Abstract
Nitric oxide (NO) and hydrogen sulfide (H2S) have emerged as dominant redox regulators of numerous aspects of cellular and physiological functions within several organ systems included cardiovascular, immune and neurological tissues. Recent studies have begun to reveal that these two gaseous molecules may have redundant or overlapping pathophysiological functions often involving similar molecular targets. However, it remains less clear when and how NO and H2S may interact under biological and disease processes. In this graphical review, we discuss the current understanding of NO and H2S interactions and how they may functionally influence each other and what this may mean for biology and mechanisms of disease.
Keywords: Thiol, Redox biology, Cardiovascular disease, Vascular biology, Chemistry
Highlights
-
•
H2S and NO are important gaseous regulators of numerous physiological responses.
-
•
H2S and NO may target both similar and divergent signaling and molecular pathways.
-
•
H2S and NO react with protein free thiols that differentially affect protein function.
-
•
H2S and NO metabolites can react together to yield novel biochemical products.
-
•
The presence and physiological importance of these novel products remains unknown.
Introduction
Nitric oxide (NO) has been extensively studied over the last three decades for its role in vascular functions and as a signaling molecule [1]. Nobel winning works from the trio Furchgott, Murad and Ignarro has placed NO as a central endothelial-derived relaxing factor (EDRF) and a key regulator of cardiovascular pathophysiological responses. However, the role of this gaseous molecule is being re-evaluated with the appreciation of a new gasotransmitter hydrogen sulfide (H2S) that also serves many important regulatory roles in physiological systems. Like NO, H2S was once thought to simply be a toxic gas but it is now believed to be an important redox-signaling molecule. A decade of studies on H2S biology have elucidated its role in regulation of vascular homeostasis, neurological function, cytoprotection, anti-inflammation, revascularization and therapeutic angiogenesis; along with modulation of cell survival responses, which is similar to many physiological roles of NO.
Production of either molecule occurs through enzymatic and non-enzymatic pathways. Fig. 1 illustrates H2S formation via the transulfuration pathway involving CBS and CSE along with cysteine catabolism via MST. It is also possible that H2S may be obtained through reductive chemistry on thiosulfate, thiocystine and other molecules. Similarly, NO formation predominantly occurs through nitric oxide synthases (NOS's); however, it is increasingly apparent that non-enzymatic generation of NO via various nitrite/nitrate reduction mechanisms also critically regulates bioavailability. The physiological functions of NO [2–4] and H2S [5–7] have been extensively studied and reviewed in the literature. However, the interrelation of NO–H2S and their subsequent biochemical interactions are complex and currently unclear. While some studies have shown that NO/H2S positively affect each other's production and function [8–10]; other studies report contrarian, if not directly opposite findings [11–13]. Thus, significant ambiguity remains regarding NO–H2S chemical interactions and subsequent biological effects. This graphical review discusses the latest understanding of the relationship between these two gaseous signaling molecules and their roles in regulating several biological functions along with important future directions for research.
Fig. 1.
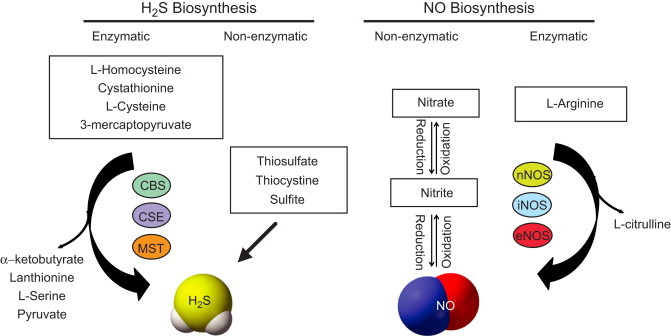
Biosynthesis of NO and H2S: NO and H2S are enzymatically synthesized by three enzymes. H2S is generated from oxidation of the substrates l-homocysteine, cystathionine, l-cysteine and 3-mercaptopyruvate through the enzymes cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (3-MST). α-ketobutyrate, lanthionine, l-serine and pyruvate are the secondary products formed. NO is produced by three NOS isoforms neuronal, inducible and endothelial NO synthase (nNOS, iNOS and eNOS) that catalyze the oxidation of l-arginine to l-citrulline; Alternatively, production of H2S occurs non-enzymatically from various storage forms of sulfur like thiosulfate, thiocysteine and sulfite; whereas NO is produced through reduction of nitrite/nitrate under low oxygen conditions.
