Abstract
Background
Fetal-to-neonatal transition is associated with oxidative stress. In preterm infants, immaturity of the antioxidant system favours supplemental oxygen-derived morbidity and mortality.
Objectives
To assess if prolonging in utero-like oxygenation during the fetal-to-neonatal transition limits oxidative stress in the lung and brain, improving postnatal adaptation of mice pups.
Material and methods
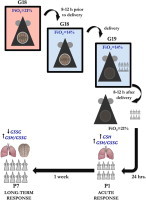
Inspiratory oxygen fraction (FiO2) in pregnant mice was reduced from 21% (room air) to 14% (hypoxia) 8–12 h prior to delivery and reset to 21% 6–8 h after birth. The control group was kept at 21% during the procedure. Reduced (GSH) and oxidized (GSSG) glutathione and its precursors [γ-glutamyl cysteine (γ-GC) and L-cysteine (CySH)] content and expression of several redox-sensitive genes were evaluated in newborn lung and brain tissue 1 (P1) and 7 (P7) days after birth.
Results
As compared with control animals, the GSH/GSSG ratio was increased in the hypoxic group at P1 and P7 in the lung, and at P7 in the brain. In the hypoxic group a significant increase in the mRNA levels of NAD(P)H:quinone oxidoreductase 1 (noq1), Sulfiredoxin 1 (srnx1) and Glutathione Peroxidase 1 (gpx) was found in lung tissue at P1, as well as a significant increase in gpx in brain tissue at P7.
Conclusions
Delaying the increase in tissue oxygenation to occur after birth reduces short-and-long-term oxidative stress in the lung. Similar yet more subtle effects were found in the brain. Apparently, the fetal-to-neonatal transition under hypoxic conditions appears to have protective qualities.
Abbreviations: CySH, L-cysteine; CyS–NEM, cysteine covalently bonded to N-ethylmaleimide; FiO2, inspiratory oxygen fraction; gapdh, glyceraldehyde-3-phosphate dehydrogenase gene; GCL, glutamylcysteine ligase; gclm, glutamylcysteine ligase modifier subunit gene; γ-GC, gamma-glutamyl cysteine; γ-GC–NEM, gamma-glutamyl cysteine covalently bonded to N-ethylmaleimide; gpx1, glutathione peroxidase 1 gene; GSH, reduced glutathione; GS–NEM, reduced glutathione covalently bonded to N-ethylmaleimide; gsr, glutathione reductase gene; GSSG, oxidized glutathione; G18, 18th day of gestation; g6pdx, glucose 6 phosphate dehydrogenase gene; LC–MS/MS, liquid chromatography coupled to tandem mass spectrometry; m/z, mass-to-charge ratio; noq1, NAD(P)H:quinone oxidoreductase 1; me1, malic enzyme 1 gene; NEM, N-ethylmaleimide; O14, hypoxia group (FiO2=14%); O21, normoxia group (FiO2=21%); paO2, partial pressure of oxygen; pgd, phosphogluconate dehydrogenase gene; P1, 24 h after birth; P7, 1 week after birth; SpO2, oxygen saturation; srnx1, sulfiredoxin 1 gene; trxnd1, thioredoxin reductase 1 gene
Keywords: Fetal-to-neonatal transition, Oxygen, Oxidative stress, Redox regulation, Glutathione
Graphical abstract
Highlights
-
•
The present study describes a mouse model meant to study redox biology of the fetal-to-neonatal transition under hypoxia.
-
•
Lung protection against oxidative stress was induced at day 1 after birth.
-
•
Improvement in lung and brain redox environments 7 days after birth was observed.
-
•
Changes detected in lung and brain were subtle, however significant, under physiologic conditions.
-
•
The applicability of our model under pathophysiologic conditions (e.g. postnatal hyperoxia) should be tested.
Introduction
Birth amounts to a significant oxidative stress to the newborn, given the marked increase in tissue oxygen exposure. Of all organs, the lungs are subjected to the greatest increase in oxygen exposure. As a consequence, a physiologic increase in the production of reactive oxygen species (ROS) by the electron transport chain is expected [1]. Mammalian newborns benefit from the physiological role of ROS for organ development by positively influencing protein functionality through reversible protein oxidation, involving cell signaling and intracellular redox regulation. However, an excessive and abrupt burst of ROS may induce oxidative damage to proteins, lipids, carbohydrates or DNA. This can cause disruption of thiol redox circuits, leading to aberrant cell signaling and dysfunctional redox control, altogether referred to as oxidative stress [2].
Birth associated oxidative stress is tolerated by the newborn because of the activation of the antioxidant defense system (ADS), a process that begins with gestation through the neonatal period [3–7]. Accordingly, an increased oxygen tension in organs which come into direct contact with enviromental oxygen or in active oxygen-consuming organs like the lung or brain respectively, induces the expression of antioxidant and other redox regulation enzymes such as thioredoxin (TRX), thioredoxin reductase (TXNRD) or peroxiredoxin I (PRX I) [6–10]. Many of these enzymes are transcriptionally induced by Nuclear factor (erythroid-derived 2)-like 2 (NRF2), the primary cellular defense against the cytotoxic effects of oxidative stress activated by redox fluctuations and ROS accumulation [11].
Little is known about the factors accounting for the fetal tolerance to lower oxygen delivery, as compared with postnatal life. Yet, it is known that maintenance of a low arterial oxygen tension postnatally in infants with congenital cyanotic heart conditions is compatible with life. Improved outcome of premature infants allowed to reach an adequate blood oxygenation over a longer than traditional period of immediate postnatal life suggests that there might be a biological advantage to a gradual oxygen transition from fetal to neonatal life [12].
