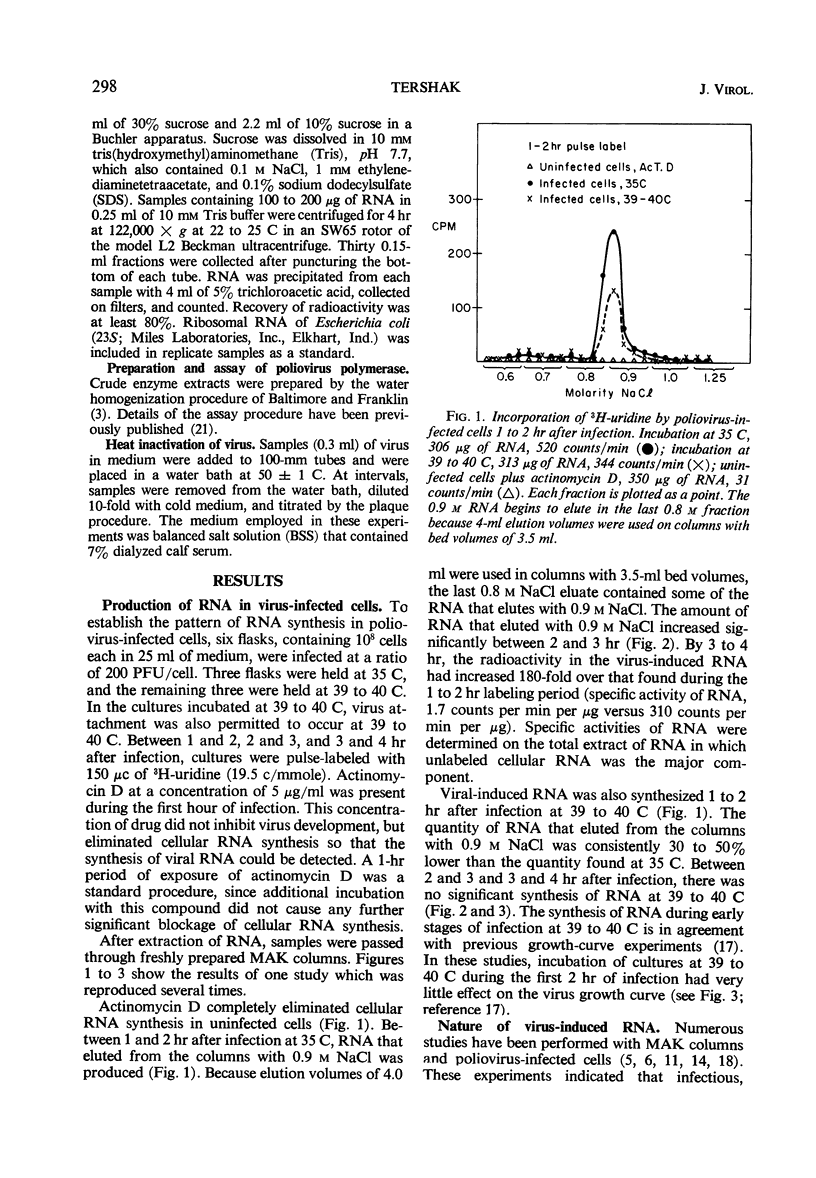
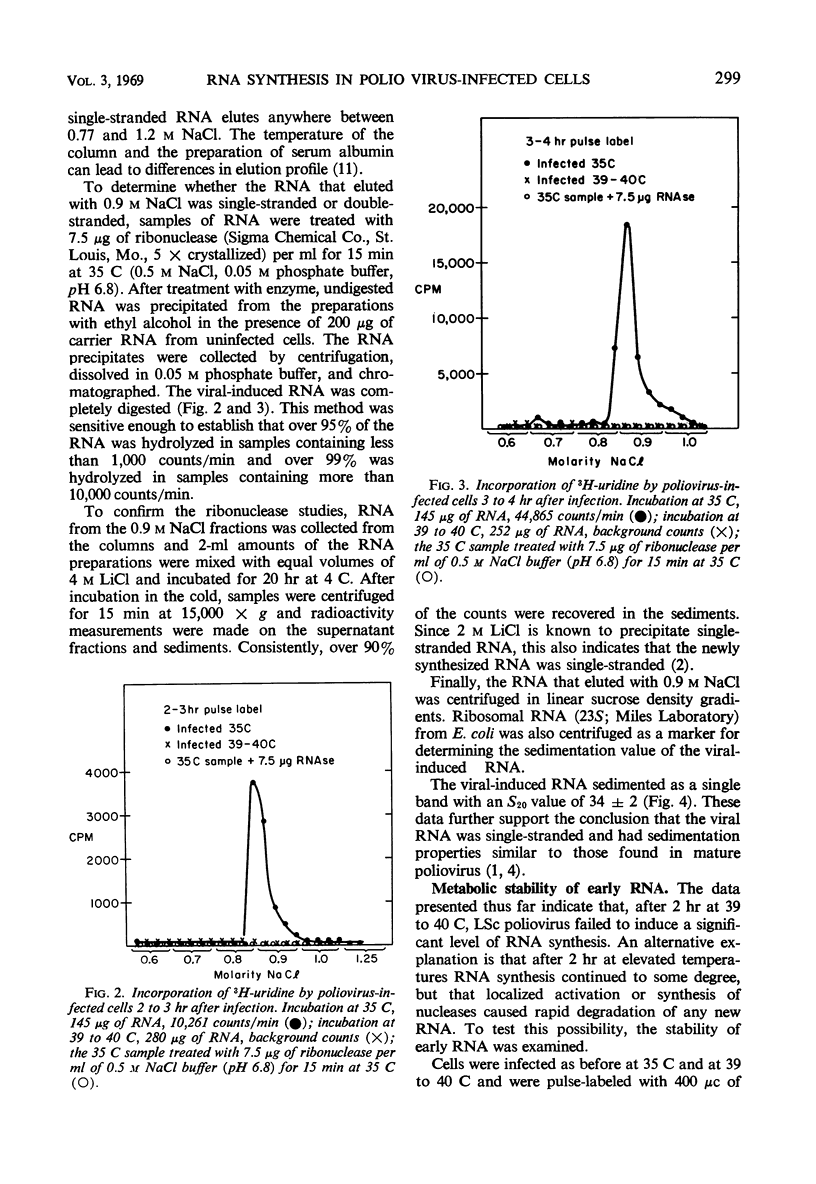
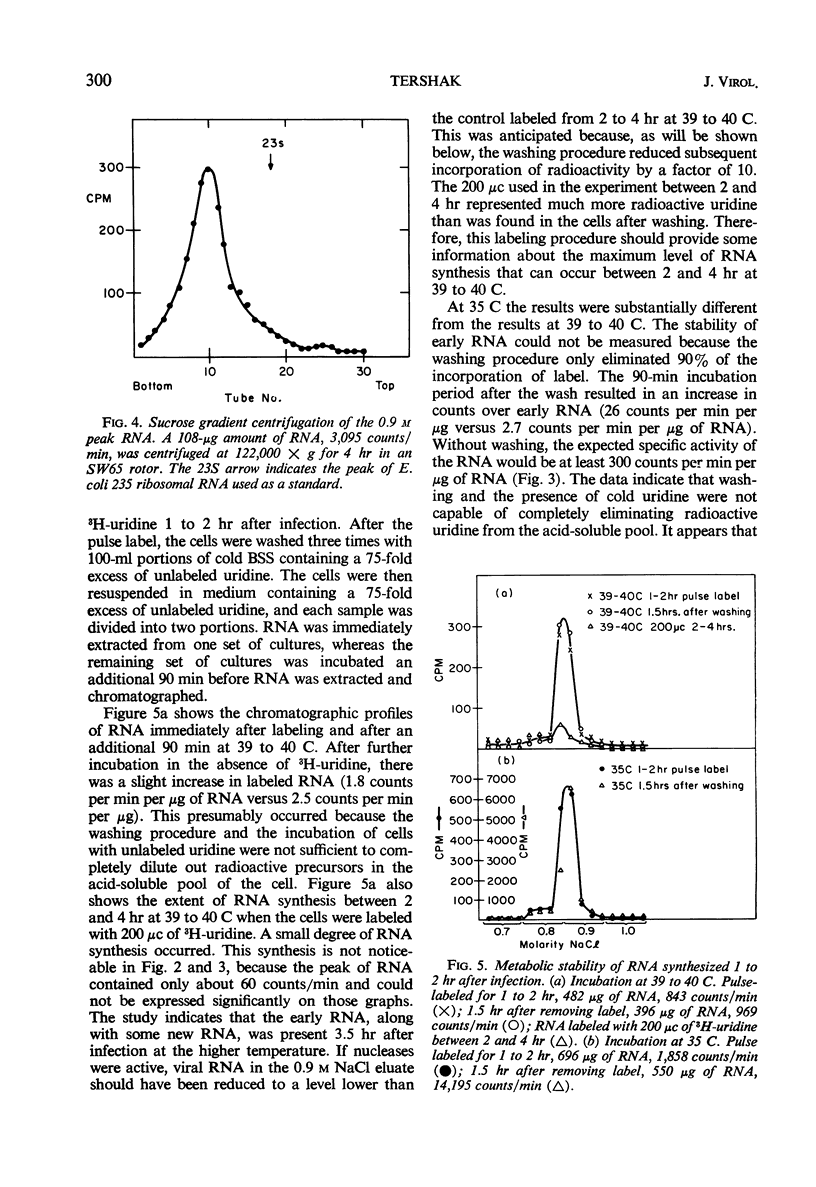
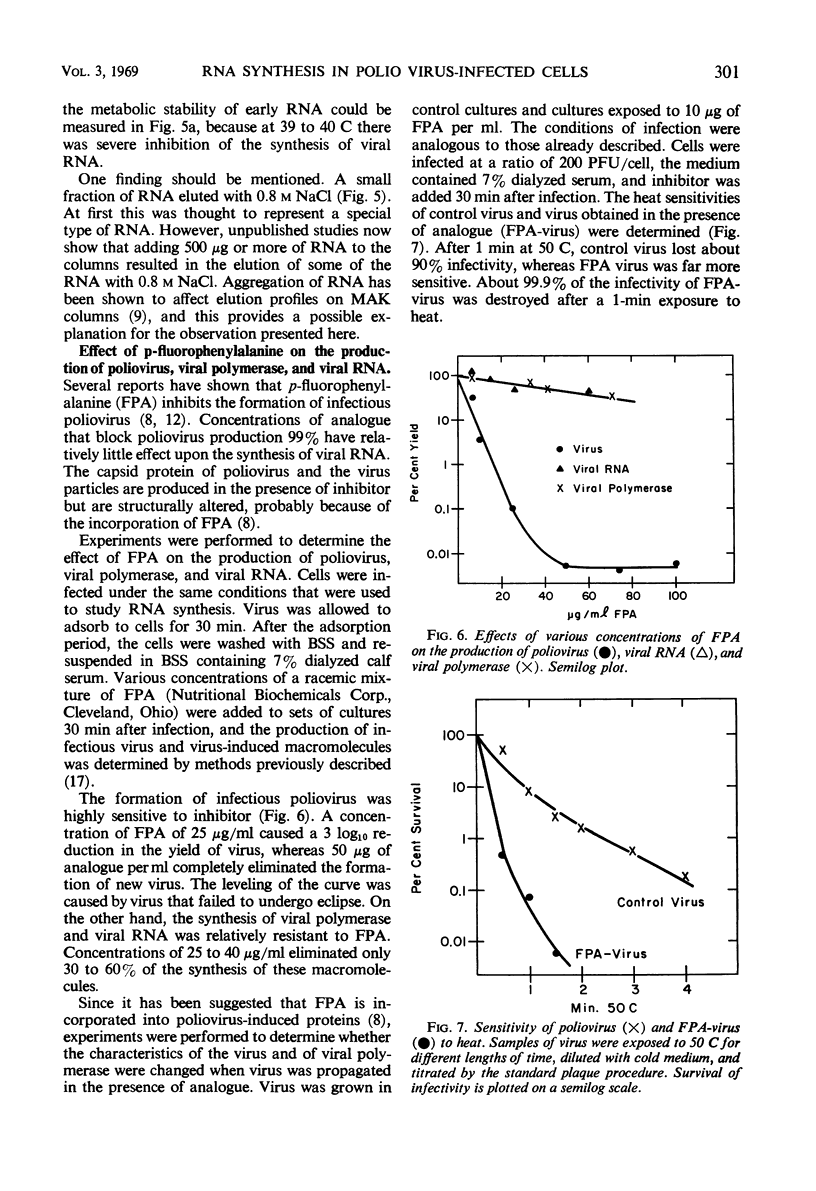
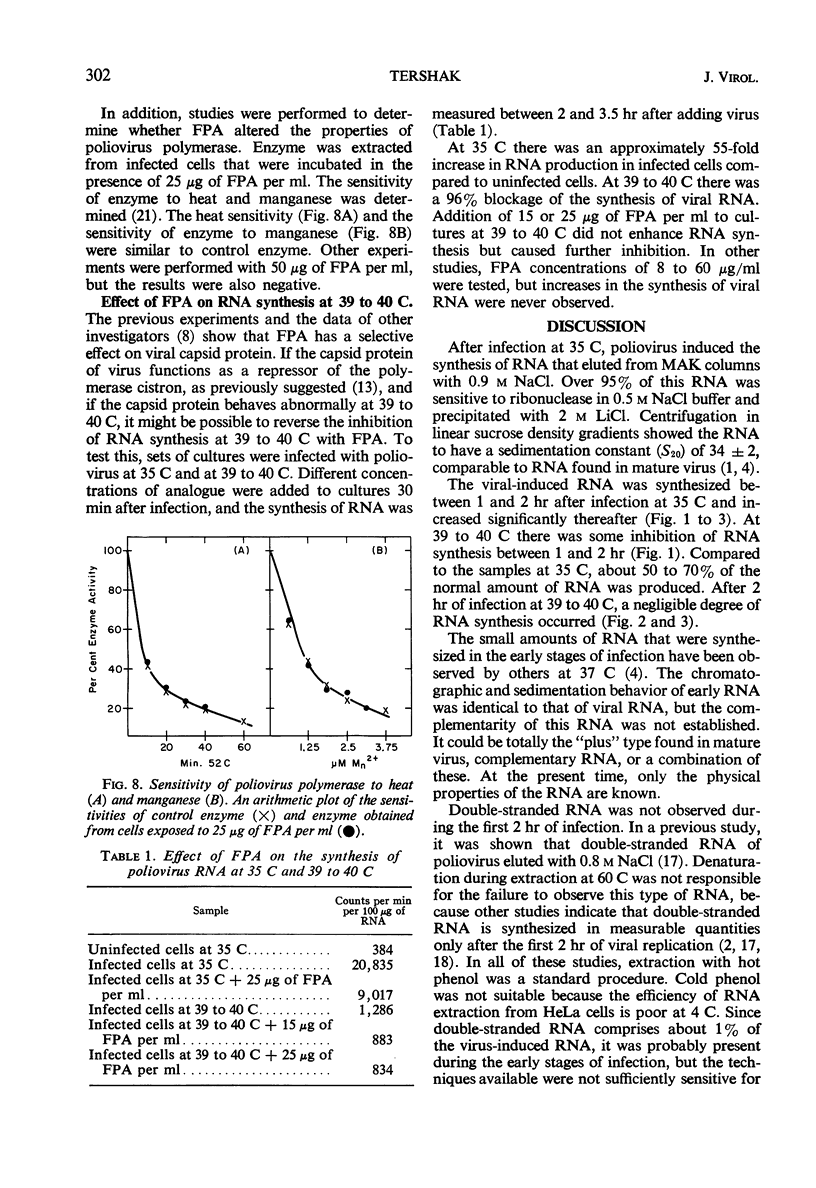
The synthesis of viral ribonucleic acid (RNA) was detected within 2 hr after infection with LSc poliovirus at 35 C. This RNA eluted as a single peak with 0.9 m NaCl on methylated albumin celite columns, was sensitive to ribonuclease, precipitated in the presence of 2 m LiCl, and had an S20 value at 34 ± 2 in linear sucrose gradients. When cells were infected at 39 to 40 C, there was also early synthesis of RNA. However, 2 hr after infection this synthesis was drastically inhibited. The absence of net RNA synthesis at 39 to 40 C during the late stages of infection was not caused by rapid degradation of newly formed RNA, since the RNA produced between 1 and 2 hr at 39 to 40 C was still present 3.5 hr after infection. There was a 3 log10 inhibition in the production of infectious virus when p-fluorophenylalanine was present in the medium at a