NO–H2S signaling
To date, only a small number of reports suggest that NO–H2S molecules may influence each other in their production and pathophysiological functions [5,14]. Studies demonstrate a common signaling pathway where NO–H2S crosstalk mediates their effects on vascular functions such as vasodilation, vascular remodeling (migration and proliferation) and angiogenesis [10,14–16]. Recent studies demonstrate H2S mediated upregulation of NO and vice-versa in regulating angiogenesis and attenuation of ischemia reperfusion (I/R) injury [14,15,17,18]. Fig. 2 illustrates that pro-angiogenic and I/R injury protection of H2S and its donors may occur through induction of VEGF/VEGFR2 signaling and its downstream effectors such as PI3K/Akt/eNOS in the vascular endothelial cells [8,10,19,20]. Moreover, H2S has been reported to prevent eNOS degradation and induce eNOS phosphorylation with subsequent NO production via PI3K/Akt activity [21,22] and p38 MAPK pathways [23]. H2S therapy can also preserve mitochondrial function and modulate cardioprotection through attenuation of oxidative stress via VEGF/Akt/eNOS/NO/cGMP pathway [8]. Reciprocally, pharmacological donors of NO can up-regulate substrate bioavailability for and expression of the H2S synthesis enzyme cystathionine gamma lyase (CGL/CSE) resulting in H2S production eliciting vasodilatory effects [14,24–26]. However, it has been reported that use of an NO donor can inhibit CBS expression counteracting what has been shown for CGL [13]. Finally, studies have shown that H2S has opposing effects on NOS/NO metabolism in that H2S can down regulate expression or inhibit activity of eNOS and subsequent NO production involving altered l-arginine/BH4, increased HO-1/CO and other unknown mechanisms [11,12,27,28].
Fig. 2.
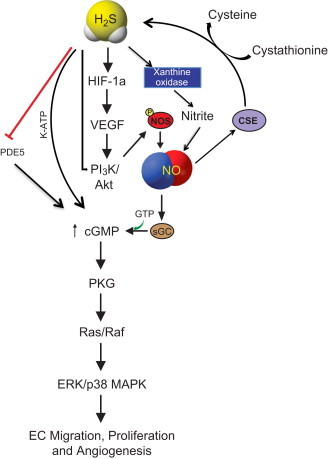
Common signaling pathways of H2S and NO: H2S and NO mediated vascular remodeling aspects through common pathway that include VEGF, HIF-1α, PI3K/AKT upregulated by H2S. PI3K/AKT induces NOS/NO. H2S directly effects NO through XO mediated nitrite. Both NO and H2S are independently involved in upregulating cGMP; H2S acts through K-ATP and PDE5, NO activates enzyme sGC to increase cGMP production that has downstream signaling effects of EC migration, proliferation and angiogenesis via PKG/Ras–Raf/ERK-p38 MAPK axis.
Hydrogen sulfide also potently regulates cellular redox balance necessary for cytoprotection and inhibition of oxidative stress (Fig. 3). Studies have shown that exogenous H2S stimulates Nrf2 activation leading to increased anti-oxidant defense responses [29,30]. Moreover, H2S therapy has been reported to blunt NOX1 expression and activity thus alleviating oxidative stress [31]. H2S exerts anti-inflammatory protection of organs exposed to I/R injury through an eNOS/NO and p38 MAPK dependent mechanism. Importantly, this protective effect of H2S is not evident in eNOS−/− mice [32]. Similar observations are found upon inhibition of eNOS and subsequent NO production attenuating H2S-mediated vascular responses including vasorelaxation and angiogenesis [17]. These observations indicate that H2S can mediate its effects through a NO dependent pathway. Likewise, chemical inhibition of CSE inhibits NO-mediated vascular functions, suggesting its interdependency on H2S [17]. However, our group has found that H2S therapy is beneficial for ischemic vascular remodeling in eNOS−/− mice involving alternative non-enzymatic generation of NO via xanthine oxidase (XO) mediated nitrite reduction that increases tissue VEGF, cGMP production and angiogenesis activity under ischemic conditions [15].
Fig. 3.
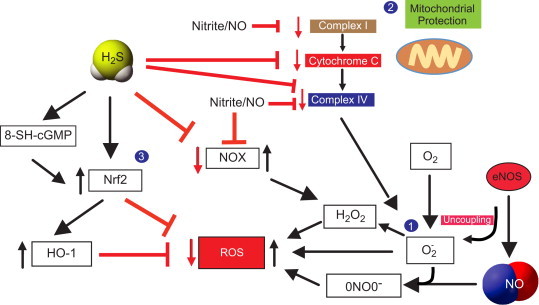
NO and H2S redox regulations and cytoprotection: (1) Reactive oxygen species are collectively formed from hydrogen peroxide (H2O2), peronynitrite (ONOO−) and superoxide radical (•O2−). Superoxide radical is formed from the mitochondrial complexes and uncoupling of eNOS, which further reacts with NO to form peroxynitrite. Nitrite/NO regulates NOX that upregulates H2O2 thereby contributing to ROS. (2) Both H2S and nitrite/NO are involved in cytoprotection by inhibiting mitochondrial complexes I and IV and the intermediary component cytochrome C that generate ROS. (3) H2S regulate NRF2 directly and via cGMP variant 8-SH-cGMP to reduce ROS production. Nrf2 also induces HO-1 mediated inhibition of ROS.
