Abstract
Objective
Ovarian senescence affects many tissues and produces a variety of symptoms and signs. We hypothesized that estrogens may also influence circulating redox balance by regulating activity of the cellular antioxidative enzyme system. We aimed to explore the impact of surgical estrogen deprivation and replacement (ERT) on the glutathione balance and antioxidant enzymes expression in fertile women.
Study design
Nineteen healthy premenopausal women who underwent total hysterectomy with bilateral salpingo-oophorectomy were evaluated at baseline, 30 days after surgery without ERT and 30 days after ERT. Redox balance was determined by measuring blood reduced (GSH) and oxidized (GSSG) glutathione, as well as the GSSG/GSH ratio. Antioxidant status was evaluated by measuring serum estrogen (E2) levels and mRNA expression of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px) and glutathione S-transferase (GST) in peripheral blood mononuclear cells.
Results
Serum E2 significantly lowered after surgery, and increased in 12 out of 19 patients after 30 days of ERT (Responders). In such patients, an increase in oxidative stress was observed after surgery that resolved after ERT. Oxidative stress was sustained by reduction in the mRNA expression of both SOD and GSH-Px, that recovered after 30 days of therapy in responders. CAT and GST mRNA expression were not modified by surgery and replacement therapy.
Conclusions
Menopause is associated with significant change in antioxidant gene expression that in turn affects circulating redox state. Estrogens replacement therapy is able to prevent and counteract such modifications by acting as regulators of key antioxidant gene expression. These findings suggest that antioxidant genes are, almost in part, under the control of sex hormones, and that pathophysiology of the difference in gender disease may depend on the redox biology.
Keywords: Oxidative stress, Glutathione, Estrogen replacement therapy, Menopause
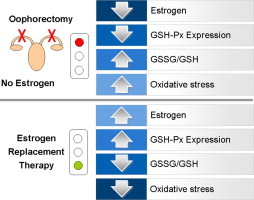
Graphical abstract
Estrogen deprivation by surgical menopause is associated with significant change in antioxidant gene expression that in turn affects circulating redox state. Estrogens replacement therapy may prevent and counteract oxidative stress by regulating key antioxidant gene expression.
Highlights
-
•
Surgical menopause is a physiological model of estrogen deprivation in women.
-
•
Menopause reduces antioxidant gene expression.
-
•
Estrogen replacement restores antioxidant gene expression.
-
•
Antioxidant genes are, almost in part, under the control of sex hormones.
-
•
Pathophysiology of the difference in gender disease may depend on the redox biology.
Introduction
Ovarian senescence occurs gradually during the fourth and fifth decades of life, leading to menopause. This process is associated with a progressive decline in estrogen level, which affects many tissues of the body and produces a variety of signs and symptoms [1]. In contrast, surgical removal of the ovaries interrupts abruptly and completely estrogen production, leading to more severe symptoms than those generated by natural menopause [2,3]. Bilateral oophorectomy at the time of hysterectomy for benign disease is commonly practiced in order to prevent the subsequent development of ovarian cancer [4]. However, premenopausal oophorectomy causes a rapid decline in circulating ovarian estrogens and androgens, since postmenopausal ovaries continue to produce significant amounts of testosterone and androstenedione which are converted to estrogen peripherally [5,6]. Estrogen depletion caused by oophorectomy is associated with coronary heart disease and hip fractures, as well as higher risk of cognitive impairment, depression and anxiety [7–9]. Symptoms and pathological manifestations associated with menopause may be, in part, related to oxidative stress [10]. Oxidative stress is defined as an imbalance between oxidants (superoxide anion radical, hydrogen peroxide, hydroxyl radical, peroxynitrite) and antioxidants (enzymatic and non enzymatic) in favor of the former [11].
Oxidative stress is higher in postmenopausal women, suggesting that antioxidant status may be related to estrogen deficit [12,13]. Surgical menopause is also associated with elevated production of oxidants, but estrogen counteracts oxidative stress, as observed during ovaries retention [14]. Estrogen exerts an antioxidant effect; in fact, estrogen level positively correlates with plasma antioxidant capacity and antioxidant enzymes expression throughout the menstrual cycle [15–17], and negatively with lipid peroxides, the product of oxidative damage [18]. Very interestingly, estrogen replacement therapy (ERT) restores total plasma antioxidant capacity and decreases lipid peroxides [18,19].
