Abstract
The reversible redox conversion of nitrite and nitric oxide (•NO) in a physiological setting is now widely accepted. Nitrite has long been identified as a stable intermediate of •NO oxidation but several lines of evidence support the reduction of nitrite to nitric oxide in vivo. In the gut, this notion implies that nitrate from dietary sources fuels the longstanding production of nitrite in the oral cavity followed by univalent reduction to •NO in the stomach. Once formed, •NO boosts a network of reactions, including the production of higher nitrogen oxides that may have a physiological impact via the post-translational modification of proteins and lipids. Dietary compounds, such as polyphenols, and different prandial states (secreting specific gastric mediators) modulate the outcome of these reactions. The gut has unusual characteristics that modulate nitrite and •NO redox interplay: (1) wide range of pH (neutral vs acidic) and oxygen tension (c.a. 70 Torr in the stomach and nearly anoxic in the colon), (2) variable lumen content and (3) highly developed enteric nervous system (sensitive to •NO and dietary compounds, such as glutamate). The redox interplay of nitrite and •NO might also participate in the regulation of brain homeostasis upon neuronal glutamatergic stimulation in a process facilitated by ascorbate and a localized and transient decrease of oxygen tension. In a way reminiscent of that occurring in the stomach, a nitrite/•NO/ascorbate redox interplay in the brain at glutamatergic synapses, contributing to local •NO increase, may impact on •NO-mediated process.
We here discuss the implications of the redox conversion of nitrite to •NO in the gut, how nitrite-derived •NO may signal from the digestive to the central nervous system, influencing brain function, as well as a putative ascorbate-driven nitrite/NO pathway occurring in the brain.
Keywords: Dietary nitrate, Nitrite, Nitric oxide, Gut, Brain, Glutamate
Highlights
-
•
Dietary nitrite converts to NO in the gut, triggering the modification of proteins and lipids.
-
•
Polyphenols and ascorbate facilitate the redox conversion of nitrite to NO in the stomach.
-
•
Dietary nitrite-derived NO triggers physiological responses.
-
•
The nitrite: NO: ascorbate interplay in brain may impact functionally upon glutamatergic stimulation.
From nitric oxide to nitrite and back
Nitric oxide (•NO), an ubiquitous free radical produced via a highly regulated enzymatic process in vivo, modulates a wide range of signaling pathways in virtually all body organs and systems [1]. Physiological mechanisms, such as the regulation of vascular tone, host response as well as learning and memory formation are tightly regulated by this diffusible free radical [2]. Upon its synthesis via NO synthases (NOS), •NO may embark in reactions with biomolecular targets, being ultimately neutralized into the supposedly inert oxidation products nitrite and nitrate [3]. Both anions have been disregarded as inert products devoid of physiological relevance but recently it was demonstrated that nitrate and nitrite might be stepwisely reduced back to •NO in vivo.
The so-called nitrate-nitrite-NO pathway, by opposition to the classical l-arginine-NOS pathway, implies that nitrate and nitrite fuel the production of •NO, a process particularly evident in the gastrointestinal compartment for nitrate from dietary sources originates nitrite in the oral cavity and •NO in the stomach [4]. The gut is therefore a primary site for the production of significantly high •NO concentrations [5,6]. The nitrite reduction to •NO has also been described in other compartments/tissues under hypoxic conditions, a condition in which several hemeproteins (e.g., myoglobin, hemoglobin, xanthine oxidase, among others) acquire a nitrite-reductase activity, ensuring •NO bioavailability under oxygen deprivation [7]. Taken together, these observations suggest a reciprocal relationship between nitrite and •NO during redox signaling mechanisms.
Nitrite and nitric oxide in the gut: luminal warfare or welfare?
The production of •NO from nitrate obligates the intermediary formation of nitrite [4]. Nitrate, obtained from the diet or endogenously produced upon •NO oxidation, is mixed with saliva in the oral cavity and travels along the gut being absorbed in the small intestine. The salivary glands then recover c.a. 20% of circulating nitrate and secrete it into the oral cavity where it is reduced to nitrite by the local microflora and mixed with saliva, thus establishing the enterosalivar circulation of nitrate [8,9].
Under the acidic conditions of the gastric juice, nitrite is protonated to nitrous acid (HNO2), which then decomposes into different nitrogen oxides, depending on the redox microenvironment and gastric content [10,11]. Under normal fasting conditions, HNO2 yields •NO and nitrogen dioxide radical (•NO2) which may trigger signaling cascades by direct interaction with hemeproteins, such as soluble guanylate clyclase in the case of •NO, or by oxidizing and nitrating proteins and lipids in the case of •NO2 [1,12]. Nitric oxide and •NO2 may also combine to produce dinitrogen trioxide (N2O3) [13], a nitrosating agent. Dinitrogen trioxide reacts with gastric chloride, phosphate and bicarbonate anions to form nitrosyl compounds that nitrosate secondary amines, a process that has been claimed to support a deleterious effect of dietary nitrite [14]. However, N2O3 hydrolysis to nitrite and HNO2 is kinetically favored rather than N-nitrosation [14]. Of note, nitrite has also been shown to induce S-nitrosation within the gastric compartment, suggesting that this posttranslational modification may also be considered as part as nitrite redox signaling [15]. Finally, two •NO2 molecules may also combine to generate dinitrogen tetraoxide (N2O4) which under aqueous solutions yields both nitrate and nitrite [16].
However, this scenario is expected to change after a meal. During a postprandial period, ascorbic acid is secreted together with gastric juice into the lumen and, upon reaction with nitrite, is oxidized to ascorbyl radical whereas nitrite is univalently reduced to •NO (Fig. 1A) [6,17]. Consequently, in the presence of endogenous reducing agents, the network of reactions is shifted towards •NO production [6,18,19]. This raised the question as to whether dietary products could modulate the intricate dynamics of nitrite and •NO in the acidic gastric lumen. Here, we highlight three mechanisms by which nitrite signals in vivo through redox chemistry.
Fig. 1.
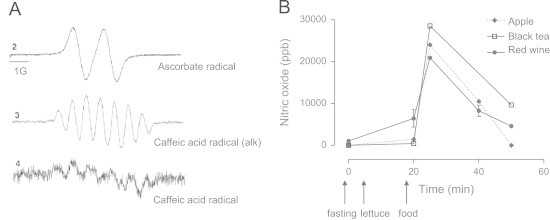
EPR analysis of the reaction between caffeic acid or ascorbic acid with nitrite. (A) EPR signal of ascorbate radical (line 1) and caffeic acid semiquinone radical (line 3) obtained under flow conditions upon mixing 2 mM of the compounds with 4 mM of nitrite at pH 2.0. Line 2 is the mixture of caffeic acid with nitrite alkalinized immediately before being pumped to the EPR cavity. (B) In vivo•NO production in the stomach of healthy volunteers following consumption of lettuce and the dietary products and beverages indicated. Reproduced from Gago B, Lundberg JO, Barbosa RM, Laranjinha J. Red wine-dependent reduction of nitrite to nitric oxide in the stomach. Free Radic Biol Med 43(9):1233–42; 2007 and Rocha BS, Gago B, Barbosa RM, Laranjinha J. Dietary polyphenols generate nitric oxide from nitrite in the stomach and induce smooth muscle relaxation. Toxicology 265(1–2):41–8; 2009 with permission from Elsevier.
Reduction by dietary polyphenols
The paradigm defining polyphenols as global antioxidants has nowadays been largely discredited for many reasons reviewed elsewhere [20]. Yet, more than 8000 different polyphenols are provided by the human diet and their health benefits are well established from the epidemiologic viewpoint, suggesting that other mechanisms operate in vivo [21]. One of them relies precisely on the nitrite-NO interplay in the gastric lumen. Foods (and beverages, including the red wine) rich in polyphenols, in a way reminiscent of ascorbate, have been shown to boost •NO production from nitrite at acidic pH in the human stomach [6,18,19]. Upon the consumption of lettuce (as a rich source of nitrite) and of red wine, apples or black tea (as rich sources of polyphenols) the intragastric •NO levels in humans rise by about three orders of magnitude (Fig. 1B) [19]. Mechanistically, we have shown that dietary nitrite is univalently reduced to •NO while the polyphenol is oxidized to the corresponding o-semiquinone radical (Fig. 1A) [6]. As a result, two radicals are produced: •NO and the phenoxyl radical. They can both combine to produce nitroso derivatives or, due to the high oxygen tension in the stomach (≈70 Torr [22]), •NO may be oxidized to •NO2 which, in turn, can combine with the phenoxyl radicals yielding nitrated phenols [23]. Of note, the trimolecular reaction of •NO with oxygen, considered to be too slow to be pertinent in vivo (k=8×106 M−1s−1) [24], acquires relevance in the gastric compartment as the steady state concentrations of both reagents are unusually high.