The role of NO as a potent vasodilator through activation of soluble guanylate cyclase (sGC) and subsequent cGMP production is well established [33]. Likewise, H2S can exhibit vasodilatory effects indirectly by delaying cGMP degeneration through PDE5 inhibition [34]. However, H2S may also trigger dose-dependent vasoconstriction or dilatory effects depending on type of the vessel and the animal species examined. This conditional response toward H2S is mediated though chemical modification of potassium channel protein thiols [5,35,36] or due to nitrosothiol formed as a result of NO/H2S interaction [36]. Studies with specific knockouts of NOS or CSE further substantiate the vasodilatory effect of NO/H2S [37,38]. Moreover, a concept is emerging whereby H2S induces eNOS/NO production by Ca2+ release that may also contribute to vasodilation [19]. In contrast, chemical inhibition of CSE attenuates NO mediated cGMP formation, vasodilation and angiogenesis [17]. Together, these reports suggest an important role for H2S in NO mediated vascular effects. Nevertheless, it appears that NO–H2S and their enzymatic pathways may be mutually interactive and influence various biological functions. Additional studies are desperately needed to understand this complex relationship that has significant potential to advance our understanding of redox regulation of vascular reactivity.
Posttranslational modifications
Protein posttranslational modifications (PTM's) play significant roles in affecting not only the structure and interaction of proteins, but also regulating physiological functions through activation of various signaling pathways [39–41]. Fig. 4 shows that thiol residues of proteins may undergo different PTM through reactions with various biochemicals (e.g. H2S/HS−, NO or GSH) thereby affecting protein function. Active Cys residues at enzymes sites with low pKa can exist predominantly as thiolate anions (S−) that are strong nucleophiles and interact with ROS species to generate sulfenic acid (SOH) (panel 4A), which can readily react with HS− or H2S to form a persulfide bond [42]. NO also participates in modulating signaling function in a similar way through protein thiol S-nitrosation. This predominantly occurs due to NO radical (or other RNS species) oxidation of protein free thiol (RSH) with a nitrosyl group at the cysteine to form S-nitrosothiol [43]. This modified S-nitrosothiol may be much less reactive than the original thiol group resulting in reduced reactivity of cysteine. Thus, H2S mediates protein modifications through persulfidation (also known as ‘sulfhydration’), similar to nitrosation by NO.
Fig. 4.
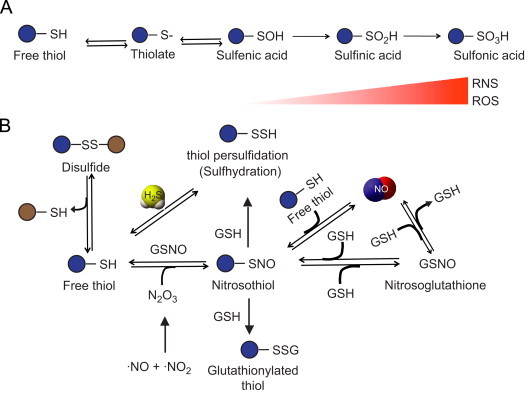
Protein modifications mediated by NO and H2S: (A) Oxidation of free thiol leads to form thiolate. A series of oxidation reactions can take place in the presence of increasing reactive nitrogen or oxygen species that can oxidize thiolate (protein-S-) to sulfenic acid (protein–SOH) further irreversibly oxidize to sulfinic (protein–SO2H) and sulfonic acid (protein–SO3H). (B) Reduction of disulfides forms thiols. A free thiol decoupled from disulfide can react with either H2S to form –SSH group through persulfidation (sulfhydration) or may form nitrosothiol by reacting with dinitrogen trioxide (N2O3) that is formed from nitrite and a NO radical (NO•). SNO formation occurs when NO reacts with a thiol and to form NO, likewise SNO can also release NO. SNO can be modified into glutathionylated thiol (protein–SSG) in presence of a GSH or can alternatively form nitrosoglutathione (GSNO). GSNO can further oxidize to form NO and GSH.