In a piglet model of hypoxia/re-oxygenation, urinary biomarkers of oxidative damage to proteins and DNA directly correlate with the inspiratory fraction of oxygen (FiO2) used during reoxygenation [13]. Oxygen supplementation in different animal models has proved to be not only toxic to the lung, but also to other organs such as heart, brain or liver. On the contrary, resuscitation in piglet and mice models using FiO2 as low as 15–18% improved outcomes [14].
In newborn infants the use of high oxygen concentrations for resuscitation causes a pro-oxidant status and hyperoxemia. Remarkably, a significant correlation was found between oxidized glutathione (GSSG), partial pressure of oxygen (paO2) and glutathione redox cycle enzymes' activity [15,16]. Preterm babies not needing resuscitation at birth remained relatively hypoxic in the first minutes of life without short or long-term sequelae [12]. Moreover, preterm infants receiving lower oxygen load after birth exhibited less oxidative stress and developed less bronchopulmonary dysplasia (BPD) [17]. Seemingly, 21% or even lower oxygen concentrations appear to be beneficial for newborn infants because they attenuate oxidative stress associated with reoxygenation after hypoxia. In well-adapted preterm infants, a transitional period of relative hypoxemia seems to be beneficial for postnatal adaptation [1].
Therefore, we hypothesized that delaying the postnatal increase in arterial oxygenation for several hours after birth would reduce short-and-long-term tissue oxidative stress.
To test this hypothesis, we allocated wild-type mice to be delivered either on room-air or in a hypoxic environment, resulting in arterial oxygen tension comparable to fetal values. Lung and brain tissue were chosen for analysis, since the former is exposed to higher enviromental oxygen concentrations and the latter is representative of a vital organ oxygen-supplied by the blood. Reduced (GSH) and oxidized (GSSG) glutathione were measured as indicators of tissue oxidative state, together with its precursors L-cysteine (CySH) and γ-glutamyl cysteine (γ-GC). Expression of relevant redox-sensitive genes such as glutathione reductase (gsr), glucose 6 phosphate dehydrogenase (g6pdx), phosphogluconate dehydrogenase (pgd), malic enzyme 1 (me1), NAD(P)H:quinone oxidoreductase 1 (nqo1), sulfiredoxin 1(srxn1), thioredoxin reductase 1 (txnrd1), glutathione peroxidase 1 (gpx1) and glutamyl cysteine Ligase Modifier Subunit (gclm) were also evaluated.
Material and methods
Chemicals and reagents
All chemicals and reagents for analytical determinations were obtained from Sigma Aldrich (St. Louis, USA) with the exception of deuterated phenylalanine (phenylalanine-d5) and methionine (methionine-D3), which were obtained from Cambridge Isotope Laboratories (MA, USA). Materials and reagents for gene expression assays employed were RNeasy mini kit (Qiagen, Hilden, Germany) and GeneAmp Gold RNA PCR Reagent kit, TaqMan® Gene Expression Assay and TaqMan® Gene Expression Master Mix (Applied Biosystems, Life Technologies Corporation, CA, USA).
Animals
All procedures were conducted according to criteria established by the Canadian Council on Animal Care and approved by the Animal Care Committee of The Hospital for Sick Children Research Institutes (Toronto, Canada).
Wild-type C57Bl/6 timed-pregnant mice and newborn pups mice were utilized. Animals were fed food and water ad-libitum and exposed to regular dark/light cycles of 12/12 h. Half the pups of each litter were sacrificed by decapitation for organ removal at 24 h (P1) of age and the rest at 1 week of life (P7). Lung and brain tissue for biochemical and RNA measurements were rapidly cleared of blood and snap-frozen in liquid nitrogen. The number of animals utilized for each experiment is reported in the figure legends.
Experimental hypoxia exposure model
At G18 (8–12 h prior to delivery) pregnant mice from the experimental group were placed in the computer-controlled oxygen concentration chamber (Oxycycler Chambers, Biospherix, Lacona, NY, USA) and maintained at FiO2=14%. Animals were closely monitored for signs of delivery. 6–8 h after pups were born, the chamber FiO2 was increased to 21%. A control group was similarly placed in the chamber at an equal time point, but maintained in room air. The dam and litter were otherwise handled accordingly, as to the animal care facility protocol. The pups were evaluated either at P1 or P7 and kept in an Oxycycler in regular cages on room air until 1 week post-partum.
Investigators evaluated postnatal adaptation of mouse pups by observing respiratory and motor activity immediately after birth (P1) and breast feeding activity thereafter, in those pups that were allowed to survive until P7.
Determination of low molecular weight thiols
GSH, GSSG, CySH and γ-GC were determined at the Analytical Unit of the Health Research Institute Hospital La Fe (IIS La Fe, Valencia, Spain) by Liquid Chromatography coupled with tandem mass spectrometry (LC–MS/MS) following the Harwood et al. procedure modified based on the Nishiyama's thiols determination [18,19]. 10 mM N-ethylmaleimide (NEM) dissolved in PBS 4:1 (v/w) was added to the lung and brain tissue and homogenized for 1 min. Samples were deproteinized with 4% v/v perchloric acid (PCA) and centrifuged at 11,000 rpm for 15 min at 4 °C. 5 μL of a standard solution of phenylalanine-D5 and methionine-D3 (3.5 µM) was added to 100 μL of supernatant and subjected to LC–MS/MS analysis using a 2795 Alliance coupled to a QuattroMicro (Waters, Manchester, UK). Chromatographic separations were carried out at 50 °C using a C8 Zorbax column (150×2.1 mm2, 3.5 µm, Agilent) and injection volume of 5 µL. A 14 min gradient elution was performed at a flow rate of 300 µL min−1. Initial conditions A (water, 0.1% v/v formic acid (HCOOH)) 100%: B (acetonitrile, 0.1% v/v HCOOH) (99:1) were kept for 2.5 min, followed by a linear gradient up to 85% B in 2.5 min, and then up to 99% B in 4.5 min. Then, isocratic condition was held for 1.5 min. Finally, a 0.5 min linear gradient was used to return to the initial conditions. Positive ion electrospray MS/MS was recorded using the following conditions: capillary voltage 3.1 kV (kV), desolvation temperature 300 °C and cone and nebulization gases were set at 750 and 50 L/h, respectively. Linear calibration curves in the 20 µM to 2.5 nM concentration range were obtained using peak area values and either phenylalanine-D5 (171.1>125.1, RT=1.8 min) or methionine-D3 as internal standards. The limits of detection and quantitation for GSH, GSSG, γ-GC and CySH were in the 5–10 nM range. Due to the relatively high concentration of GSH, samples were appropriately diluted (1:50) in mobile phase and re-analyzed for GS–NEM quantification. MS/MS ionization, fragmentation and retention conditions for each considered analyte are summarized in Table 1.