The antioxidant effect of estrogen may be related to a direct free radical scavenging activity [20]. However, the circulating level and the administered doses of estrogen are much lower than the necessary concentration of classical chemical antioxidants, indicating that its antioxidant effects is likely related to the upregulation of antioxidant enzymes [21]. Such hypothesis has been verified by in vitro and ex vivo animal studies [22,23]; nevertheless, the relationship between estrogen and the antioxidant enzymes system has been so far not addressed in humans. Thus, we aimed to investigate the impact of estrogen level change (depletion and replacement) and antioxidant gene expression and circulating redox balance in premenopausal women undergoing bilateral oophorectomy.
Materials and methods
Study design
Nineteen consecutive premenopausal women, referred the Department of Obstetrics and Gynecology of the University of Foggia for benign gynecological disease, who did not respond to standard medical treatments and with no indication for endometrial ablation, underwent hysterectomy and bilateral oophorectomy.
Exclusion criteria were Body Mass Index (BMI) ≥30 kg/m2, a previous hospital admission related to cardiovascular disease, and a previous diagnosis of angina, hypercholesterolemia, diabetes, alcoholism, thyroid or any other endocrine disease. In addition, none of them had smoking habits or had taken medications, as well as contraceptive drugs, vitamin supplements and soy derivatives in the 6 months before the study. The subjects were asked to record any consumption of drugs not included in the experimental design. Study participants were assigned to a 60-day experimental period: 30 days after oophorectomy, they received 50 micrograms/day of continuous trans dermal in the form of patches (Dermestril, Rottapham S.p.A., Milan, Italy). The study protocol was approved by the institutional review board and the local Ethics Committee, and all patients gave written informed consent. Evaluation of treatment compliance was ascertained by weekly interview.
Blood sampling and analysis
Blood samples were collected the day before the surgical oophorectomy (Baseline time), 30 days after oophorectomy and before starting estrogen replacement therapy (Menopause time) and 30 days after starting therapy (ERT time) from an antecubital vein between 8:00 and 9:00 AM, with subjects in the supine position after an overnight fast. Blood samples for estradiol measurement were collected in 8 mL-Vacutainer tubes containing Z Serum Sep Clot Activator (Greiner Bio-one Gmbh, Frickenhausen, Germany) and centrifuged for 10 min at 1600×g at room temperature to isolate serum. Blood samples for RNA isolation were collected into tubes containing K-ethylenediaminetetraacetic acid (EDTA) and processed within 30 min.
Oxidized (GSH) and reduced (GSSG) glutathione were determined in whole blood as previously described [25]. Briefly, blood samples were treated with an equal volume of 6% (v/v) perchloric acid containing 1 mM EDTA to determine GSH or with 6% perchloric acid containing 50 mM N-ethylmaleimide and 1 mM EDTA to determine GSSG by high-performance liquid chromatography (HPLC). Afterwards, samples were centrifuged for 10 min at 1500×g and the acidic supernatants were neutralized and used for determination of metabolites.
Serum concentration of estrogen (E2), progesterone, follicle stimulating hormone (FSH) and luteinizing hormone (LH) were measured using commercial chemiluminescence immunoassays (Vitros Estradiol, Johnson & Johnson Medical S.p.A., Milan, Italy).