H2S modifies Cys residues of the proteins where the sulfhydryl group of cysteine is converted to an –SSH group (panel 4B). This modified cysteine residue is highly reactive and increases the catalytic activity of targeted proteins. For instance, Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), a glycolytic enzyme can be modified both by S-nitrosylation and S-sulfhydration at Cys-150 [40]. While, NO inhibits catalytic activity of GAPDH, H2S increases it. Interestingly, other enzymes such as protein tyrosine phosphatases (PTP) are also regulated by NO and H2S through protein modifications. NO modifies PTP-1B by S-nitrosation at cysteine 215 residue, which prevents inactivation by H2O2 induced irreversible oxidation [44]. It has been reported that H2S typically activates target proteins through sulfhydration of cysteine; however, with PTP1B Cys persulfide formation inactivates the enzyme in a reversible manner contributing to ER stress response effects on PTP enzyme function. Importantly, PTP1B persulfidation also occurs on cysteine 215, which is preferentially reversed by thioredoxin/thioredoxin reductase versus other intracellular reduction pathways highlighting the thiol persulfidation (sulfhydration) may act in a sensitive manner to control cellular physiology [45]. Together, these recent studies suggest that persulfidation may be more prevalent than nitrosylation and possibly equivalent to phosphorylation in regulating various biological functions; however, comprehensive studies examining thiol modification states are necessary in order to understand the magnitude and degree of various thiol PTM [40,45–47]. It is intriguing that both NO and H2S can target the same reactive Cys residues of a given protein that diversely impacts catalytic activity and function of the protein revealing complex regulatory mechanisms that are not fully understood. Additionally, it is also unclear what role post-translational thiol modification by glutathione plays in modulating nitrosation versus persulfidation in an ever-evolving paradigm of redox signaling. It is clear that more work is needed to better understand when these various PTM's form, the prevalence of them in biological systems, and their functional effects under physiological and pathological conditions.
Novel molecules from NO–H2S interaction
Both NO and H2S are chemically reactive gaseous molecules that are generated and distributed across various tissues. The nucleophilic properties of H2S and metabolites and electrophilic characteristics of NO metabolites suggest a possible cross talk between metabolite products of these two gaseous molecules forming new intermediates (Fig. 5). Although, it is important to understand that direct reaction between H2S and NO is chemically unfavorable thus opening the possibility of ionic metabolite interactions. Early observations from Kimura and co-workers suggested that H2S and NO serve a coordinated relationship in regulating the vascular tone [48]. Later, Whiteman and colleagues reported potential antioxidant effects of H2S through its interaction with peroxynitrite (ONOO−) [49]. A follow up study from this group further suggested that NO and H2S may react to form a novel nitrosothiol that has not been further characterized [50,51]. However, more recent studies have demonstrated that NO/H2S metabolites can react forming novel chemical products that could uniquely influence biochemical and physiological responses [52–56].
Fig. 5.
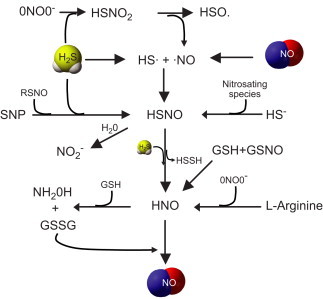
Potential NO–H2S chemical interactions: Radicals of NO (NO•) and H2S (HS•) leads to formation of thionitrous acid (HSNO). HSNO formation can also occur from reaction between sodium nitroprusside (SNP) either with RSNO or H2S. Alternatively the anionic form of H2S, hydrosulfide ion (HS−) can react with a nitrosating species leading to form HSNO. Peroxynitrite reacts with H2S to form sulfinyl nitrite (HSNO2), which further dissociate into NO• and HSO•. HSNO can react with H2S to form nitroxyl (HNO), and HSNO upon hydration (H2O) can lead to nitrite formation. HNO is also formed from l-arginine through a reaction of peroxynitrite. Finally, HNO can further dissociate into hydroxylamine on reacting with glutathione (GSH), releasing GSSG in this process, which can in turn react with HNO forming NO.
NO can react with oxygen radicals to form secondary reactive nitrogen species such as N2O3 or ONOO−, as well as S-nitrosation reactions with protein and small molecular weight thiols resulting in S-nitrosothiols (RSNO) affecting numerous redox dependent processes. NO oxidation products such as nitrite, nitrate, N2O3, nitrosothiols and other NO metabolites such as electrophilic-nitrated fatty acids can react with H2S. While it is most unlikely that a direct interaction between H2S and NO occurs; HS− may react with either oxidized form of NO, NO• or nitrosating species (formed through reaction with NO•, O2−• and ONOO−) or SNO/GSNO to form novel molecules like nitrosothiol, sulfinyl nitrite (HS(O)NO or HSNO2) or nitroxyl (HNO) with less clear physiological implications. Importantly H2S, at therapeutic concentrations (low to mid micromolar) inhibit cytotoxic effects of peroxynitrite possibly through formation sulfinyl nitrite, a precursor that may also form HNO. This novel product of H2S and ONOO− has the potential to release NO while simultaneously neutralizing pro-apoptotic and oxidative effects of peroxynitrite [55].
H2S by itself may react with RSNO to form thionitrous acid (HSNO) [27,51]. Intracellular formation of HSNO, under physiological conditions is still debatable. However, its production through NO [57], cytochrome c [58] and heme containing enzymes [59] has been reported. HSNO has been shown in vitro to diffuse intracellularly and facilitate transnitrosation of proteins such as hemoglobin. HSNO can be metabolized to generate NO and other NO species like HNO that has presumably longer half-life and may have physiological roles in oxygen delivery and cardioprotection [53].