Table 1.
Main parameters for MS data acquisition.
| Analyte | Cone (V) | Collision (eV) | Transitions m/z | Internal standard | Retention time (min) |
|---|---|---|---|---|---|
| GS–NEM | 20 | 22 | 433.4>201.3 | Phe–Ala-D5 | 9.5 |
| GSSG | 20 | 22 | 613.2>355.2 | Methionine-D3 | 5.85 |
| CyS–NEM | 25 | 20 | 247.3>158.2 | Phe–Ala-D5 | 8.84 |
| γ-GC–NEM | 20 | 25 | 376>230.3 | Phe–Ala-D5 | 9.53 |
| Methionine-D3 | 20 | 22 | 153.2>63.8 | n.a. | 3.88 |
| Phenylalanine-D5 | 20 | 15 | 171.1>125.1 | n.a. | 9.07 |
GS–NEM, CyS–NEM and γ-GC–NEM means N-ethylmaleimide (NEM) covalently bonded through a thioester linkage to reduced glutathione (GSH), Cysteine (CySH) and gamma-glutamylcysteine (γ-GC) respectively.
RNA extraction and cDNA synthesis
Total RNA was isolated using a RNeasy mini kit (Qiagen, Hilden, Germany) following the manufacturer's protocol. RNA concentration was quantified by spectrophotometry at 260 nm. Then RNA was reverse transcribed to cDNA using the GeneAmp Gold RNA PCR Reagent kit (Applied Biosystems, Carlsbad, CA, USA) following manufacturer's instructions. All samples were treated (reverse transcribed and PCR amplified) simultaneously to avoid batch-to-batch bias.
Real-time PCR
The expression of redox sensitive genes was evaluated by real-time PCR using a commercial thermal-cycler (Applied Biosystems ViiA™ 7 Real-Time PCR System; Life Technologies Corporation, Carlsbad, CA, USA) using TaqMan® gene expression assays and the TaqMan® 2× PCR Master Mix (Applied Biosystems, Life Technologies Corporation, Carlsbad, CA, USA). A list of analyzed genes and TaqMan probes is presented in Table 2. The glyceraldehyde 3-phosphate dehydrogenase (gapdh) gene was used as housekeeping gene.
Table 2.
TaqMan® gene expression assays (Applied Biosystems, Life Technologies Corporation, Carlsbad, CA, USA) for several Nrf2 target genes and for glyceraldehyde-3-phosphate dehydrogenase (gapdh) studied in lungs and brains of mice pups.
| Gene name | Gene symbol | Applied Biosystems TaqMan®gene expression assays |
|---|---|---|
| Glutathione reductase | gsr | Mm00439154_m1 |
| Glucose 6 phosphate dehydrogenase | g6pdx, g6pd | Mm00656735_g1 |
| Phosphogluconate dehydrogenase | pgd | Mm00503037_m1 |
| Malic enzyme 1 | me1 | Mm00782380_s1 |
| NAD(P)H:quinone oxidoreductase 1 | nqo1 | Mm01253561_m1 |
| Sulfiredoxin 1 | srxn1 | Mm00769566_m1 |
| Thioredoxin reductase 1 | txnrd1 | Mm00443675_m1 |
| Glutathione peroxidase 1 | gpx1 | Mm00656767_g1 |
| Glutamylcysteine ligase modifier subunit | gclm | Mm00514996_m1 |
| Glyceraldehyde-3-phosphate dehydrogenase | gapdh | Mm99999915_g1 |
Real time PCR was performed with one cycle of 2 min at 50 °C, required for optimal UNG activity, tailed by one cycle of denaturation of 10 min at 95°C, followed by 40 cycles of 15 s denaturation at 95 °C and 60 s annealing at 60 °C.
Data analysis
To assess relative abundance of different genes in the lung and brain, the mRNA fold change expression was calculated by the 2(−ΔΔCt) method, where Ct is the threshold cycle of each sample, using gapdh as the reference gene and the group of 1 day old mice pups born under normoxia, as calibrator [20].
Statistical analysis
Statistical analysis of both metabolites and gene data was performed using the Wilkoxon rank sum test for equal medians employing the Statistics Toolbox running in MATLAB R2001b (Mathworks; Natick, MA, USA). The Wilkoxon rank sum test is a two-sided test of the hypothesis where two independent samples come from distributions with equal medians. Differences were considered to be significant at p<0.05.
Results
Postnatal adaptation
Mice pups adapted well to the hypoxic atmosphere (FiO2=14%) immediately after birth. Respiratory activity and movement was not dissimilar to that of the control group. Pups that were allowed to survive exhibited a normal motor and breastfeeding activity. Hence, no differences in behavior or physical adaptation were noted between the experimental and control groups.
GSH and GSSG levels and GSH/GSSG ratio in lung of newborn mice
We determined GSH and GSSG levels and calculated the GSH/GSSG ratio as a reliable marker of general redox status in lungs of P1 and P7 experimental and control pups.