RNA isolation and quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR)
Total cellular RNA was isolated from peripheral blood mononuclear cell (PBMC) samples using the RNAeasy Kit (Qiagen, Hilden, Germany) according to the manufacturer instructions. To ensure minimum in vitro impact on the activation status of the cells, we employed a modified gradient separation. PBMC were immediately isolated by a rapid Ficoll–Hystopaque centrifugation for 30 min at 900×g. Total cellular RNA was extracted with RNeasy kit and immediately stored at −80 °C. Samples were quantified by absorption spectrophotometry, and RNA integrity was confirmed using nondenaturing agarose gel electrophoresis. cDNA was obtained using a random hexamer primer and a SuperScript III Reverse Transcriptase kit as described by the manufacturer (Invitrogen, Frederick, MD, USA). A PCR master mix containing the specific primers (superoxide dismutase 1 (SOD1): forward, TGT GGG GAA GCA TTA AAG G; reverse, CCG TGT TTT CTG GAT AGA GG; catalase (CAT): forward, GCC ATT GCC ACA GGA AAG TA; reverse, CCA ACT GGG ATG AGA GGG TA; glutathione peroxidase (GSH-Px): forward, GGA GAC CTC ACC CTG TAC C; reverse, GTC ATT CAC CAT GTC CAC C; glutathione S-transferase (GST): forward, ACC TCC ACC GTA TAT TTG AG; reverse, TTG CCC CAG ACA GCC ATC TT; glyceraldehyde-3-phosphate dehydrogenase (GAPDH): forward, CAA GGC TGA GAA CGG GAA; reverse: 59-GCA TCG CCC CAC TTG ATT TT-39) was added, along with AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA, USA). Real-time quantification of mRNA was performed with a SYBR Green I assay, and evaluated using an iCycler detection system (Bio-Rad Laboratories). The threshold cycle (CT) was determined, and the relative genes expression was subsequently calculated as follows: fold change=2−Δ(ΔCT), where ΔCT=CTtarget−CThousekeeping and Δ(ΔCT)=ΔCTtreated−ΔCTcontrol.
Statistical analysis
Data were expressed as mean±standard deviation of the mean (SDM). For biochemical and real-time RT-PCR data, the one way analysis of variance (ANOVA) for repeated measures was performed to compare results obtained before vs. after oophorectomy and ERT. Bonferroni's multiple comparison test was performed as post-hoc test. The student's t-test was used for paired data. A linear regression model was used to analyze the association between serum E2 levels and SOD, CAT, GSTP, GSTM levels, as well as GSH, GSSG and GSSG/GSH ratio, at all the times studied. A value of p<0.05 was considered statistically significant. The Statistical Package for Social Sciences (SPSS v. 15) was used to perform all the statistical analysis.
Results
Baseline patients characteristics
Baseline characteristics of patients are shown in Table 1. Nine patients (47%) were overweight (BMI>25 kg/m2), four patients (21%) presented history of arterial hypertension (systolic blood pressure≥140 mm Hg and/or diastolic blood pressure≥90 mm Hg). Six patients (31%) had a plasma hemoglobin value <12 g/dL at baseline; these patients reported to have irregular menstrual cycle with metrorrhagia. Of note, no association was found between BMI and serum estrogen level in all the subjects studied. The compliance of women to ERT was complete.
Table 1.
Clinical and biochemical features of patients enrolled in the study at the baseline time point.
| (n=19) | |
|---|---|
| Age | |
| Mean±SD | 48±3.9 |
| Weight | |
| Mean±SD | 65±7.5 |
| Range | 50–79 |
| Body mass index (kg/m2) | |
| Mean±SD | 26.0±2.9 |
| Range | 19.8–29.8 |
| Plasma hemoglobin (g/dL) | |
| Mean±SD | 12.5±1.5 |
| Range | 10.8–13.9 |
| Serum estradiol (pg/mL) | |
| Mean±SD | 156.6±94.8 |
| Range | 50–350 |
| Serum progesterone (ng/mL) | |
| Mean±SD | 3.6±4.8 |
| Range | 0.2–11.9 |
| Serum FSH (U/L) | |
| Mean±SD | 8.5±5.2 |
| Range | 1.8–17.5 |
| Serum LH (nmol/L) | |
| Mean±SD | 10.9±8.7 |
| Range | 2.8–33.1 |
FSH, follicle stimulating hormone; LH, luteinizing hormone.
Effect of surgery on circulating redox balance and antioxidant enzymes expression
Table 2 shows the change of glutathione status 30 days after oophorectomy as compared to the baseline. We observed both a decrease in GSH and an increase in GSSG blood level, and consequently an increase in GSSG/GSH ratio, consistent with an impaired redox balance. Moreover, surgical menopause was associated with a reduction in SOD and GSH-Px mRNA gene expression, while no modifications were observed in the expression of CAT and GST (Table 2).
Table 2.