Although thermodynamically feasible [25], the mechanistic details of nitrite reduction to •NO at the expenses of polyphenols in the gastric environment still need further exploration. Overall, it is now important to recognize that the interaction of nitrite with polyphenols in the gastric lumen produces •NO, which diffuses toward deep regions of the gastric mucosa [26,27], participating in distinct physiological mechanisms such as smooth muscle relaxation [19], regulation of mucosal blood flow and mucus thickness [28,29] and also in the eradication of pathogens such as Helicobacter pylori [30].
Protein nitrosation
Wine has been identified as one of the polyphenol-containing dietary sources that promote •NO production from nitrite in the stomach (See Fig. 1B). Along with polyphenols, ethanol is a major component of wine that can interact with nitrite under gastric conditions [31]. The reaction of aliphatic alcohols, with nitrite-derived reactive nitrogen species, such as HNO2 and N2O3 may lead to the formation of alkyl nitrites of the corresponding alcohols via O-nitrosation [32]. This family of organic nitrites are known for their potent vasodilator activity [31]. Along these lines we have demonstrated that, under acidic gastric conditions, ethyl nitrite is formed in micromolar concentrations from the reaction of red wine or distilled alcoholic drinks with nitrite at concentrations that can be easily found physiologically [31]. Of note, ethyl nitrite is as a potent nitrosating agent [33] and may indirectly mediate •NO effects, as supported by relaxation experiments with blood vessels and gastric strips [31].
Several chemical groups other than alcohols can be nitrosated as a result of the nitrite chemistry in the stomach, namely aromatic compounds, amines and thiols, yielding C-, N-, S- nitroso species [34,35] along with heme moieties that produce heme-nitrosyls (heme-nitrosylation) [36,37]. A further level in the complex chemistry that may take place in the gastric compartment is added by the observation that different physiological/pathophysiological conditions may redirect the gastric chemistry such as, for instance, an achlorhydric stomach, where the neutral pH facilitates the formation of N-nitroso compounds (reviewed in [38]). The biological significance of many of these modifications remains obscure but S-nitrosation has received particular attention. The posttranslational modification of a critical cysteine residue in a protein by S-nitrosation can be relevant on the regulation of protein function [39] and has been implicated in controlling oxygen delivery to tissues, modulating the function or activity of transcription factors, enzymes, membrane receptors and ion channels [37]. Mechanistically, S-nitrosation may occur between •NO and a thiol group, if a thyil radical is formed in the cysteine residue [40] or, more importantly, by the action nitrogen oxides (formally addition of a nitrosonium equivalent, NO+), such as N2O3 formed, for instance, by the reaction of •NO with oxygen [41]. It may be also considered that two different thiols can undergo a fast transnitrosation reaction, which may explain in part the high liability of S-nitrosothiols [42,43].
The stomach is a proper environment for protein S-nitrosation for diet, saliva, gastric secretions (containing glutathione) and mucus glycoproteins (cysteine-rich mucins) constitute potential targets for S-nitrosation [28]. Considering the perfect timing (nitrite consumption) and place for mucins nitrosation, we have tried to elucidate the effects of the nitrite rich chemistry on the mucus layer from a physiological and pathophysiological point a view. A nitrosation pattern in the mucus proteins was found and, moreover, can be modulated with diet components and endogenous reductants (unpublished observations). It is tempting to speculate that several •NO-like effects of nitrite described in the stomach, such as stimulation of the mucosal blood flow and mucus generation, might proceed via intermediate formation of S-nitrosothiols which can act as stable carriers of •NO. Finally, it must be added that many of the compounds formed in the gastric lumen from acidified nitrite are fairly stable and readily absorbed which suggests systemic effects [4].
Protein and lipid nitration
In the gastric lumen, due to the low pH (c.a. 2 under fasting) and unusually high nitrite concentrations (1–2 mM upon a nitrate load [44]), nitrite triggers a complex network of chemical reactions yielding oxidizing and nitrating agents. Different nitrite-dependent nitrating pathways have been pointed out but •NO2 seems to be the main intermediate in most of them [45,46]. In the stomach, two important sources of •NO2 may be forwarded: •NO autoxidation and peroxynitrous acid (ONOOH) formation. As aforementioned, the high concentrations of •NO and oxygen achieved in the gastric lumen afford physiological significance to an apparently irrelevant reaction in vivo (•NO autoxidation). In addition, •NO and superoxide radical (O2•−) combine at near limit diffusion rate (k=6.7×109 M−1s−1) to produce ONOOH [47]. At physiological pH, ONOOH exists in equilibrium with peroxynitrite anion (ONOO−) (pKa=6.8) but in the acidic gastric juice ONOOH is the predominant form [48]. Peroxynitrous acid undergoes homolytic cleavage to produce •NO2 and hydroxyl radical (•OH) [49]. To this pathway aids the high carbon dioxide (CO2) tension in the headspace and bicarbonate in the gastric juice that via the production of nitrosoperoxycarbonate (ONOOCOO) drives the formation of not only •NO2 but also carbonate radical (CO3•−), which is an oxidant stronger than •NO2 [50,51]. This facilitates the univalent oxidation or tyrosine to tyrosil radical (tyr-O•) (the first step of protein tyrosine nitration) and further radical combination of tyr-O• and •NO2 (k=3.0×109 M−1s−1) [51].
Given this complex web of reactions, it is likely that dietary nitrite activates oxidative signaling pathways in the stomach trough protein posttranslational modifications. Accordingly, we have observed nitrite-dependent protein (tyrosine) nitration in vivo in the stomach [46]. We have observed that pepsin, a gastric protease involved in the breakdown of dietary and mucosal proteins, is nitrated by both inorganic nitrite and human saliva upon a nitrate load [52]. Pepsin nitration is inhibited by urate, pointing to •NO2 as the nitrating agent arising from nitrite. Moreover, this posttranslational modification is associated with a decrease of the proteolytic activity of the enzyme. These results strongly support the hypothesis of nitrite, from dietary sources, to signal through nitration reactions with functional and physiological implications.
In addition to endogenous protein nitration, dietary compounds may also be targeted for nitrite-dependent nitration, notoriously dietary lipids, which have already been described to go through structural modifications upon exposure to acidified nitrite in vitro [53]. Therefore, lipid nitration afforded by gastric nitrite is a hypothesis that requires further investigation [46].
Taken together, these considerations support the concept that at acidic pH, protein and lipid nitration may constitute a yet poorly characterized pathway accounting for nitrite signaling in vivo [46].
Redox signaling by nitrite and nitric oxide from gut mucosa and beyond
Once formed in the acidic gastric lumen, and in addition to reactions with radicals, nitrogen oxides or macromolecules, •NO diffuses towards the gastric mucosa [26,27], hence triggering physiological responses. It increases mucus thickness (diets supplemented with nitrate have been shown to increase MUC6 expression, [54]), enhances mucosal blood flow (likely by binding to endothelial guanylate clyclase in vascular smooth muscle cells) and induces smooth muscle relaxation, influencing gastric emptying rates (reviewed in [10]). There is a vast collection of biochemical reactions in which •NO participates [11] that go beyond the scope of this review but we would like to emphasize that •NO can be also oxidized to nitrite and nitrate, contributing to a pool of these anions in vivo [55]. However, the documented increase of plasma and tissue nitrate and nitrite upon a nitrate load [56] supports a dietary contribution to the in vivo pool of those anions via, for instance, the enterosalivary recirculation.
In tissues, nitrite may trigger distinct signaling pathways, either via •NO production or in a •NO-independent manner. An interesting example of the later is myeloperoxidase (MPO), that can use nitrite as a substrate to produce oxidizing (compound I and II) and nitrating (•NO2) agents [57]. This reaction is of particular interest throughout the gut mucosa due to the continuous recruitment of polymorphonuclear cells that express this peroxidase as part of their antimicrobial arsenal [58]. Therefore, nitrite may be expected to trigger protein or lipid nitration reactions (at pH 7.4) through MPO activation.