Lung GSH/GSSG ratios were significantly higher in the experimental group, as compared with values from control animals at 24 h of age (P1) and this difference was still present on time-points (Fig. 1A) on day 7 of life (P7). The observed higher GSH/GSSG ratio at P1 in the experimental group, as compared with control animals, was mostly due to higher GSH levels (Fig. 1B). However, at P7, the group differences in the GSH/GSSG ratios reflected lower levels of GSSG in the experimental group (Fig. 1C).
Fig. 1.
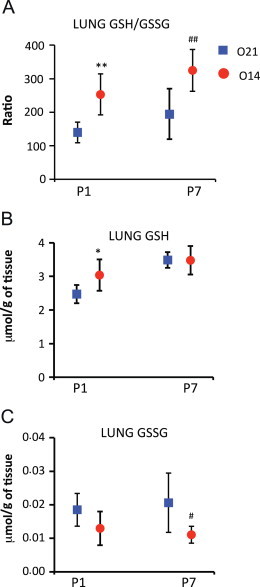
Reduced and oxidized glutathione levels at P1 and P7 in lungs of mice pups born under hypoxic conditions (O14; FiO2=14%; n=6) or under normoxic conditions (O21; FiO2=21%; n=7). Panel A shows the reduced (GSH) to oxidized (GSSG) glutathione ratio (GSH/GSSG). Panel B represents reduced glutathione (GSH) levels. Panel C shows oxidized glutathione (GSSG) levels. Analytical determinations were performed using ultra-high performance liquid chromatography coupled to mass spectrometry (LC–MS/MS). Comparisons between groups were made using the Wilcoxon rank sum test. Statistical differences are expressed: ⁎⁎p<0.01 vs. O21 at P1; ⁎p<0.05 vs. O21 group at P1; and ## p<0.01 vs. O21 group at P7.
CySH and γ-GC levels in lungs of newborn mice
The main rate limiting step upon GSH synthesis is the activity of γ-glutamyl-cysteine ligase (GCL) followed by the availability of CySH [21]. We analyzed lung levels of CySH and γ-GC substrate and product of GCL, respectively. Lower levels for γ-GC at P1 were found in the lung for the experimental group. No significant change for lung CySH levels in either group was found (see Table 3).
Table 3.
Gamma-glutamylcysteine (γ-GC) and L-cysteine (CySH) levels at P1 and P7 in lungs and brains of mice pups born under hypoxic conditions (O14; FiO2=14%; n=6) or under normoxic conditions (O21; FiO2=21%; n=7). Analytical determinations were performed using ultra-high performance liquid chromatography coupled to mass spectrometry (LC–MS/MS). Comparisons between groups were made using Wilcoxon rank sum test.
| Tissue (μmol/g) | Lung levels |
Brain levels |
||
|---|---|---|---|---|
| γ-GC | CySH | γ-GC | CySH | |
| O21_1 day | 0.009±0.001 | 0.24±0.055 | 0.002±001 | 0.04±0.015 |
| O14_1 day | 0.007±0.001 | 0.28±0.038 | 0.002±0.0004 | 0.05±0.011 |
| O21_7 days | 0.011±0.003 | 0.30±0.075 | 0.004±0.001 | 0.05±0.016 |
| O14_7 days | 0.011±0.001 | 0.27±0.041 | 0.003±0.0004⁎⁎ | 0.05±0.015 |
p<0.01 vs. O21 at P1.
GSH and GSSG levels and GSH/GSSG ratio in brains of newborn mice
GSH and GSSG were determined and the GSH/GSSG ratio calculated in brains of experimental and control mice pups at P1 and P7 (Fig. 2A).
Fig. 2.
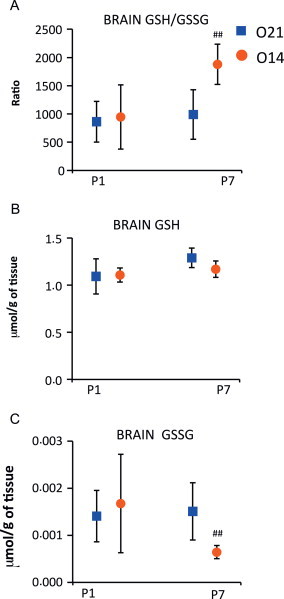
Reduced and oxidized glutathione levels at P1 and P7 in the brains of mice pups born under hypoxic conditions (O14; FiO2=14%; n=7) or under normoxic conditions (O21; FiO2=21%; n=7). Panel A shows the reduced (GSH) to oxidized (GSSG) glutathione ratio (GSH/GSSG). Panel B shows reduced glutathione (GSH) levels. Panel C shows oxidized glutathione (GSSG) levels. Analytical determinations were performed using ultra-high performance liquid chromatography coupled to mass spectrometry (LC–MS/MS). Comparisons between groups were made using the Wilcoxon rank sum test. Statistical differences are expressed are: ⁎p<0.05 vs. O21 group at P1; and ## p<0.01 vs. O21 group at P7.
In contrast to the lung, no group differences on GSH, GSSG and their ratio were observed at P1 in the brain (Fig. 2B). Yet, at 7 days of age, the experimental animals showed evidence of reduced brain tissue oxidative stress manifested by a significantly lower GSSG content and increased GSH/GSSG (Fig. 2C).
CySH and γ-GC levels in brains of newborn mice
No significant differences among CySH and γ-GC levels between both groups were found at P1 in brain tissue. However, low γ-GC levels were found at P7 in the experimental group as compared to those found in the control group (see Table 3).
Redox-sensitive gene expression in lungs of newborn mice
The redox potential represented by the GSH/GSSG ratio was significantly increased at P1 and P7 in mice pups' lungs born in a hypoxic atmosphere (FiO2=14%). In addition to metabolic studies, the expression of several genes involved in antioxidant activity and maintenance of redox couples (i.e., GSH/GSSG, NADPH/NADP, and cysteine/cystine) was determined. Hence, mRNA expression of antioxidant genes including gpx1, gclm, nqo1 and others in the upper scaffolds of the redox network such as gsr, trxnd1 and srnx1, as well as genes involved in NADPH recycling such as me1, g6pdx and pgd, were analyzed.