Nitroxyl (HNO), a protonated form of NO, is highly reactive to nucleophiles such as thiols. Recent studies demonstrate that HNO can have distinct physiological functions such as vasodilation and cardioprotection especially at low concentrations [20,60]. The effects of HNO in cardioprotection may also occur through cGMP [61]. On the contrary, there are also reports suggesting that HNO can be neurotoxic, cause inflammation and arrhythmia, DNA oxidation and thiol loss at much higher concentrations than what would be considered a therapeutic dose [20,62,63]. These studies suggest careful use considering their significant toxicity at high concentrations. Extensive studies of these various reaction products between H2S and NO require further experimentation to understand complex biochemical signaling mechanisms between these mediators for various biological functions.
Conclusion
It is evident from the literature that the two “gasotransmitters” NO and H2S perform a variety of homeostatic physiological functions. Both of these molecules are implicated in signaling of many complex pathways under physiological and pathological conditions. Though there are few studies demonstrating NO–H2S interplay, it is also unclear whether the kinetics of NO–H2S reaction and formation of novel compounds may be biologically significant compared to the presence and amount of other molecules (e.g. GSH). Considering the concentration dependent effects of NO and H2S, careful attention to bioavailable levels of these molecules will be critical to determine the likelihood that various reactions or interactions may occur to influence molecular and cellular physiological responses. Moreover, it will also be important for studies focusing on NO–H2S interactions to evolve from theoretical and test tube levels to cellular and pathophysiological models if we are to truly understand the relationship between NO and H2S interactions. Given the novelty of this area, it is likely that new and exciting discoveries will be revealed detailing gaseous mediator regulation of redox biology.
Acknowledgments
This work was supported by NIH Grant HL113303 to C.G.K and by a fellowship from the Malcolm Feist Cardiovascular Research Endowment, LSU Health Sciences Center-Shreveport to G.K.K.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Marsh N., Marsh A. A short history of nitroglycerine and nitric oxide in pharmacology and physiology. Clinical and Experimental Pharmacology and Physiology. 2000;27:313–319. doi: 10.1046/j.1440-1681.2000.03240.x. [DOI] [PubMed] [Google Scholar]
- 2.Kolluru G.K., Siamwala J.H., Chatterjee S. eNOS phosphorylation in health and disease. Biochimie. 2010;92:1186–1198. doi: 10.1016/j.biochi.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nature Reviews Drug Discovery. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 4.Moncada S., Palmer R.M., Higgs E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacological Reviews. 1991;43:109–142. [PubMed] [Google Scholar]
- 5.Li L., Rose P., Moore P.K. Hydrogen sulfide and cell signaling. Annual Review of Pharmacology and Toxicology. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 6.Szabo C., Papapetropoulos A. Hydrogen sulphide and angiogenesis: mechanisms and applications. British Journal of Pharmacology. 2011;164:853–865. doi: 10.1111/j.1476-5381.2010.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiological Reviews. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 8.Kondo K., Bhushan S., King A.L., Prabhu S.D., Hamid T., Koenig S., Murohara T., Predmore B.L., Gojon G., Sr, Gojon G., Jr, Wang R., Karusula N., Nicholson C.K., Calvert J.W., Lefer D.J. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 2013;127:1116–1127. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M.J., Cai W.J., Li N., Ding Y.J., Chen Y., Zhu Y.C. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxidants and Redox Signaling. 2010;12:1065–1077. doi: 10.1089/ars.2009.2945. [DOI] [PubMed] [Google Scholar]
- 10.Cai W.J., Wang M.J., Moore P.K., Jin H.M., Yao T., Zhu Y.C. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovascular Research. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Kubo S., Kurokawa Y., Doe I., Masuko T., Sekiguchi F., Kawabata A. Hydrogen sulfide inhibits activity of three isoforms of recombinant nitric oxide synthase. Toxicology. 2007;241:92–97. doi: 10.1016/j.tox.2007.08.087. [DOI] [PubMed] [Google Scholar]
- 12.Li X.H., Du J.B., Bu D.F., Tang X.Y., Tang C.S. Sodium hydrosulfide alleviated pulmonary vascular structural remodeling induced by high pulmonary blood flow in rats. Acta Pharmacologica Sinica. 2006;27:971–980. doi: 10.1111/j.1745-7254.2006.00353.x. [DOI] [PubMed] [Google Scholar]