Below, we will briefly address some of nitrite-dependent signaling pathways.
Mitochondrial nitric oxide-nitrite cycle
Nitric oxide is known to regulate mitochondrial function through mechanisms that include mitochondrial biogenesis [59], reactive oxygen species production [60], cytochrome c release [61] and inhibition of mitochondrial Complex I (NADH-ubiquinone oxireductase) and Complex IV (cytochrome c oxidase, CcOX) [62]. CcOX, in turn, is endowed with a nitrite-reductase activity [63,64] as well with a nitric oxide oxidase activity, particularly under conditions of low electron flux and in the presence of oxygen (reviewed in [65]), indicating that the enzyme contributes to nitrite physiological levels. In connection with these observations it is interesting to note that recently, nitrite was shown to regulate mitochondrial biogenesis in a •NO- and soluble guanylate cyclase-independent manner [66].
Complex I can be inhibited by •NO via S-nitrosation after a long exposure to high •NO concentrations [67] and crucially depends on the structural conformation of the enzyme which, in turn, depends on the availability of oxygen and NADH. Under hypoxic conditions, Complex I undergoes a transition between the active form and the de-activated form, exposing a critical cysteine residue that when S-nitrosated fully inhibits the enzyme [68,69]. Apparently, during re-oxygenation, the inhibited S-nitrosated Complex I has a fundamental contribution for cell protection, preventing the accumulation of reducing equivalents and ROS (reactive oxygen species) production [70]. Regarding the inhibition of complex IV (CcOX) by •NO and related species, two mechanisms have generated consensus [71,72]. A first reaction pathway involves the formation of a NO-bound (nitrosyl) enzyme in the ferrous heme a3 center and a second one produces nitrite-bound CcOX on the ferric heme a3 center. Both pathways lead to the reversible inhibition of the enzyme, and CcOX can restore its activity via dissociation of both, •NO, the process being slow and light-sensitive, and nitrite, the process being more rapid and light-sensitive. Sarti and co-workers, showed that both mechanisms can occur. Under conditions of low electron flux and high oxygen concentration, the ‘nitrite’ uncompetitive inhibition pathway prevails, whereas the electron flux is increased, and the oxygen concentration reduced, the oxygen-competitive ‘nitrosyl’ pathway tends to take over [71,72]. Recent evidences in favor of a nitrite reductase activity of CcOX [73] under acidic conditions and low oxygen tension, producing •NO, support that such a nitrite-reductase activity plays a role in hypoxic cell signaling.
Taken together, the reaction of mitochondrial complexes I and IV with •NO may trigger physiological or pathological events [67,74] and it is of note that •NO bioavailability is intimately related to the oxygen tension for conditions in which oxygen decreases in tissues below normoxic conditions, the activity of NOSs to produce •NO is progressively slowed. However, the fact that the anoxic environment promotes tissue acidification, favouring the reduction of nitrite to •NO, highlights the importance of the tissue/cell distribution of nitrite concerning the •NO chemistry [4,75].
Hypoxic signaling
The nitrite-dependent redox signaling under low oxygen tension is largely dependent on its reduction to •NO by several enzymes that acquire nitrite-reductase activity under these circumstances (for a recent review see [7]).
Hemoglobin and myoglobin
Several enzymes ensure a nitrite-reductase activity along the oxygen gradient but deoxygenated hemoglobin (plasma) and myoglobin (tissues) are of foremost importance. Although at different rates, both proteins may participate in the production •NO by the same mechanism [76]. Nitrite interacts with the heme group of deoxyhemoglobin [deoxyHb(Fe2+)], producing •NO and methemoglobin [metHb(Fe3+)] [77,78] at rates that increase when the protein conformation is changing from the R-to-T state (from oxy to deoxy), being maximized when hemoglobin is 50% saturated with oxygen [79]. It has been described that •NO hence formed in the erythrocyte may originate N2O3 via its combination with •NO2 which is present as a ferrous nitrogen dioxide [•NO2(Fe2+)] in equilibrium with [NO2−(Fe3+)] [80]. The functional significance of this reaction is that N2O3 is much more stable than •NO in the heme environment and therefore can escape the erythrocyte and may then transduce •NO signal in the vessel or endothelial compartment via, for instance, its ability to nitrosate thiols [80]. Likewise, deoxymyoglobin acts as a nitrite reductase, reducing nitrite to •NO faster than deoxyhemoglobin due to its lower heme redox potential. This mechanism underlies some of the mitochondrial effects of nitrite, as myoglobin:nitrite-derived •NO, being produced in proximity to mitochondria, inhibits CcOX and regulates mitochondrial respiration [81]. Such an effect on oxygen bioavailability has been shown to positively impact in the performance of healthy volunteers under submaximal exercise upon dietary supplementation with nitrate [82]. In the heart, under hypoxic conditions, •NO production via deoxymyoglobin reduction of nitrite, downregulates the cardiac energy status, oxygen consumption and tissue contractility [83], highlighting the homeostatic effects of nitrite under particularly disfavored conditions.
Xanthine oxidase (XO)
Besides the catabolism of purines and pyrimidines, XO is also able to reduce oxygen to O2•− and hydrogen peroxide (H2O2). However, it can also reduce nitrite to •NO at the molybdenum site under hypoxic conditions in the presence of NADH as electron donor [84]. The reaction is obviously affected by the tissue levels of the enzyme but, additionally, its activity is critically controlled by nitrite that, given the high value of Km (km=2.5 mM), acts as the limiting substrate [7]. Therefore, since the Km for nitrite exceed the typical levels on nitrite by two orders of magnitude, it is likely that only when nitrite levels rise above basal (such as when the gastric mucosa is exposed to intense •NO fluxes upon a nitrate load) this reaction may acquire physiological relevance. Alternatively, under ischemic conditions, when the pH drops and nitrite is converted into HNO2, •NO production by this pathway is favored [7] and has been suggested to protect against myocardial infarction [85]. Interestingly, for 70 µM oxygen, XO can univalently reduce both, oxygen to O2•− and nitrite to •NO, and further considering that both radicals can combine to produce ONOO− under normoxic physiological conditions [86] one may envisage that nitrite may also be implicated in ONOO− biological impact. Peroxynitrite is a short-lived oxidant implicated in a wide range of diseases [87] and therefore nitrite can be regarded as a Janus-faced molecule because depending on the local microenvironment (e.g., oxygen levels) it can activate protective pathways against ischemia or trigger the formation of deleterious oxidants.
The enteric nervous system
Gastrointestinal mucosa houses submucosal and myenteric plexuses that integrate gut and nervous functions, bridging the enteric and central nervous systems [88]. Diet composition modulates not only gastrointestinal homeostasis but also the gut-brain signaling through diverse mechanisms. For instance, there is an operative amino acid sensing system within the gastric mucosa that recognizes dietary glutamate [89]. Albeit being the major excitatory neurotransmitter in the central nervous system, this amino acid also modulates important gut functions such as secretion, motility and metabolism [90] via mechanisms that include the generation of •NO and serotonin [91]. Given the high steady state concentrations of •NO achieved in the gastric lumen and mucosa upon nitrite reduction, glutamate-dependent pathways in the gastrointestinal tract are likely to be modulated by dietary nitrate.
Nitrite and nitric oxide in the central nervous system
Presley et al. have shown that a high nitrate diet increases the blood flow in specific brain areas in older subjects [92], introducing the notion that nitrate available from the diet may impact on brain homeostasis. However, the mechanistic details that bridge dietary nitrate and brain function remain largely elusive. As aforementioned, upon a nitrate load there is an increase of both nitrate and nitrite concentration in blood and tissues, but how (and if) is nitrite converted into •NO in the brain and what is the impact in brain functions such as cognition and memory formation remains to be elucidated.
Given the critical role of •NO in the brain, a particular challenging question is whether one can identify a brain selective or favored mechanism that might affect the redox conversion between nitrite and •NO and, thereby •NO-mediated process. When considering the extremely high concentration of ascorbate in neurons and the occurrence of glutamate:ascorbate exchangers it is tempting to speculate that ascorbate might, under conditions of excitatory stimuli, participate in nitrite-dependent •NO production in the brain. Before addressing this hypothesis we will briefly review biochemical and physiological features of •NO in the brain.