A significant increase in the mRNA levels of gpx1, srnx1 and nqo1 in the experimental group was found at P1, compared to the control group (Fig. 3). However, no significant differences in the expression of these three genes were detected at P7 (Fig. 3). Conversely, no significant statistical differences in mRNA levels for the rest of the genes were found between control and experimental groups at P1 nor P7 (see Table 4).
Fig. 3.
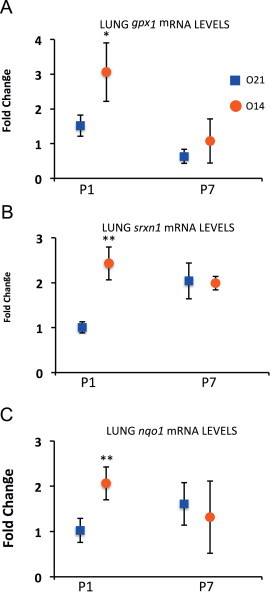
RT-PCR expression of genes related to redox regulation in lung tissue at P1 and P7 in mice pups born under hypoxic conditions (O14; FiO2=4%; n=3) or under normoxic conditions (O21; FiO2=21%; n=4). Panel A shows fold expression of glutathione peroxidase 1 (gpx1). Panel B shows fold expression of sulfiredoxin 1 (srnx1). Panel C shows fold expression for NAD(P)H:quinone oxidoreductase 1 (nqo1). Comparisons between groups were made using the Wilcoxon rank sum test Statistical differences are expressed as: ⁎⁎p<0.01 vs. O21 group at P1. Glyceraldehyde 3-phosphate dehydrogenase (gapdh) was used as housekeeping gene.
Table 4.
RT-PCR expression of genes related to redox regulation in lung tissue at P1 and P7 in mice pups born under hypoxic conditions (O14; FiO2=14%; n=3) or under normoxic conditions (O21; FiO2=21%; n=4). The table shows fold expression of Glucose 6 phosphate dehydrogenase (g6pdx), phosphogluconate dehydrogenase (pgd), malic enzyme 1 (me1), glutathione reductase (gsr) and thioredoxin reductase 1 (txnrd1). Comparisons between groups were made using the Wilcoxon rank sum test. Glyceraldehyde 3-phosphate dehydrogenase (gapdh) gene was used as housekeeping gene.
| Fold change | g6pdx | pgd | me1 | gsr | txnrd1 |
|---|---|---|---|---|---|
| O21_1 day | 1.02±0.24 | 1.01±0.17 | 1.41±0.34 | 1.22±0.25 | 1.05±0.38 |
| O14_1 day | 0.94±0.14 | 1.47±0.12 | 1.01±0.11 | 1.17±0.15 | 0.54±0.16 |
| O21_7 days | 1.20±0.24 | 1.65±0.35 | 1.37±0.34 | 1.14±0.30 | 1.04±0.37 |
| O14_7 days | 0.65±0.13 | 0.95±0.20 | 1.54±0.46 | 1.09±0.25 | 0.72±0.47 |
⁎⁎p<0.01 vs. O21 group at P1.
Redox-sensitive gene expression in brains of newborn mice
Expression for the same genes as reported for the lung was determined in the brain. At P1 gpx1 levels were significantly higher in the experimental group compared to the control group. Besides, significantly lower expression for gsr was found at P7 in the experimental group (Fig. 4).
Fig. 4.
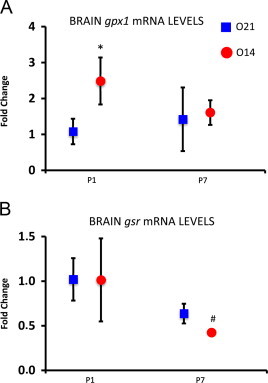
RT-PCR expression of genes related to redox regulation in lung tissue at P1 and P7 in mice pups born under hypoxic conditions (O14; FiO2=14%; n=3) or under normoxic conditions (O21; FiO2=21%; n=4). Panel A shows fold expression of malic enzyme 1 (me1). Panel B shows fold expression of glutathione reductase (gsr). Panel C shows fold expression of interleukin 6. Comparisons between groups were made using the Wilcoxon rank sum test. Statistical differences are expressed as: # p<0.05 vs. O21 group at P7. Glyceraldehyde 3-phosphate dehydrogenase (gapdh) was used as housekeeping gene.
By contrast, no significant change in the mRNA expression in the rest of the analyzed genes at P1 or P7 was found (see Table 5).
Table 5.
RT-PCR expression of genes related to redox regulation in brain tissue at P1 and P7 in mice pups born under hypoxic conditions (O14; FiO2=14%; n=3) or under normoxic conditions (O21; FiO2=21%; n=4). The table shows fold expression of Glucose 6 phosphate dehydrogenase (g6pdx), phosphogluconate dehydrogenase (pgd), malic enzyme 1 (me1), glutathione reductase (gsr), thioredoxin reductase 1 (txnrd1), NADPH:quinone oxidoreductase 1 (nqo1) and sulfiredoxin 1(srxn1). Comparisons between groups were made using the Wilcoxon rank sum test. Glyceraldehyde 3-phosphate dehydrogenase (gapdh) was used as housekeeping gene.
| Fold change | g6pdx | pgd | me1 | gsr | txnrd1 | nqo1 | srxn1 |
|---|---|---|---|---|---|---|---|
| O21_1 day | 1.04±0.35 | 1.02±0.23 | 1.01±0.17 | 1.02±0.24 | 1.02±0.25 | 1.03±0.29 | 1.04±0.33 |
| O14_1 day | 1.03±0.29 | 1.30±0.63 | 0.99±0.38 | 1.01±0.47 | 0.92±0.39 | 0.86±0.53 | 0.89±0.34 |
| O21_7 days | 0.60±0.14 | 0.75±0.28 | 0.57±0.18 | 0.64±0.11 | 0.55±0.11 | 0.44±0.14 | 1.95±0.31 |
| O14_7 days | 0.52±0.15 | 0.73±0.24 | 0.88±0.15 | 0.42±0.03# | 0.68±0.49 | 0.49±0.22 | 1.79±0.37 |
#p<0.05.