concentration of 25 μg/ml. This concentration of analogue had little effect upon the production of viral polymerase and viral RNA. Virus grown in the presence of analogue at a concentration of 10 μg/ml exhibited increased heat sensitivity compared to control virus. However, viral polymerase exhibited no change in sensitivity to heat or manganese when cells were exposed to 25 μg of p-fluorophenylalanine per ml during infection. p-Fluorophenylalanine had a relatively selective effect on viral capsid protein but did not reverse the inhibition of synthesis of viral RNA at 39 to 40 C.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALTIMORE D., FRANKLIN R. M. A NEW RIBONUCLEIC ACID POLYMERASE APPEARING AFTER MENGOVIRUS INFECTION OF L-CELLS. J Biol Chem. 1963 Oct;238:3395–3400. [PubMed] [Google Scholar]
- BALTIMORE D. IN VITRO SYNTHESIS OF VIRAL RNA BY THE POLIOVIRUS RNA POLYMERASE. Proc Natl Acad Sci U S A. 1964 Mar;51:450–456. doi: 10.1073/pnas.51.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Girard M., Darnell J. E. Aspects of the synthesis of poliovirus RNA and the formation of virus particles. Virology. 1966 Jun;29(2):179–189. doi: 10.1016/0042-6822(66)90024-9. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Purification and properties of poliovirus double-stranded ribonucleic acid. J Mol Biol. 1966 Jul;18(3):421–428. doi: 10.1016/s0022-2836(66)80034-7. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Koch G. Purification and characterization of poliovirus-induced infectious double-stranded ribonucleic acid. J Biol Chem. 1967 Apr 25;242(8):1736–1743. [PubMed] [Google Scholar]
- COCITO C., PRINZIE A., DE SOMER P. Chromatographic analysis of infectious ribonucleic acid from poliovirus. Nature. 1961 Aug 5;191:573–575. doi: 10.1038/191573a0. [DOI] [PubMed] [Google Scholar]
- HUMMELER K., WECKER E. INFLUENCE OF P-FLUOROPHENYLALANINE ON POLIOVIRUS PARTICLES. Virology. 1964 Nov;24:456–460. doi: 10.1016/0042-6822(64)90184-9. [DOI] [PubMed] [Google Scholar]
- Holland J. J. Virus-directed protein synthesis in different animal and human cells. Science. 1968 Jun 21;160(3834):1346–1348. doi: 10.1126/science.160.3834.1346. [DOI] [PubMed] [Google Scholar]