Basic tenets of nitric oxide in the brain: production, actions, diffusion and inactivation
Upon neuronal activation, •NO production in the brain is related to glutamatergic activity, involving the activation of ionotropic glutamate receptors (iGluR), particularly NMDA subtype, and the consequent influx of Ca2+ that activates neuronal isoform of NOS (nNOS), thus promoting the conversion of l-arginine to l-citrulline and •NO, in an oxygen dependent way [93,94]. Following its production, •NO, among other targets, may activate soluble guanylate cyclase, [95–98], triggering physiological responses that are tissue and cell specific and include neuronal excitability, synaptic plasticity, modulation of neurotransmitter release, learning and memory processes and neurovascular coupling [93,98–100].
It is of note that •NO rapidly diffuses in the brain across cell membranes with a coefficient of 2. 2×10−5 cm2/s [101], interacting with multiple targets, especially heme and other iron proteins at relatively fast reactions (in the case of myo and hemoglobin rate constants are of the order of 107 M−1 s−1) [102]. In the absence of specific interaction with receptors, •NO actions are dependent on its spatiotemporal profile which, in turn, is dependent on the balance between its synthesis and inactivation. Regarding the latter process, on basis of selective microelectrodes stereotaxically inserted in the rat brain in vivo [103–107], we have recently shown that •NO has a half life of 0.64 s in rat cortex [101] and that scavenging by erythrocytes represents the major inactivaction pathway in the brain.
Where nitric oxide and nitrite come together
Nitric oxide synthesized by the neuronal isoform of NOS (nNOS) can not only regulate oxygen availability in tissues (via mitochondrial actions described above) but also bridges neuronal activity with local changes of blood flow in the microcirculation of the brain, a process known as neurovascular coupling [108]. Under hypoxia, it is expectable that the activity of NOS (and, in particular nNOS) is highly attenuated and, consequently, we would expect that the decreased production of •NO translates into subsequent impairment of blood flow and compromised vasodilation. However, apparently, NOS inactivation during hypoxia does not block vasodilation [109]. This observation adds to several others, including the viability of mice lacking all three isoforms of NOS [110], to suggest the occurrence of alternative and/or complementary pathways to ensure that tissues get the needed amount of •NO, regardless of the functional state of the enzymes usually involved in its synthesis. In this scenario, the possibility that dietary nitrite/nitrate may contribute to basal levels of •NO signaling emerges as a plausible possibility yet poorly investigated in the brain. This interaction becomes particularly relevant in maintaining the balance between •NO production, oxygen consumption and neurovascular coupling in the brain. Nitrite has shown to increase blood flow preferentially in hypoxic conditions, allowing blood flow to increase precisely where it is needed most [111–120]. This shift from the classical view of nitrite as a simple by product of •NO oxidation to a molecule with physiological impact, as discussed ahead, is associated with redox mechanisms leading to •NO formation when enzymatic production is somehow compromised.
Based on preliminary data and on the rationale discussed ahead we will propose a critical role for ascorbate in •NO biochemistry in the brain via the univalent reduction of nitrite.
Nitric oxide/ nitrite/ ascorbate cycle in the brain
Ascorbate is a powerful reducing agent highly concentrated in the brain, both in the extracellular fluid and cells, namely neurons and astrocytes. Ascorbate is present at even higher concentrations in areas such as the hippocampus where •NO has been shown to play critical roles in learning and memory processes [121–123]. Ascorbate release from neuronal cells to the extracellular fluid occurs in connection with glutamatergic activity, and a putative glutamate/ascorbate heteroexchange mechanism has been proposed to regulate this flow outwards the cells following stimulation [124]. However, other reports failed to find a direct correlation between glutamatergic uptake and ascorbate release [125,126].
Nitric oxide undergoes oxidation to nitrite and this amount adds to nitrite received from the diet, yielding a reservoir in the extracellular fluid. As discussed before, the reduction of nitrite promoted by univalent reductants such as dietary polyphenols and ascorbate is now well established in the gastric environment where both reagents (nitrite and ascorbate) achieve optimal conditions (low pH, high concentration) for the reduction of nitrite to •NO occur.
Years ago, Millar elegantly described an alternative mechanism for •NO production in the brain completely independent of enzymatic control, involving the reduction of nitrite to •NO by ascorbate released from neurons during increased neuronal activity [109]. In this regard, it is of note the millimolar range of ascorbate concentration in neurons and also that ascorbate, which is released following neuronal activity, could contribute to convert nitrite to •NO, thus promoting beneficial vasodilator actions on blood vessels in the brain in the absence of an enzymatic and oxygen requiring process, namely the NOS-catalyzing process. This would be particularly important in hypoxia, because (a) the low of oxygen compromises NOS activity and (b) during hypoxia, local pH may drop to values near 6.4 [127] and, as depicted in Fig. 2, when ascorbate and nitrite are in solution and pH drops from 7.4 to values near 6.5 •NO production occurs.
Fig. 2.
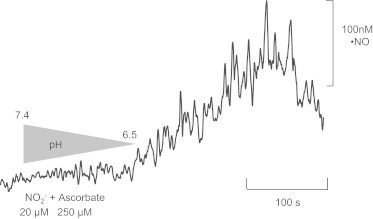
Nitric oxide production from a mixture of ascorbate and nitrite as a function of pH. The reaction vessel was supplemented with 250 µM of ascorbate and 20 µM of nitrite at a pH of 7.4. The vessel was coupled to an amiNO® sensor (selective for •NO measurements) and to a pH meter. Current was allowed to stabilize. Then, pH was slowly acidified until it reached the value of 6.51 (a value that can be reached in vivo under hypoxic conditions). At this pH value, current increased indicating that •NO was being produced. Maximum concentration of •NO obtained in these conditions was 118 nM.
However, considering that under glutamatergic stimulus local oxygen drops to a very low tension, reaching a “hypoxia like” transient status, it can be hypothesized that during this time period pH will drop sufficiently to promote nitrite reduction to •NO by the ascorbate hence released during the stimulation. In agreement with this hypothesis preliminary experiments have shown a temporal correlation between •NO and ascorbate dynamics upon glutamate stimulation of rat hippocampus in vivo (unpublished data), suggesting that ascorbate is contributing to •NO signals upon glutamatergic stimulation.
The basal nitrite concentration in the extracellular space of hippocampus is not accurately known and is likely to vary as a function of the redox environment but on basis of a microdyalisis approach in connection with a chemiluminescence measurement of nitrite-derived nitric oxide a range between 50 and 400 nanomolar was routinely found in male Wistar rats found [128]. On the other hand, nitrite concentration in the cerebrospinal fluid (of humans and rats), was reported to be circa 1 microM [129,130]. Under these conditions, the increase of •NO and ascorbate concentration in the extracellular space concurrently with a drop on the local pH (since oxygen concentration also drops) might set the conditions for nitrite reduction to •NO. The physiological outcome is that nitrite/•NO/ascorbate redox interplay could contribute to •NO-mediated processes such as the neurovascular coupling and the subsequent blood flow increase.
In summary, the redox couple nitrite:•NO in the brain might acquire relevance under physiological conditions in which the stimulation of glutamatergic terminals sets the environment and facilitates the chemical conditions for nitrite univalent reduction to •NO by ascorbate in a way reminiscent of what occurs in the stomach. The notion of nitrite as •NO reservoir acquires a particular interest in the brain because •NO production is achieved under physiological process, without the need to propose hypoxic or ischemic conditions for •NO production. However, although challenging, the hypothesis of the nitrite/•NO/ascorbate redox interplay with functional consequences in the brain still needs to be robustly substantiated.