**p<0.01 vs. O21 group at P1.
Discussion
The present study shows that oxidative stress in lung and brain tissue is increased in mice born in room air compared to mice born in a hypoxic environment resembling in utero oxygenation conditions. The consequences of enviromental oxygen difference upon tissue oxidative status, confirms that newborns are subjected to oxidative stress during physiologic fetal-to-neonatal transition and postnatal air breathing. It also suggests that delaying exposure to air after birth may reduce birth-associated oxidative stress.
Postnatal adaptation to atmospheric respiration requires rapid changes in lung, airways and cardiopulmonary circulation [1]. Normal-term newborn infants need around 5 min and well-adapted preterm infants need 10 or more minutes to achieve a stable arterial oxygen saturation (SpO2) and heart rate. Seemingly, the time needed for postnatal stabilization inversely correlates with gestational age [12]. Interestingly, the use of supplemental oxygen for resuscitation has been associated with increased levels of oxidative stress biomarkers and higher mortality and morbidity such as BPD [17,22].
In experiments performed in isolated hepatocytes of Whistar rats, fetal to neonatal transition was associated with a 15–20-fold decrease in the GSH/GSSG ratio, mainly caused by a GSSG accumulation [23]. We hypothesized that delaying exposure to 21% oxygen after birth keeping an in utero-like oxygenation (14%) would reduce the generation of reactive oxygen species, oxidative stress and favor postnatal adaptation. To prove this hypothesis, we studied metabolic adaptation of mice pups delivered in a hypoxic atmosphere (14%) and performed the transition to environmental oxygen (21%) 6–8 h after birth. Notably, we found that the GSH/GSSG ratio was preserved in the lung on the first day after birth and in the lung and brain 1 week thereafter in the experimental group. GSH/GSSG is the most relevant sulfide/di-sulfide couple responsible for cellular redox status, and therefore relevant to regulation of differentiation, division, apoptosis and necrosis [24]. The reduced cellular environment in the lung during postnatal adaptation cannot be explained by increased de novo synthesis of GSH, and therefore high levels may indicate a reduction in its metabolic utilization. Accordingly, we did not find differences in the concentration of CySH in the lungs of hypoxic and control groups. Furthermore, concentration of γ-GC was lower in the hypoxic group than in the control group in lungs on day 1 after birth (P1), which may be explained by a feed-back inhibition of glutamyl-cysteine ligase (GCL); the rate-limiting enzyme for the GSH synthesis, by GSH [25]. One week after birth (P7), γ-GC levels in the brain were also diminished in the hypoxic group, suggesting lower GCL activity due to a further reduced environment. Our findings suggest that keeping a period of relative hypoxia after birth stimulates the presence of a suitable redox environment, both in the lung and brain in mice pups at birth and 1 week thereafter. Accordingly, hypoxic newborn piglets were satisfactorily resuscitated and brain activity adequately recovered using 15–18% oxygen. Seemingly, adjusting oxygen supply to the minimal requirements to the brain after an intense hypoxic episode allows an adequate hypoxanthine clearance and recovery of electroencephalographic activity [26]. To the contrary, the use of high FiO2 causes oxidative stress and substantial damage to the lung and brain in different animal models [14].
To seek a mechanistic explanation for hypoxic-derived redox protection in our experimental model, we studied NRF2-dependent gene expression both in the lung and brain. NRF2 is considered the first line of defense against oxidative injury and it is responsible for the expression of numerous antioxidant enzymes. We found up-regulation of nqo1 and srnx1and gpx1 in lung tissue and gpx1 in brain tissue of the hypoxic group, during postnatal adaptation under hypoxic conditions. NQO1 antioxidant capacity derives from its ability to divert quinone electrophiles from reactions that could lead to sulfhydryl depletion and in addition, is capable of detoxifying superoxide anion (O2−) [27–29]. Srnx1 is another antioxidant gene induced by NRF2. SRNX1 catalyzes reactivation of the 2-Cys-peroxyredoxins (Prxs) subfamily (Prx I-IV) after their over-oxidation during ROS detoxification. Prxs rely on a cysteine residue at the active site to reduce hydrogen peroxide (H2O2), alkyl-hydroperoxide (ROOH) or peroxynitrite (ONOO−) [30]. Srnx1 may not only reduce hyperoxidation of the active-site cysteine from sulfinic (Cys–SO2H) to sulfenic (Cys–SOH) acid, but it may also play a part in the specific de-glutathionylation of Prxs [31]. Furthermore, gpx1, a relevant antioxidant gene, was up-regulated in the lungs as well as in the brains of mice pups born under hypoxia at P1. The main GPX biological function, akin to that of PRXs, is to reduce lipid hydroperoxides to their corresponding alcohols and reduce free hydrogen peroxide to water, using GSH as cofactor and forming GSSG. GPX activity is couple to the GSR activity, which reduces GSSG to GSH; what is known as the GSH redox cycle [32]. Brain gsr expression is reduced in the hypoxic group at P7, reinforcing the idea that the redox environment was more suitable at P7 in the hypoxic group.
Our results show that keeping mice pups in a hypoxic environment (FiO2=14%) for 6–8 h after birth, favored a reducing state in both lung and brain immediately after birth, but also 1 week thereafter, suggesting that the experimental intervention also induced a long-lasting redox effect.