- Ingle J., Key J. L. A re-evaluation of the fractionation of high molecular weight RNA by MAK chromatography. Biochem Biophys Res Commun. 1968 Mar 27;30(6):711–716. doi: 10.1016/0006-291x(68)90571-8. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUBINSKI H., KOCH G. On the separation of poliovirus ribonucleic acid from cellular ribonucleic acid. Virology. 1962 May;17:219–221. doi: 10.1016/0042-6822(62)90109-5. [DOI] [PubMed] [Google Scholar]
- LEVINTOW L., THOREN M. M., DARNELL J. E., Jr, HOOPER J. L. Effect of p-fluorophenylalanine and puromycin on the replication of poliovirus. Virology. 1962 Mar;16:220–229. doi: 10.1016/0042-6822(62)90241-6. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Zinder N. D. Mutants of the bacteriophage f2. 8. Control mechanisms for phage-specific syntheses. J Mol Biol. 1966 Aug;19(2):333–348. doi: 10.1016/s0022-2836(66)80008-6. [DOI] [PubMed] [Google Scholar]
- MAES R., WECKER E. FRACTIONATION OF NUCLEIC ACIDS FROM POLIO-INFECTED HE LA CELLS. Z Naturforsch B. 1964 Jan;19:43–45. doi: 10.1515/znb-1964-0110. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- Nichol F. R., Tershak D. R. Rescue of temperature-sensitive poliovirus. J Virol. 1968 May;2(5):415–420. doi: 10.1128/jvi.2.5.415-420.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONS M. INFECTIOUS DOUBLE-STRANDED POLIOVIRUS RNA. Virology. 1964 Nov;24:467–473. doi: 10.1016/0042-6822(64)90186-2. [DOI] [PubMed] [Google Scholar]
- SUEOKA N., CHENG T. Y. Fractionation of nucleic acids with the methylated albumin column. J Mol Biol. 1962 Mar;4:161–172. doi: 10.1016/s0022-2836(62)80048-5. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Nakada D. Control of translation of MS2 RNA cistrons by MS2 coat protein. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1744–1750. doi: 10.1073/pnas.57.6.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tershak D. R. Effect of 5-fluorouracil on poliovirus-induced RNA polymerase. J Mol Biol. 1966 Oct 28;21(1):43–50. doi: 10.1016/0022-2836(66)90078-7. [DOI] [PubMed] [Google Scholar]