Acknowledgments
This work was supported by grants PTDC/AGR-ALI/115744/2009, PTDC/SAU-NEU/108992/2008 and PTDC/SAU-NEU/103538/2008 from Fundação para a Ciência e Tecnologia (Portugal) and COMPETE. Grant PEst-C/SAU/LA0001/2011 from CNC is also acknowledged.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Moncada S., Palmer R.M., Higgs E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacological Reviews. 1991;43(2):109–142. [PubMed] [Google Scholar]
- 2.Moncada S. Nitric oxide: discovery and impact on clinical medicine. Journal of the Royal Society of Medicine. 1999;92(4):164–169. doi: 10.1177/014107689909200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moncada S., Higgs A. The l-arginine-nitric oxide pathway. The New England Journal of Medicine. 1993;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 4.Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nature Reviews Drug Discovery. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 5.Lundberg J.O., Weitzberg E., Lundberg J.M., Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35(11):1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gago B., Lundberg J.O., Barbosa R.M., Laranjinha J. Red wine-dependent reduction of nitrite to nitric oxide in the stomach. Free Radical Biology and Medicine. 2007;43(9):1233–1242. doi: 10.1016/j.freeradbiomed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 7.van Faassen E., Bahrami S., Feelisch M., Hogg N., Kelm M., Kim-Shapiro D., Kozlov A., Li H., Lundberg J., Mason R., Nohl H., Rassaf T., Samouilov A., Slama-Schwok A., Shiva S., Vanin A., Weitzberg E., Zweier J., Gladwin M. Nitrite as regulator of hypoxic signaling in mammalian physiology. Medicinal Research Reviews. 2009;29(5):683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner D.A., Schultz D.S., Deen W.M., Young V.R., Tannenbaum S.R. Metabolic fate of an oral dose of 15N-labeled nitrate in humans: effect of diet supplementation with ascorbic acid. Cancer Research. 1983;43(4):1921–1925. [PubMed] [Google Scholar]
- 9.Spiegelhalder B., Eisenbrand G., Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food and Cosmetics Toxicology. 1976;14(6):545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 10.Rocha B.S., Gago B., Pereira C., Barbosa R.M., Bartesaghi S., Lundberg J.O., Radi R., Laranjinha J. Dietary nitrite in nitric oxide biology: a redox interplay with implications for pathophysiology and therapeutics. Current Drug Targets. 2011;12(9):1351–1363. doi: 10.2174/138945011796150334. [DOI] [PubMed] [Google Scholar]
- 11.Lundberg J.O., Weitzberg E. Biology of nitrogen oxides in the gastrointestinal tract. Gut. 2012 doi: 10.1136/gutjnl-2011-301649. [DOI] [PubMed] [Google Scholar]
- 12.Rubbo H., Radi R. Protein and lipid nitration: role in redox signaling and injury. Biochimica Biophysica Acta. 2008;1780(11):1318–1324. doi: 10.1016/j.bbagen.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Archer S. Measurement of nitric oxide in biological models. FASEB Journal. 1993;7(2):349–360. doi: 10.1096/fasebj.7.2.8440411. [DOI] [PubMed] [Google Scholar]
- 14.Caulfield J.L., Singh S.P., Wishnok J.S., Deen W.M., Tannenbaum S.R. Bicarbonate inhibits N-nitrosation in oxygenated nitric oxide solutions. Journal of Biological Chemistry. 1996;271(42):25859–25863. doi: 10.1074/jbc.271.42.25859. [DOI] [PubMed] [Google Scholar]
- 15.Richardson G., Hicks S.L., O'Byrne S., Frost M.T., Moore K., Benjamin N., McKnight G.M. The ingestion of inorganic nitrate increases gastric S-nitrosothiol levels and inhibits platelet function in humans. Nitric Oxide. 2002;7(1):24–29. doi: 10.1016/s1089-8603(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 16.Doyle M.P., Hoekstra J.W. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. Journal of Inorganic Biochemistry. 1981;14(4):351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 17.Tannenbaum S.R., Wishnok J.S., Leaf C.D. Inhibition of nitrosamine formation by ascorbic acid. The American Journal of Clinical Nutrition. 1991;53(Suppl. 1):247S–250S. doi: 10.1093/ajcn/53.1.247S. [DOI] [PubMed] [Google Scholar]
- 18.Peri L., Pietraforte D., Scorza G., Napolitano A., Fogliano V., Minetti M. Apples increase nitric oxide production by human saliva at the acidic pH of the stomach: a new biological function for polyphenols with a catechol group? Free Radical Biology and Medicine. 2005;39(5):668–681. doi: 10.1016/j.freeradbiomed.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Rocha B.S., Gago B., Barbosa R.M., Laranjinha J. Dietary polyphenols generate nitric oxide from nitrite in the stomach and induce smooth muscle relaxation. Toxicology. 2009;265(1–2):41–48. doi: 10.1016/j.tox.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Fraga C.G. Plant polyphenols: how to translate their in vitro antioxidant actions to in vivo conditions. IUBMB Life. 2007;59(4–5):308–315. doi: 10.1080/15216540701230529. [DOI] [PubMed] [Google Scholar]
- 21.Fraga C.G., Galleano M., Verstraeten S.V., Oteiza P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Molecular Aspects of Medicine. 2010;31(6):435–445. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 22.He G., Shankar R.A., Chzhan M., Samouilov A., Kuppusamy P., Zweier J.L. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(8):4586–4591. doi: 10.1073/pnas.96.8.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pannala A.S., Mani A.R., Rice-Evans C.A., Moore K.P. pH-dependent nitration of para-hydroxyphenylacetic acid in the stomach. Free Radical Biology and Medicine. 2006;41(6):896–901. doi: 10.1016/j.freeradbiomed.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Ramezanian M.S., Padmaja S., Koppenol W.H. Nitration and hydroxylation of phenolic compounds by peroxynitrite. Chemical Research in Toxicology. 1996;9(1):232–240. doi: 10.1021/tx950135w. [DOI] [PubMed] [Google Scholar]
- 25.Jovanovic S.V.S.S., Simic M., Hara Y. Antioxidant properties of flavonoids: reduction potentials and electron transfer reactions of flavonoid radicals. In: Rice-Evans C.A., Packer L., editors. Flavonoids in Health and Disease. Marcel Dekker; New York: 1998. pp. 137–161. [Google Scholar]
- 26.Asanuma K., Iijima K., Sugata H., Ohara S., Shimosegawa T., Yoshimura T. Diffusion of cytotoxic concentrations of nitric oxide generated luminally at the gastro-oesophageal junction of rats. Gut. 2005;54(8):1072–1077. doi: 10.1136/gut.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocha B.S., Gago B., Barbosa R.M., Laranjinha J. Diffusion of nitric oxide through the gastric wall upon reduction of nitrite by red wine: physiological impact. Nitric Oxide. 2010;22(3):235–241. doi: 10.1016/j.niox.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Bjorne H.H., Petersson J., Phillipson M., Weitzberg E., Holm L., Lundberg J.O. Nitrite in saliva increases gastric mucosal blood flow and mucus thickness. Journal of Clinical Investigation. 2004;113(1):106–114. doi: 10.1172/JCI200419019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersson J., Phillipson M., Jansson E.A., Patzak A., Lundberg J.O., Holm L. Dietary nitrate increases gastric mucosal blood flow and mucosal defense. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2007;292(3):G718–G724. doi: 10.1152/ajpgi.00435.2006. [DOI] [PubMed] [Google Scholar]
- 30.Dykhuizen R.S., Fraser A., McKenzie H., Golden M., Leifert C., Benjamin N. Helicobacter pylori is killed by nitrite under acidic conditions. Gut. 1998;42(3):334–337. doi: 10.1136/gut.42.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gago B., Nystrom T., Cavaleiro C., Rocha B.S., Barbosa R.M., Laranjinha J., Lundberg J.O. The potent vasodilator ethyl nitrite is formed upon reaction of nitrite and ethanol under gastric conditions. Free Radical Biology and Medicine. 2008;45(4):404–412. doi: 10.1016/j.freeradbiomed.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 32.Allen A.D. Studies in the hydrolysis and alcoholysis of some organic nitrites. Journal of the Chemical Society. 1954:1968–1974. [Google Scholar]
- 33.Lundberg J.O., Weitzberg E., Shiva S., Gladwin M.T. The Nitrate–Nitrite–Nitric Oxide Pathway in Mammals. In: Bryan N.S., Loscalzo J., editors. Nitrite and Nitrate in Human Health and Disease. Humana Press; 2011. pp. 21–48. [Google Scholar]
- 34.Butler A.R., Rhodes P. Chemistry, analysis, and biological roles of S-nitrosothiols. Analytical Biochemistry. 1997;249(1):1–9. doi: 10.1006/abio.1997.2129. [DOI] [PubMed] [Google Scholar]
- 35.K.M. Wink DAM, M.G. Espey, J.B. Mitchell, M.B. Grisham, J. Fukuto, M. Feelisch, The Chemical Biology of Nitric Oxide. Balancing Nitric Oxide with Oxidative and Nitrosative Stress. In: S.B. Heidelberg, editor, Mayer, B2000. pp. 7–29.