Obviously, there are limitations to this study; rodents have evolved to being highly resistant to oxygen-derived free radicals and therefore, it is difficult to assess if such a brief period of mild hypoxia as experienced in our study has preconditioned mice pups to better resist a highly stressful situation such as a hyperoxic stress, which we plan to investigate in forthcoming experiments [33]. Our aim was to reproduce the clinical situation experienced in the delivery room where present guidelines recommend keeping preterm babies within a low oxygen saturation range during the first minutes of life without oxygen supplementation and especially to evaluate how this approach affects the oxidant status in two vital organs, such as the lung and brain [34].
In summary, postnatal redox adaptation of normal-term mice born in a low oxygen atmosphere (14%) and kept for several hours after birth has been studied. An increased GSH/GSSG ratio in lungs and brains of the hypoxic pups was discovered. This reducing state was generated as a consequence of GSH sparing in the immediate postnatal period and decreased production of GSSG 1 week after birth. From a translational point of view, these results strengthen the idea that delaying the oxygenation switch from in utero to ex utero conditions after birth apparently promotes a reducing state that could be protective to the lung and brain. Babies who did not need postnatal interventions in the delivery room and pointedly preterm infants achieved a stable arterial oxygen saturation (>90%) 10 or more minutes after birth [12]. In preterm infants endowed with an immature antioxidant defense system, resuscitation maneuvers to rapidly increase postnatal oxygenation could be deleterious and should be carefully applied [1,3,7,8]. Further research should be performed in this regard.
Acknowledgments
This study was supported by FISPI11/0313 and RD08/0022/0072 grants to MV, JE, JK and EC; Sara Borrell postdoctoral fellowships CD11/00154 to JE, CD12/00667 to JK and the Rio Hortega fellowship CM12/00187 to EC, and PFIS fellowship FI12/00109 to IT-C; all of them from the Instituto Carlos III (Spanish Ministry of Economy and Innovation). In addition, a Linde Healthcare and INO Therapeutics AB grant to EC, and a CSD2007-00020 grant to JS by the Spanish Ministry of Economy and Innovation and from the Canadian Institutes of Health Research (MOP 93710) to JB.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Vento M., Escobar J., Cernada M., Escrig R., Aguar M. The use and misuse of oxygen during the neonatal period. Clinics in Perinatology. 2012;39:165–176. doi: 10.1016/j.clp.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Jones D.P. Radical-free biology of oxidative stress. American Journal of Physiology: Cell Physiology. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vento M., Aguar M., Escobar J., Arduini A., Escrig R., Brugada M., Izquierdo I., Asensi M.A., Sastre J., Saenz P., Gimeno A. Antenatal steroids and antioxidant enzyme activity in preterm infants: influence of gender and timing. Antioxidants & Redox Signaling. 2009;11:2945–2955. doi: 10.1089/ars.2009.2671. [DOI] [PubMed] [Google Scholar]
- 4.Perrone S., Negro S., Tataranno M.L., Buonocore G. Oxidative stress and antioxidant strategies in newborns. Journal of Maternal-Fetal and Neonatal Medicine. 2010;(Suppl. 3):63–65. doi: 10.3109/14767058.2010.509940. [DOI] [PubMed] [Google Scholar]
- 5.Mariucci G., Ambrosini M.V., Colarieti L., Bruschelli G. Differential changes in Cu, Zn and Mn superoxide dismutase activity in developing rat brain and liver. Experientia. 1990;46:753–755. doi: 10.1007/BF01939957. [DOI] [PubMed] [Google Scholar]
- 6.Gitto E., Pellegrino S., Gitto P., Barberi I., Reiter R.J. Oxidative stress of the newborn in the pre- and postnatal period and the clinical utility of melatonin. Journal of Pineal Research. 2009;46:128–139. doi: 10.1111/j.1600-079X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 7.Davis J.M., Auten R.L. Maturation of the antioxidant system and the effects of preterm birth. Seminars in Fetal and Neonatal Medicine. 2010;15:191–195. doi: 10.1016/j.siny.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 8.J.W. Lee, J.M. Davis, Future applications of antioxidants in premature infants. Current Opinion in Pediatrics 2011;23:1612–1616. [DOI] [PMC free article] [PubMed]
- 9.Das K.C., Guo X.L., White C.W. Induction of thioredoxin and thioredoxin reductase gene expression in lungs of newborn primates by oxygen. American Journal of Physiology. 1999;276:L530–L539. doi: 10.1152/ajplung.1999.276.3.L530. [DOI] [PubMed] [Google Scholar]
- 10.Das K.C., Pahl P.M., Guo X.L., White C.W. Induction of peroxiredoxin gene expression by oxygen in lungs of newborn primates. American Journal of Respiratory Cell and Molecular Biology. 2001;25:226–232. doi: 10.1165/ajrcmb.25.2.4314. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra D., Portales-Casamar E., Singh A, Srivastava S., Arenillas D., Happel C., Shyr C., Wakabayashi N., Kensler T.W., Wasserman W.W., Biswal S. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Research. 2010;38:5718–5734. doi: 10.1093/nar/gkq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson J.A., Kamlin C.O., Vento M., Wong C., Cole T.J., Donath S.M., Davis P.G., Morley C.J. Defining the reference range for oxygen saturation for infants after birth. Pediatrics. 2010;125:e1340–e1347. doi: 10.1542/peds.2009-1510. [DOI] [PubMed] [Google Scholar]