Blood level of GSSG/GSH ratio, reduced (GSH) and oxidized (GSSG) glutathione, mRNA expression of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px) and glutathione-S-transferase (GST) in peripheral blood mononuclear cells from 19 women at baseline and 30 days after surgical menopause (Menopause). Statistical differences were assessed using Student's t-test for repeated measures.
| Baseline (mean±SD) | Menopause (mean±SD) | |
|---|---|---|
| Blood GSSG/GSH ratio (%) | 9.46±2.15 | 14.28±5.64⁎⁎ |
| Blood GSH (nmol/L) | 54.69±12.28 | 39.28±10.42⁎ |
| Blood GSSG (nmol/L) | 4.98±1.32 | 7.40±2.63⁎ |
| SOD mRNA expression (%) | 100±24.48 | 64.20±29.56⁎⁎ |
| CAT mRNA expression (%) | 100±36.72 | 89.31±31.55 |
| GSH-Px mRNA expression (%) | 100±31.94 | 42.66±25.88⁎⁎ |
| GST mRNA expression (%) | 100±29.33 | 88.43±21.28 |
p<0.05 vs. T0.
p<0.01 vs. T0.
ERT restored redox balance by inducing antioxidant genes expression
Administration of estrogen for 30 days in surgical post-menopausal women induced a significant increase in the circulating E2 level as reported in Fig. 1. The mean level of E2 was 157.63±89.97 pg/mL before the surgery and 13.01±9.40 pg/ml after ovariectomy. After ERT, patients with E2 levels of at least 50 pg/mL were considered responders. The analysis of the cases evidenced that the level of E2 reached 50 pg/mL in 12 out 19 patients (63%) after 30 days replacement treatment; the remaining seven patients (37%) were considered non-responders (Fig. 1). The analysis of circulating redox balance revealed a significant consumption of reduced glutathione and a production of oxidized glutathione suggesting that oophorectomy induced oxidative stress that was counteracted by ERT (Fig. 1B and D). To better analyze the impact of estrogen deprivation on the circulating redox balance the responders and non responders women were compared in terms of redox state. In women who responded to the ERT the level of reduced glutathione resulted higher and the GSSG lower as compared to non responders; as a consequence, the blood GSSG/GSH ratio decreased 30 days after ERT (Fig. 2).
Fig. 1.
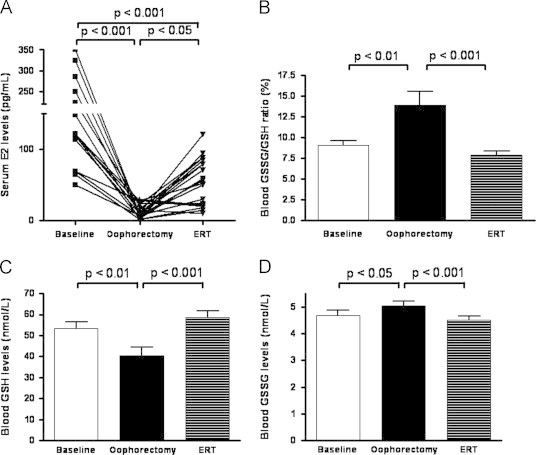
(A) Serum level of estrogen (E2) in 19 women that underwent hysterectomy and bilateral oophorectomy at baseline (B), after surgical menopause (SM) and after 30 days of estrogen replacement therapy (ERT). (B–D) Blood level of reduced (GSH) and oxidized (GSSG) glutathione, and GSSG/GSH ratio in 12 women that underwent hysterectomy and bilateral oophorectomy and responded to estrogen replacement therapy (ERT) at baseline (B), 30 days after surgical menopause (SM) and 30 days after ERT. Statistical differences were assessed using one-way ANOVA for repeated measures with Bonferroni's multiple comparison test as post-hoc test.
Fig. 2.
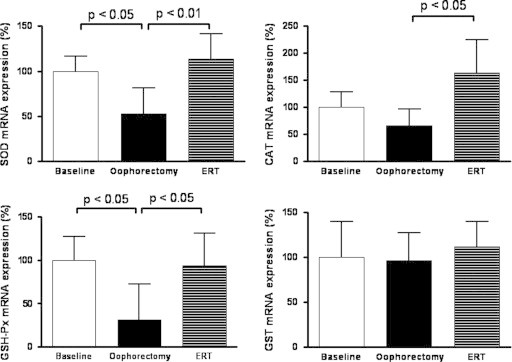
Blood level of reduced (GSH) and oxidized (GSSG) glutathione, and GSSG/GSH ratio in responder (n=12) and non responder (n=7) women in surgical menopause to 30 days of estrogen replacement therapy. Data are expressed as mean±standard deviation of the mean. Statistical differences were assessed using Student's t-test for unpaired measures.