- 36.Lancaster J.R., Jr., Hibbs J.B., Jr. EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proceedings of the National Academy of Sciences USA. 1990;87(3):1223–1227. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jourd'heuil F.L., Lowery A.M., Melton E.M., Mnaimneh S., Bryan N.S., Fernandez B.O., Park J.H., Ha C.E., Bhagavan N.V., Feelisch M., Jourd'heuil D. Redox-sensitivity and site-specificity of S- and N- denitrosation in proteins. PLoS One. e14400. 2010;2010;5(12) doi: 10.1371/journal.pone.0014400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bryan N.S., Alexander D.D., Coughlin J.R., Milkowski A.L., Boffetta P. Ingested nitrate and nitrite and stomach cancer risk: An updated review. Food and Chemical Toxicology. 2012;50(10):3646–3665. doi: 10.1016/j.fct.2012.07.062. [DOI] [PubMed] [Google Scholar]
- 39.Stamler J.S., Lamas S., Fang F.C. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001;106(6):675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Ruiz A., Lamas S. Signaling by NO-induced protein S-nitrosylation and S-glutathionylation: convergences and divergences. Cardiovascular Research. 2007;75(2):220–228. doi: 10.1016/j.cardiores.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Wink D.A., Darbyshire J.F., Nims R.W., Saavedra J.E., Ford P.C. Reactions of the bioregulatory agent nitric oxide in oxygenated aqueous media: determination of the kinetics for oxidation and nitrosation by intermediates generated in the NO/O2 reaction. Chemical Research in Toxicology. 1993;6(1):23–27. doi: 10.1021/tx00031a003. [DOI] [PubMed] [Google Scholar]
- 42.Rossi R., Lusini L., Giannerini F., Giustarini D., Lungarella G., Di Simplicio P. A method to study kinetics of transnitrosation with nitrosoglutathione: reactions with hemoglobin and other thiols. Analytical Biochemistry. 1997;254(2):215–220. doi: 10.1006/abio.1997.2424. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z., Rudd M.A., Freedman J.E., Loscalzo J. S-Transnitrosation reactions are involved in the metabolic fate and biological actions of nitric oxide. Journal of Pharmacology and Experimental Therapeutics. 1998;284(2):526–534. [PubMed] [Google Scholar]
- 44.McKnight G.M., Smith L.M., Drummond R.S., Duncan C.W., Golden M., Benjamin N. Chemical synthesis of nitric oxide in the stomach from dietary nitrate in humans. Gut. 1997;40(2):211–214. doi: 10.1136/gut.40.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Archives of Biochemistry and Biophysics. 1998;356(1):1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 46.Rocha B.S., Gago B., Barbosa R.M., Lundberg J.O., Radi R., Laranjinha J. Intragastric nitration by dietary nitrite: implications for modulation of protein and lipid signaling. Free Radical Biology and Medicine. 2012;52(3):693–698. doi: 10.1016/j.freeradbiomed.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 47.Beckman J.S. Oxidative damage and tyrosine nitration from peroxynitrite. Chemical Research in Toxicology. 1996;9(5):836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- 48.Radi R., Beckman J.S., Bush K.M., Freeman B.A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. Journal of Biological Chemistry. 1991;266(7):4244–4250. [PubMed] [Google Scholar]
- 49.Beckman J.S., Beckman T.W., Chen J., Marshall P.A., Freeman B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proceedings of the National Academy of USA. 1990;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denicola A., Freeman B.A., Trujillo M., Radi R. Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated oxidations. Archives of Biochemistry and Biophysics. 1996;333(1):49–58. doi: 10.1006/abbi.1996.0363. [DOI] [PubMed] [Google Scholar]
- 51.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proceedings of the National Academy of USA. 2004;101(12):4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rocha B.S., Gago B., Barbosa R.M., Lundberg J.O., Mann G.E., Radi R., Laranjinha J. Pepsin is nitrated in the rat stomach, acquiring antiulcerogenic activity: a novel interaction between dietary nitrate and gut proteins. Free Radical Biology and Medicine. 2012;8:26–34. doi: 10.1016/j.freeradbiomed.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 53.Napolitano A., Panzella L., Savarese M., Sacchi R., Giudicianni I., Paolillo L., d'Ischia M. Acid-induced structural modifications of unsaturated fatty acids and phenolic olive oil constituents by nitrite ions: a chemical assessment. Chemical Research in Toxicology. 2004;17(10):1329–1337. doi: 10.1021/tx049880b. [DOI] [PubMed] [Google Scholar]
- 54.Jansson E.A., Petersson J., Reinders C., Sobko T., Bjorne H., Phillipson M., Weitzberg E., Holm L., Lundberg J.O. Protection from nonsteroidal anti-inflammatory drug (NSAID)-induced gastric ulcers by dietary nitrate. Free Radical Biology and Medicine. 2007;42(4):510–518. doi: 10.1016/j.freeradbiomed.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 55.Ignarro L.J., Fukuto J.M., Griscavage J.M., Rogers N.E., Byrns R.E. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from l-arginine. Proceedings of the National Academy of USA. 1993;90(17):8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Govoni M., Jansson E.A., Weitzberg E., Lundberg J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19(4):333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Burner U., Furtmuller P.G., Kettle A.J., Koppenol W.H., Obinger C. Mechanism of reaction of myeloperoxidase with nitrite. Journal of Biological Chemistry. 2000;275(27):20597–20601. doi: 10.1074/jbc.M000181200. [DOI] [PubMed] [Google Scholar]
- 58.Wallace J.L. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn't the stomach digest itself? Physiological Reviews. 2008;88(4):1547–1565. doi: 10.1152/physrev.00004.2008. [DOI] [PubMed] [Google Scholar]
- 59.Nisoli E., Falcone S., Tonello C., Cozzi V., Palomba L., Fiorani M., Pisconti A., Brunelli S., Cardile A., Francolini M., Cantoni O., Carruba M.O., Moncada S., Clementi E. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proceedings of the National Academy of USA. 2004;101(47):16507–16512. doi: 10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poderoso J.J., Carreras M.C., Lisdero C., Riobo N., Schopfer F., Boveris A. Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Archives of Biochemistry and Biophysics. 1996;328(1):85–92. doi: 10.1006/abbi.1996.0146. [DOI] [PubMed] [Google Scholar]
- 61.Brookes P.S., Salinas E.P., Darley-Usmar K., Eiserich J.P., Freeman B.A., Darley-Usmar V.M., Anderson P.G. Concentration-dependent effects of nitric oxide on mitochondrial permeability transition and cytochrome c release. Journal of Biological Chemistry. 2000;275(27):20474–20479. doi: 10.1074/jbc.M001077200. [DOI] [PubMed] [Google Scholar]
- 62.Brown G.C., Cooper C.E. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Letters. 1994;356(2–3):295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 63.Castello P.R., Woo D.K., Ball K., Wojcik J., Liu L., Poyton R.O. Oxygen-regulated isoforms of cytochrome c oxidase have differential effects on its nitric oxide production and on hypoxic signaling. Proceedings of the National Academy of Sciences USA. 2008;105(24):8203–8208. doi: 10.1073/pnas.0709461105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poyton R.O., Castello P.R., Ball K.A., Woo D.K., Pan N. Mitochondria and hypoxic signaling. Annals of the New York Academy of Sciences. 2009;1177(1):48–56. doi: 10.1111/j.1749-6632.2009.05046.x. [DOI] [PubMed] [Google Scholar]
- 65.Sarti P., Forte E., Mastronicola D., Giuffre A., Arese M. Cytochrome c oxidase and nitric oxide in action: molecular mechanisms and pathophysiological implications. Biochimica Biophysica Acta. 2012;1817(4):610–619. doi: 10.1016/j.bbabio.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 66.Mo L., Wang Y., Geary L., Corey C., Alef M.J., Beer-Stolz D., Zuckerbraun B.S., Shiva S. Nitrite activates AMP kinase to stimulate mitochondrial biogenesis independent of soluble guanylate cyclase. Free Radical Biology and Medicine. 2012;53(7):1440–1450. doi: 10.1016/j.freeradbiomed.2012.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clementi E., Brown G.C., Feelisch M., Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proceedings of the National Academy of Sciences USA. 1998;95(13):7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galkin A., Moncada S. S-nitrosation of mitochondrial complex I depends on its structural conformation. Journal of Biological Chemistry. 2007;282(52):37448–37453. doi: 10.1074/jbc.M707543200. [DOI] [PubMed] [Google Scholar]