- 13.Solberg R., Andresen J.H., Escrig R., Vento M., Saugstad O.D. Resuscitation of hypoxic newborn piglets with oxygen induces a dose-dependent increase in markers of oxidative stress. Pediatric Research. 2007;62(5):559–563. doi: 10.1203/PDR.0b013e318156e8aa. [DOI] [PubMed] [Google Scholar]
- 14.Saugstad O.D., Sejersted Y., Solberg R., Wollen E.J., Bjøras M. Oxygenation of the newborn: a molecular approach. Neonatology. 2012;101:315–325. doi: 10.1159/000337345. [DOI] [PubMed] [Google Scholar]
- 15.Vento M., Asensi M., Sastre J., Garcia-Sala F., Pallardo F.V., Viña J. Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics. 2001;107(4):642–647. doi: 10.1542/peds.107.4.642. [DOI] [PubMed] [Google Scholar]
- 16.Vento M., Asensi M., Sastre J., Lloret A., Garcia-Sala F., Viña J. Oxidative stress in asphyxiated term infants resuscitated with 100% oxygen. Journal of Pediatrics. 2003;142(3):240–246. doi: 10.1067/mpd.2003.91. [DOI] [PubMed] [Google Scholar]
- 17.Vento M., Moro M., Escrig R., Arruza L., Villar G., Izquierdo I., Roberts L.J., 2nd, Arduini A., Escobar J.J., Sastre J., Asensi M.A. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics. 2009;124:e439–e449. doi: 10.1542/peds.2009-0434. [DOI] [PubMed] [Google Scholar]
- 18.Harwood D.T., Kettle A.J., Brennan S., Winterbourn C.C. Simultaneous determination of reduced glutathione, glutathione disulphide and glutathione sulphonamide in cells and physiological fluids by isotope dilution liquid chromatography-tandem mass spectrometry. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 2009;877(28):3393–3399. doi: 10.1016/j.jchromb.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Nishiyama J., Kuninori T. Assay of thiols and disulfides based on the reversibility of N-ethylmaleimide alkylation of thiols combined with electrolysis. Analytical Biochemistry. 1992;200:230–234. doi: 10.1016/0003-2697(92)90457-i. [DOI] [PubMed] [Google Scholar]
- 20.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Quintana-Cabrera R., Fernandez-Fernandez S., Bobo-Jimenez V., Escobar J., Sastre J., Almeida A., Bolaños J.P. γ-glutamyl-cysteine detoxifies reactive oxygen species by acting as glutathione peroxidase-1 cofactor. Nature Communications. 2012;3:718. doi: 10.1038/ncomms1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saugstad O.D., Ramji S., Soll R.F., Vento M. Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology. 2008;94:176–182. doi: 10.1159/000143397. [DOI] [PubMed] [Google Scholar]
- 23.Pallardo F.V., Sastre J., Asensi M., Rodrigo F., Estrela J.M., Viña J. Physiological changes in glutathione metabolism in foetal and newborn rat liver. Biochemical Journal. 1991;274:891–893. doi: 10.1042/bj2740891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones D.P., Go Y.M., Anderson C.L., Ziegler T.R., Kinkade J.M., Jr, Kirlin W.G. Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signalling and control. FASEB Journal. 2004;18:1246–1248. doi: 10.1096/fj.03-0971fje. [DOI] [PubMed] [Google Scholar]
- 25.McBean G.J. The transsulfuration pathway: a source of cysteine for glutathione in astrocytes. Amino Acids. 2012;42:199–205. doi: 10.1007/s00726-011-0864-8. [DOI] [PubMed] [Google Scholar]
- 26.Feet B.A., Brun N.C., Hellström-Westas L., Svenningsen N.W., Greisen G., Saugstad O.D. Early cerebral metabolic and electrophysiological recovery during controlled hypoxemic resuscitation in piglets. Journal of Applied Physiology. 1998;84:1208–1216. doi: 10.1152/jappl.1998.84.4.1208. [DOI] [PubMed] [Google Scholar]
- 27.Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annual Review of Pharmacology and Toxicology. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 28.Dinkova-Kostova A.T., Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Archives of Biochemistry and Biophysics. 2010;501:116–123. doi: 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegel D., Gustafson D.L., Dehn D.L., Han J.Y., Boonchoong P., Berliner L.J., Ross D. NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Molecular Pharmacology. 2004;65:1238–1247. doi: 10.1124/mol.65.5.1238. [DOI] [PubMed] [Google Scholar]
- 30.Zhu H., Jia Z., Mahaney J.E., Ross D., Misra H.P., Trush M.A., Li Y. The highly expressed and inducible endogenous NAD(P)H:quinone oxidoreductase 1 in cardiovascular cells acts as a potential superoxide scavenger. Cardiovascular Toxicology. 2007;7:202–211. doi: 10.1007/s12012-007-9001-z. [DOI] [PubMed] [Google Scholar]
- 31.Jeong W., Bae S.H., Toledano M.B., Rhee S.G. Role of sulfiredoxin as a regulator of peroxiredoxin function and regulation of its expression. Free Radical Biology and Medicine. 2012;53:447–456. doi: 10.1016/j.freeradbiomed.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 32.Circu M.L., Aw T.Y. Glutathione and apoptosis. Free Radical Research. 2008;42:689–706. doi: 10.1080/10715760802317663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gelfand S.L., Vento M., Sastre J., Lust W.D., Smith M.A., Perry G., Walsh M., Martin R. A new model of oxidative stress in rat pups. Neonatology. 2008;94:293–299. doi: 10.1159/000151649. [DOI] [PubMed] [Google Scholar]
- 34.Kattwinkel J., Perlman J.M., Aziz K., Colby C., Fairchild K., Gallagher J., Hazinski M.F., Halamek L.P., Kumar P., Little G., McGowan J.E., Nightengale B., Ramirez M.M., Ringer S., Simon W.M., Weiner G.M., Wyckoff M., Zaichkin J. Part 15; neonatal resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl. 3):S909–S919. doi: 10.1161/CIRCULATIONAHA.110.971119. [DOI] [PubMed] [Google Scholar]