- 13.Prathapasinghe G.A., Siow Y.L., Xu Z., O K. Inhibition of cystathionine-beta-synthase activity during renal ischemia-reperfusion: role of pH and nitric oxide. American Journal of Physiology—Renal Physiology. 2008;295:F912–F922. doi: 10.1152/ajprenal.00040.2008. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y.F., Mainali P., Tang C.S., Shi L., Zhang C.Y., Yan H., Liu X.Q., Du J.B. Effects of nitric oxide and hydrogen sulfide on the relaxation of pulmonary arteries in rats. Chinese Medical Journal (England) 2008;121:420–423. [PubMed] [Google Scholar]
- 15.Bir S.C., Kolluru G.K., McCarthy P., Shen X., Pardue S., Pattillo C.B., Kevil C.G. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1α and vascular endothelial growth factor-dependent angiogenesis. Journal of the American Heart Association. 2012 doi: 10.1161/JAHA.112.004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu Q., Wang B., Zhang X.F., Ma Y.P., Liu J.D., Wang X.Z. Contribution of hydrogen sulfide and nitric oxide to exercise-induced attenuation of aortic remodeling and improvement of endothelial function in spontaneously hypertensive rats. Molecular and Cellular Biochemistry. 2013;375:199–206. doi: 10.1007/s11010-012-1542-1. [DOI] [PubMed] [Google Scholar]
- 17.Coletta C., Papapetropoulos A., Erdelyi K., Olah G., Modis K., Panopoulos P., Asimakopoulou A., Gero D., Sharina I., Martin E., Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proceedings of the National Academy of Sciences of USA. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Predmore B.L., Kondo K., Bhushan S., Zlatopolsky M.A., King A.L., Aragon J.P., Grinsfelder D.B., Condit M.E., Lefer D.J. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. American Journal of Physiology. Heart and Circulatory Physiology. 2012;302:H2410–H2418. doi: 10.1152/ajpheart.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kida M., Sugiyama T., Yoshimoto T., Ogawa Y. Hydrogen sulfide increases nitric oxide production with calcium-dependent activation of endothelial nitric oxide synthase in endothelial cells. European Journal of Pharmaceutical Sciences. 2013;48:211–215. doi: 10.1016/j.ejps.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Yong Q.C., Hu L.F., Wang S., Huang D., Bian J.S. Hydrogen sulfide interacts with nitric oxide in the heart: possible involvement of nitroxyl. Cardiovascular Research. 2010;88:482–491. doi: 10.1093/cvr/cvq248. [DOI] [PubMed] [Google Scholar]
- 21.Lei Y.P., Liu C.T., Sheen L.Y., Chen H.W., Lii C.K. Diallyl disulfide and diallyl trisulfide protect endothelial nitric oxide synthase against damage by oxidized low-density lipoprotein. Molecular Nutrition and Food Research. 2010;54(Suppl 1):S42–S52. doi: 10.1002/mnfr.200900278. [DOI] [PubMed] [Google Scholar]
- 22.Yong Q.C., Lee S.W., Foo C.S., Neo K.L., Chen X., Bian J.S. Endogenous hydrogen sulphide mediates the cardioprotection induced by ischemic postconditioning. American Journal of Physiology. Heart and Circulatory Physiology. 2008;295:H1330–H1340. doi: 10.1152/ajpheart.00244.2008. [DOI] [PubMed] [Google Scholar]
- 23.Sojitra B., Bulani Y., Putcha U.K., Kanwal A., Gupta P., Kuncha M., Banerjee S.K. Nitric oxide synthase inhibition abrogates hydrogen sulfide-induced cardioprotection in mice. Molecular and Cellular Biochemistry. 2012;360:61–69. doi: 10.1007/s11010-011-1044-6. [DOI] [PubMed] [Google Scholar]
- 24.Eto K., Kimura H. A novel enhancing mechanism for hydrogen sulfide-producing activity of cystathionine beta-synthase. Journal of Biological Chemistry. 2002;277:42680–42685. doi: 10.1074/jbc.M205835200. [DOI] [PubMed] [Google Scholar]
- 25.Li H., Marshall Z.M., Whorton A.R. Stimulation of cystine uptake by nitric oxide: regulation of endothelial cell glutathione levels. American Journal of Physiology. 1999;276:C803–C811. doi: 10.1152/ajpcell.1999.276.4.C803. [DOI] [PubMed] [Google Scholar]
- 26.Liew H.C., Khoo H.E., Moore P.K., Bhatia M., Lu J., Moochhala S.M. Synergism between hydrogen sulfide (H(2)S) and nitric oxide (NO) in vasorelaxation induced by stonustoxin (SNTX), a lethal and hypotensive protein factor isolated from stonefish Synanceja horrida venom. Life Science. 2007;80:1664–1668. doi: 10.1016/j.lfs.2007.01.058. [DOI] [PubMed] [Google Scholar]
- 27.Geng B., Cui Y., Zhao J., Yu F., Zhu Y., Xu G., Zhang Z., Tang C., Du J. Hydrogen sulfide downregulates the aortic l-arginine/nitric oxide pathway in rats. American Journal of Physiology—Regulatory, Integrative, and Comparative Physiology. 2007;293:R1608–R1618. doi: 10.1152/ajpregu.00207.2006. [DOI] [PubMed] [Google Scholar]