- 69.Gavrikova E.V., Vinogradov A.D. Active/de-active state transition of the mitochondrial complex I as revealed by specific sulfhydryl group labeling. FEBS Letters. 1999;455(1–2):36–40. doi: 10.1016/s0014-5793(99)00850-9. [DOI] [PubMed] [Google Scholar]
- 70.Zweier J.L., Samouilov A., Kuppusamy P. Non-enzymatic nitric oxide synthesis in biological systems. Biochimica Biophysica Acta. 1999;1411(2–3):250–262. doi: 10.1016/s0005-2728(99)00018-3. [DOI] [PubMed] [Google Scholar]
- 71.Sarti P., Giuffre A., Forte E., Mastronicola D., Barone M.C., Brunori M. Nitric oxide and cytochrome c oxidase: mechanisms of inhibition and NO degradation. Biochemical and Biophysical Research Communications. 2000;274(1):183–187. doi: 10.1006/bbrc.2000.3117. [DOI] [PubMed] [Google Scholar]
- 72.Mastronicola D., Genova M.L., Arese M., Barone M.C., Giuffre A., Bianchi C., Brunori M., Lenaz G., Sarti P. Control of respiration by nitric oxide in Keilin–Hartree particles, mitochondria and SH-SY5Y neuroblastoma cells. Cellular and Molecular Life Sciences. 2003;60(8):1752–1759. doi: 10.1007/s00018-003-3127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castello P.R., David P.S., McClure T., Crook Z., Poyton R.O. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metabolism. 2006;3(4):277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 74.Cleeter M.W., Cooper J.M., Darley-Usmar V.M., Moncada S., Schapira A.H. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Letters. 1994;345(1):50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 75.Shiva S., Gladwin M.T. Nitrite mediates cytoprotection after ischemia/reperfusion by modulating mitochondrial function. Basic Research in Cardiology. 2009;104(2):113–119. doi: 10.1007/s00395-009-0009-3. [DOI] [PubMed] [Google Scholar]
- 76.Gladwin M.T., Kim-Shapiro D.B. The functional nitrite reductase activity of the heme-globins. Blood. 2008;112(7):2636–2647. doi: 10.1182/blood-2008-01-115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doyle M.P., LePoire D.M., Pickering R.A. Oxidation of hemoglobin and myoglobin by alkyl nitrites inhibition by oxygen. Journal of Biological Chemistry. 1981;256(23):12399–12404. [PubMed] [Google Scholar]
- 78.Brooks J. The action of nitrite on haemoglobin in the absence of oxygen. Proceedings of the Royal Society of Medicine. 1937;137:368–382. [Google Scholar]
- 79.Huang Z., Shiva S., Kim-Shapiro D.B., Patel R.P., Ringwood L.A., Irby C.E., Huang K.T., Ho C., Hogg N., Schechter A.N., Gladwin M.T. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. Journal of Clinical Investigation. 2005;115(8):2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Basu S., Grubina R., Huang J., Conradie J., Huang Z., Jeffers A., Jiang A., He X., Azarov I., Seibert R., Mehta A., Patel R., King S.B., Hogg N., Ghosh A., Gladwin M.T., Kim-Shapiro D.B. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nature Chemical Biology. 2007;3(12):785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 81.Shiva S., Huang Z., Grubina R., Sun J., Ringwood L.A., MacArthur P.H., Xu X., Murphy E., Darley-Usmar V.M., Gladwin M.T. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circulation Research. 2007;100(5):654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 82.Larsen F.J., Schiffer T.A., Borniquel S., Sahlin K., Ekblom B., Lundberg J.O., Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metabolism. 2011;13(2):149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 83.Rassaf T., Flogel U., Drexhage C., Hendgen-Cotta U., Kelm M., Schrader J. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circulation Research. 2007;100(12):1749–1754. doi: 10.1161/CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- 84.Millar T.M., Stevens C.R., Benjamin N., Eisenthal R., Harrison R., Blake D.R. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Letters. 1998;427(2):225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 85.Baker J.E., Su J., Fu X., Hsu A., Gross G.J., Tweddell J.S., Hogg N. Nitrite confers protection against myocardial infarction: role of xanthine oxidoreductase, NADPH oxidase and K(ATP) channels. Journal of Molecular and Cellular Cardiology. 2007;43(4):437–444. doi: 10.1016/j.yjmcc.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Millar T.M. Peroxynitrite formation from the simultaneous reduction of nitrite and oxygen by xanthine oxidase. FEBS Letters. 2004;562(1–3):129–133. doi: 10.1016/S0014-5793(04)00218-2. [DOI] [PubMed] [Google Scholar]
- 87.Szabo C., Ischiropoulos H., Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nature Reviews Drug Discovery. 2007;6(8):662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 88.Gershon M.D. The enteric nervous system: a second brain. Hospital Practice (Minneapolis) 1999;34(7):31–32. doi: 10.3810/hp.1999.07.153. 5–8, 41–2 passim. [DOI] [PubMed] [Google Scholar]
- 89.San Gabriel A.M., Maekawa T., Uneyama H., Yoshie S., Torii K. mGluR1 in the fundic glands of rat stomach. FEBS Letters. 2007;581(6):1119–1123. doi: 10.1016/j.febslet.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 90.Mayer E.A. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47(6):861–869. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kitamura A., Tsurugizawa T., Uematsu A., Torii K., Uneyama H. New therapeutic strategy for amino acid medicine: effects of dietary glutamate on gut and brain function. Jornal of Pharrmacological Sciences. 2012;118(2):138–144. doi: 10.1254/jphs.11r06fm. [DOI] [PubMed] [Google Scholar]
- 92.Presley T.D., Morgan A.R., Bechtold E., Clodfelter W., Dove R.W., Jennings J.M., Kraft R.A., King S.B., Laurienti P.J., Rejeski W.J., Burdette J.H., Kim-Shapiro D.B., Miller G.D. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide. 2011;24(1):34–42. doi: 10.1016/j.niox.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garthwaite J., Boulton C.L. Nitric oxide signaling in the central nervous system. Annual Review of Physiology. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- 94.Bredt D.S., Glatt C.E., Hwang P.M., Fotuhi M., Dawson T.M., Snyder S.H. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991;7(4):615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- 95.East S.J., Garthwaite J. NMDA receptor activation in rat hippocampus induces cyclic GMP formation through the L-arginine-nitric oxide pathway. Neuroscience Letters. 1991;123(1):17–19. doi: 10.1016/0304-3940(91)90147-l. [DOI] [PubMed] [Google Scholar]
- 96.Garthwaite J. Glutamate, nitric oxide and cell–cell signaling in the nervous system. Trends in Neuroscience. 1991;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- 97.Southam E., Garthwaite J. Nitric oxide-cyclic GMP pathway in brain slices. Methods in Enzymology. 1996;269:129–133. doi: 10.1016/s0076-6879(96)69015-6. [DOI] [PubMed] [Google Scholar]
- 98.Garthwaite J. Concepts of neural nitric oxide-mediated transmission. European Journal of Neuroscience. 2008;27(11):2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guix F.X., Uribesalgo I., Coma M., Munoz F.J. The physiology and pathophysiology of nitric oxide in the brain. Progress in Neurobiology. 2005;76(2):126–152. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 100.Steinert J.R., Chernova T., Forsythe I.D. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist. 2010;16(4):435–452. doi: 10.1177/1073858410366481. [DOI] [PubMed] [Google Scholar]
- 101.Santos R.M., Lourenco C.F., Gerhardt G.A., Cadenas E., Laranjinha J., Barbosa R.M. Evidence for a pathway that facilitates nitric oxide diffusion in the brain. Neurochemistry International. 2011;59(1):90–96. doi: 10.1016/j.neuint.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 102.Cooper C.E. Nitric oxide and iron proteins. Biochimica Biophysica Acta. 1999;1411(2–3):290–309. doi: 10.1016/s0005-2728(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 103.Ledo A., Barbosa R.M., Frade J., Laranjinha J. Nitric oxide monitoring in hippocampal brain slices using electrochemical methods. Methods in Enzymology. 2002;359:111–125. doi: 10.1016/s0076-6879(02)59176-x. [DOI] [PubMed] [Google Scholar]
- 104.Ferreira N.R., Ledo A., Frade J.G., Gerhardt G.A., Laranjinha J., Barbosa R.M. Electrochemical measurement of endogenously produced nitric oxide in brain slices using Nafion/o-phenylenediamine modified carbon fiber microelectrodes. Analytica Chimica Acta. 2005;535(1–2):1–7. [Google Scholar]
- 105.Barbosa R.M., Lourenco C.F., Santos R.M., Pomerleau F., Huettl P., Gerhardt G.A., Laranjinha J. In vivo real-time measurement of nitric oxide in anesthetized rat brain. Methods in Enzymology. 2008;441:351–367. doi: 10.1016/S0076-6879(08)01220-2. [DOI] [PubMed] [Google Scholar]
- 106.Santos R.M., Lourenco C.F., Piedade A.P., Andrews R., Pomerleau F., Huettl P., Gerhardt G.A., Laranjinha J., Barbosa R.M. A comparative study of carbon fiber-based microelectrodes for the measurement of nitric oxide in brain tissue. Biosensors and Bioelectronics. 2008;24(4):704–709. doi: 10.1016/j.bios.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 107.Peters J.L., Miner L.H., Michael A.C., Sesack S.R. Ultrastructure at carbon fiber microelectrode implantation sites after acute voltammetric measurements in the striatum of anesthetized rats. Journal of Neuroscience Methods. 2004;137(1):9–23. doi: 10.1016/j.jneumeth.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 108.Attwell D., Buchan A.M., Charpak S., Lauritzen M., Macvicar B.A., Newman E.A. Glial and neuronal control of brain blood flow. Nature. 2010;468(7321):232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Millar J. The nitric oxide/ascorbate cycle: how neurones may control their own oxygen supply. Medical Hypotheses. 1995;45(1):21–26. doi: 10.1016/0306-9877(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 110.Milsom A.B., Fernandez B.O., Garcia-Saura M.F., Rodriguez J., Feelisch M. Contributions of nitric oxide synthases, dietary nitrite/nitrate, and other sources to the formation of NO signaling products. Antioxidants and Redox Signaling. 2012;17(3):422–432. doi: 10.1089/ars.2011.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gladwin M.T., Raat N.J., Shiva S., Dezfulian C., Hogg N., Kim-Shapiro D.B., Patel R.P. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. American Journal of Physiology: Heart and Circulatory Physiology. 2006;291(5):H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 112.Sparacino-Watkins C.E., Lai Y.C., Gladwin M.T. Nitrate-nitrite-nitric oxide pathway in pulmonary arterial hypertension therapeutics. Circulation. 2012;125(23):2824–2826. doi: 10.1161/CIRCULATIONAHA.112.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Totzeck M., Hendgen-Cotta U.B., Luedike P., Berenbrink M., Klare J.P., Steinhoff H.J., Semmler D., Shiva S., Williams D., Kipar A., Gladwin M.T., Schrader J., Kelm M., Cossins A.R., Rassaf T. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation. 2012;126(3):325–334. doi: 10.1161/CIRCULATIONAHA.111.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zuckerbraun B.S., Shiva S., Ifedigbo E., Mathier M.A., Mollen K.P., Rao J., Bauer P.M., Choi J.J., Curtis E., Choi A.M., Gladwin M.T. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation. 2010;121(1):98–109. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]
- 115.Pedersen C.L., Faggiano S., Helbo S., Gesser H., Fago A. Roles of nitric oxide, nitrite and myoglobin on myocardial efficiency in trout (Oncorhynchus mykiss) and goldfish (Carassius auratus): implications for hypoxia tolerance. Journal of Experimental Biology. 2010;213(Pt 16):2755–2762. doi: 10.1242/jeb.041624. [DOI] [PubMed] [Google Scholar]
- 116.Mikula I., Durocher S., Martasek P., Mutus B., Slama-Schwok A. Isoform-specific differences in the nitrite reductase activity of nitric oxide synthases under hypoxia. Biochemical Journal. 2009;418(3):673–682. doi: 10.1042/BJ20080987. [DOI] [PubMed] [Google Scholar]
- 117.Webb A.J., Milsom A.B., Rathod K.S., Chu W.L., Qureshi S., Lovell M.J., Lecomte F.M., Perrett D., Raimondo C., Khoshbin E., Ahmed Z., Uppal R., Benjamin N., Hobbs A.J., Ahluwalia A. Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia: role for xanthine oxidoreductase and endothelial nitric oxide synthase. Circulation Research. 2008;103(9):957–964. doi: 10.1161/CIRCRESAHA.108.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Feelisch M., Fernandez B.O., Bryan N.S., Garcia-Saura M.F., Bauer S., Whitlock D.R., Ford P.C., Janero D.R., Rodriguez J., Ashrafian H. Tissue processing of nitrite in hypoxia: an intricate interplay of nitric oxide-generating and scavenging systems. Journal of Biological Chemistry. 2008;283(49):33927–33934. doi: 10.1074/jbc.M806654200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Piknova B., Kocharyan A., Schechter A.N., Silva A.C. The role of nitrite in neurovascular coupling. Brain Research. 2011;1407:62–68. doi: 10.1016/j.brainres.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rifkind J.M., Nagababu E., Barbiro-Michaely E., Ramasamy S., Pluta R.M., Mayevsky A. Nitrite infusion increases cerebral blood flow and decreases mean arterial blood pressure in rats: a role for red cell NO. Nitric Oxide. 2007;16(4):448–456. doi: 10.1016/j.niox.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 121.Rice M.E. Ascorbate regulation and its neuroprotective role in the brain. Trends in Neuroscience. 2000;23(5):209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- 122.Grunewald R.A. Ascorbic acid in the brain. Brain Research Reviews. 1993;18(1):123–133. doi: 10.1016/0165-0173(93)90010-w. [DOI] [PubMed] [Google Scholar]
- 123.Corti A., Casini A.F., Pompella A. Cellular pathways for transport and efflux of ascorbate and dehydroascorbate. Archives of Biochemistry and Biophysics. 2010;500(2):107–115. doi: 10.1016/j.abb.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 124.Rebec G.V., Pierce R.C. A vitamin as neuromodulator: ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Progress in Neurobiology. 1994;43(6):537–565. doi: 10.1016/0301-0082(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 125.Portugal C.C., Miya V.S., Calaza Kda C., Santos R.A., Paes-de-Carvalho R. Glutamate receptors modulate sodium-dependent and calcium-independent vitamin C bidirectional transport in cultured avian retinal cells. Journal of Neurochemistry. 2009;108(2):507–520. doi: 10.1111/j.1471-4159.2008.05786.x. [DOI] [PubMed] [Google Scholar]
- 126.Kulagina N.V., Shankar L., Michael A.C. Monitoring glutamate and ascorbate in the extracellular space of brain tissue with electrochemical microsensors. Analytical Chemistry. 1999;71(22):5093–5100. doi: 10.1021/ac990636c. [DOI] [PubMed] [Google Scholar]
- 127.van der Toorn A., Sykova E., Dijkhuizen R.M., Vorisek I., Vargova L., Skobisova E., van Lookeren Campagne M., Reese T., Nicolay K. Dynamic changes in water ADC, energy metabolism, extracellular space volume, and tortuosity in neonatal rat brain during global ischemia. Magnetic Resonance in Medical Sciences. 1996;36(1):52–60. doi: 10.1002/mrm.1910360110. [DOI] [PubMed] [Google Scholar]
- 128.Watts J., Whitton P.S., Pearce B. Unexpected effects of nitric oxide synthase inhibitors on extracellular nitrite levels in the hippocampus in vivo. Pharmacology. 2005;74(3):163–168. doi: 10.1159/000085774. [DOI] [PubMed] [Google Scholar]
- 129.Cross A.H., Manning P.T., Keeling R.M., Schmidt R.E., Misko T.P. Peroxynitrite formation within the central nervous system in active multiple sclerosis. Journal of Neuroimmunology. 1998;88(1–2):45–56. doi: 10.1016/s0165-5728(98)00078-2. [DOI] [PubMed] [Google Scholar]
- 130.Jobgen W.S., Jobgen S.C., Li H., Meininger C.J., Wu G. Analysis of nitrite and nitrate in biological samples using high-performance liquid chromatography. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 2007;851(1–2):71–82. doi: 10.1016/j.jchromb.2006.07.018. [DOI] [PubMed] [Google Scholar]