- 28.Oh G.S., Pae H.O., Lee B.S., Kim B.N., Kim J.M., Kim H.R., Jeon S.B., Jeon W.K., Chae H.J., Chung H.T. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radical Biology and Medicine. 2006;41:106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Calvert J.W., Jha S., Gundewar S., Elrod J.W., Ramachandran A., Pattillo C.B., Kevil C.G., Lefer D.J. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circulation Research. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peake B.F., Nicholson C.K., Lambert J.P., Hood R.L., Amin H., Amin S., Calvert J.W. Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner. American Journal of Physiology. Heart and Circulatory Physiology. 2013;304:H1215–H1224. doi: 10.1152/ajpheart.00796.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muzaffar S., Shukla N., Bond M., Newby A.C., Angelini G.D., Sparatore A., Del Soldato P., Jeremy J.Y. Exogenous hydrogen sulfide inhibits superoxide formation, NOX-1 expression and Rac1 activity in human vascular smooth muscle cells. Journal of Vascular Research. 2008;45:521–528. doi: 10.1159/000129686. [DOI] [PubMed] [Google Scholar]
- 32.Yusof M., Kamada K., Kalogeris T., Gaskin F.S., Korthuis R.J. Hydrogen sulfide triggers late-phase preconditioning in postischemic small intestine by an NO- and p38 MAPK-dependent mechanism. American Journal of Physiology. Heart and Circulatory Physiology. 2009;296:H868–H876. doi: 10.1152/ajpheart.01111.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friebe A., Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circulation Research. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- 34.Bucci M., Papapetropoulos A., Vellecco V., Zhou Z., Pyriochou A., Roussos C., Roviezzo F., Brancaleone V., Cirino G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- 35.Dombkowski R.A., Russell M.J., Schulman A.A., Doellman M.M., Olson K.R. Vertebrate phylogeny of hydrogen sulfide vasoactivity. American Journal of Physiology—Regulatory, Integrative, and Comparative Physiology. 2005;288:R243–R252. doi: 10.1152/ajpregu.00324.2004. [DOI] [PubMed] [Google Scholar]
- 36.Webb G.D., Lim L.H., Oh V.M., Yeo S.B., Cheong Y.P., Ali M.Y., El Oakley R., Lee C.N., Wong P.S., Caleb M.G., Salto-Tellez M., Bhatia M., Chan E.S., Taylor E.A., Moore P.K. Contractile and vasorelaxant effects of hydrogen sulfide and its biosynthesis in the human internal mammary artery. Journal of Pharmacology and Experimental Therapeutics. 2008;324:876–882. doi: 10.1124/jpet.107.133538. [DOI] [PubMed] [Google Scholar]
- 37.Huang P.L., Huang Z., Mashimo H., Bloch K.D., Moskowitz M.A., Bevan J.A., Fishman M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 38.Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A.K., Mu W., Zhang S., Snyder S.H., Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnelle D.R., Stamler J.S. NO+, NO, and NO− donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Archives of Biochemistry and Biophysics. 1995;318:279–285. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- 40.Mustafa A.K., Gadalla M.M., Sen N., Kim S., Mu W., Gazi S.K., Barrow R.K., Yang G., Wang R., Snyder S.H. H2S signals through protein S-sulfhydration. Science Signaling. 2009;2 doi: 10.1126/scisignal.2000464. ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stamler J.S., Simon D.I., Osborne J.A., Mullins M.E., Jaraki O., Michel T., Singel D.J., Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proceedings of the National Academy of Sciences of USA. 1992;89:444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finkel T. From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Science Signaling. 2012;5 doi: 10.1126/scisignal.2002943. pe10. [DOI] [PubMed] [Google Scholar]
- 43.Lancaster J.R., Jr. Protein cysteine thiol nitrosation: maker or marker of reactive nitrogen species-induced nonerythroid cellular signaling? Nitric Oxide. 2008;19:68–72. doi: 10.1016/j.niox.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y.Y., Chu H.M., Pan K.T., Teng C.H., Wang D.L., Wang A.H., Khoo K.H., Meng T.C. Cysteine S-nitrosylation protects protein–tyrosine phosphatase 1B against oxidation-induced permanent inactivation. Journal of Biological Chemistry. 2008;283:35265–35272. doi: 10.1074/jbc.M805287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krishnan N., Fu C., Pappin D.J., Tonks N.K. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Science Signaling. 2011;4 doi: 10.1126/scisignal.2002329. ra86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paul B.D., Snyder S.H. H(2)S signalling through protein sulfhydration and beyond. Nature Reviews Molecular Cell Biology. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 47.Sen N., Paul B.D., Gadalla M.M., Mustafa A.K., Sen T., Xu R., Kim S., Snyder S.H. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Molecular Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hosoki R., Matsuki N., Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochemical and Biophysical Research Communications. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 49.Whiteman M., Armstrong J.S., Chu S.H., Jia-Ling S., Wong B.S., Cheung N.S., Halliwell B., Moore P.K. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? Journal of Neurochemistry. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 50.Ali M.Y., Ping C.Y., Mok Y.Y., Ling L., Whiteman M., Bhatia M., Moore P.K. Regulation of vascular nitric oxide in vitro and in vivo: a new role for endogenous hydrogen sulphide? British Journal of Pharmacology. 2006;149:625–634. doi: 10.1038/sj.bjp.0706906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whiteman M., Li L., Kostetski I., Chu S.H., Siau J.L., Bhatia M., Moore P.K. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochemical and Biophysical Research Communications. 2006;343:303–310. doi: 10.1016/j.bbrc.2006.02.154. [DOI] [PubMed] [Google Scholar]
- 52.Bruce King S. Potential biological chemistry of hydrogen sulfide (H2S) with the nitrogen oxides. Free Radical Biology and Medicine. 2013;55:1–7. doi: 10.1016/j.freeradbiomed.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Filipovic M.R., Eberhardt M., Prokopovic V., Mijuskovic A., Orescanin-Dusic Z., Reeh P., Ivanovic-Burmazovic I. Beyond H(2)S and NO interplay: hydrogen sulfide and nitroprusside react directly to give nitroxyl (HNO). A new pharmacological source of HNO. Journal of Medicinal Chemistry. 2013 doi: 10.1021/jm3012036. [DOI] [PubMed] [Google Scholar]
- 54.Filipovic M.R., Ivanovic-Burmazovic I. The kinetics and character of the intermediates formed in the reaction between sodium nitroprusside and hydrogen sulfide need further clarification. Chemistry. 2012;18:13538–13540. doi: 10.1002/chem.201103644. [DOI] [PubMed] [Google Scholar]
- 55.Filipovic M.R., Miljkovic J., Allgauer A., Chaurio R., Shubina T., Herrmann M., Ivanovic-Burmazovic I. Biochemical insight into physiological effects of H(2)S: reaction with peroxynitrite and formation of a new nitric oxide donor, sulfinyl nitrite. Biochemical Journal. 2012;441:609–621. doi: 10.1042/BJ20111389. [DOI] [PubMed] [Google Scholar]
- 56.Filipovic M.R., Miljkovic J., Nauser T., Royzen M., Klos K., Shubina T., Koppenol W.H., Lippard S.J., Ivanovic-Burmazovic I. Chemical characterization of the smallest S-nitrosothiol, HSNO: cellular cross-talk of H2S and S-nitrosothiols. Journal of the American Chemical Society. 2012;134:12016–12027. doi: 10.1021/ja3009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmidt H.H., Hofmann H., Schindler U., Shutenko Z.S., Cunningham D.D., Feelisch M. No NO from NO synthase. Proceedings of the National Academy of Sciences of USA. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharpe M.A., Cooper C.E. Reactions of nitric oxide with mitochondrial cytochrome c: a novel mechanism for the formation of nitroxyl anion and peroxynitrite. Biochemical Journal. 1998;332(Pt 1):9–19. doi: 10.1042/bj3320009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Donzelli S., Espey M.G., Flores-Santana W., Switzer C.H., Yeh G.C., Huang J., Stuehr D.J., King S.B., Miranda K.M., Wink D.A. Generation of nitroxyl by heme protein-mediated peroxidation of hydroxylamine but not N-hydroxy-L-arginine. Free Radical Biology and Medicine. 2008;45:578–584. doi: 10.1016/j.freeradbiomed.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Irvine J.C., Ritchie R.H., Favaloro J.L., Andrews K.L., Widdop R.E., Kemp-Harper B.K. Nitroxyl (HNO): the Cinderella of the nitric oxide story. Trends in Pharmacological Sciences. 2008;29:601–608. doi: 10.1016/j.tips.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Lin E.Q., Irvine J.C., Cao A.H., Alexander A.E., Love J.E., Patel R., McMullen J.R., Kaye D.M., Kemp-Harper B.K., Ritchie R.H. Nitroxyl (HNO) stimulates soluble guanylyl cyclase to suppress cardiomyocyte hypertrophy and superoxide generation. PLoS One. 2012;7:e34892. doi: 10.1371/journal.pone.0034892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hewett S.J., Espey M.G., Uliasz T.F., Wink D.A. Neurotoxicity of nitroxyl: insights into HNO and NO biochemical imbalance. Free Radical Biology and Medicine. 2005;39:1478–1488. doi: 10.1016/j.freeradbiomed.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 63.Paolocci N., Jackson M.I., Lopez B.E., Miranda K., Tocchetti C.G., Wink D.A., Hobbs A.J., Fukuto J.M. The pharmacology of nitroxyl (HNO) and its therapeutic potential: not just the janus face of NO. Pharmacology and Therapeutics. 2007;113:442–458. doi: 10.1016/j.pharmthera.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