Taken together our data suggest that estrogen depletion decreases the antioxidant capacity of the woman that may be counteracted by estrogen replacement. To verify the mechanism accountable for such effect, the analysis of the mRNA expression of genes encoding for several antioxidant enzymes (SOD, CAT, GSH-Px and GST) was performed by real time RT-PCR in the responder group. As reported in Fig. 3, the expression of the SOD and GSH-Px genes was completely recovered after treatment. In contrast, GST expression seemed to be not modulated by estrogen; however, CAT seemed to be tightly regulated by circulating estrogen change (Fig. 3).
Fig. 3.
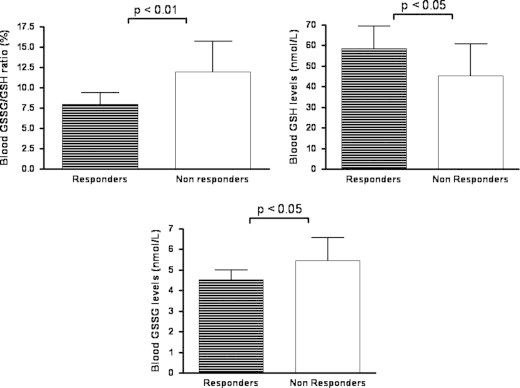
mRNA expression of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px) and glutathione-S-transferase (GST) in peripheral blood mononuclear cell of 12 women that underwent hysterectomy and bilateral oophorectomy and responded to estrogen replacement therapy (ERT) at baseline (B), 30 days after surgical menopause (SM) and 30 days after ERT. Data are expressed as mean±standard deviation of the mean. Statistical differences were assessed using one-way ANOVA for repeated measures with Bonferroni's multiple comparison test as post-hoc test.
Relationship between serum E2 levels and blood glutathione balance, as well as antioxidant gene expression
To assess the relationship between E2 changes and redox balance, a linear regression model was designed to correlate the variation of serum E2 level (independent variable) and the variation of GSH, GSSG and GSSG/GSH ratio, as well as antioxidant gene expression in the responders patients as dependent variables, and the results are reported in Fig. 4. A significant positive relationship was observed between the E2 (ΔE2) and GSH, SOD and GSH-Px; in contrast, change in E2 negatively correlated to GSSG and GSSG/GSH ratio. The change in E2 level, was, however, not able to predict modification of CAT and GST gene expression (Fig. 4).
Fig. 4.
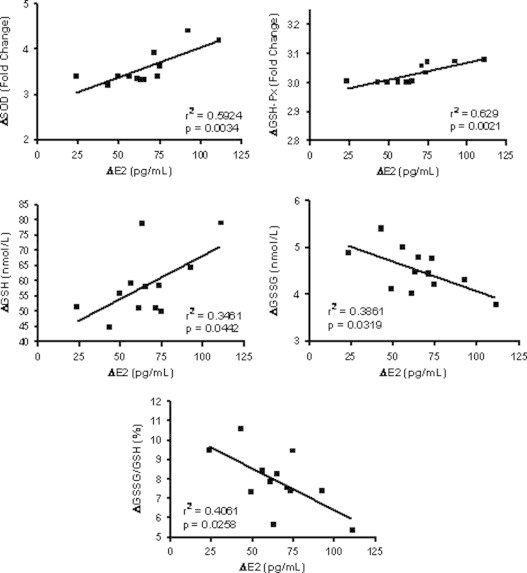
Linear regression analysis between the variations of serum E2 level (ΔE2) and superoxide dismutase (ΔSOD), glutathione peroxidase (ΔGSH-Px) mRNA expression, as well as reduced (ΔGSH) and oxidized (ΔGSSG) glutathione, and GSSG/GSH ratio (ΔGSSG/GSH), expressed as the difference between 30 days after estrogen replacement therapy (ERT) and 30 days after surgical menopause, in 12 women that underwent hysterectomy and bilateral oophorectomy and responded to ERT.
Discussion
The present study shows that in women there is a linear relationship between the level of circulating estrogen and the antioxidant status; in addition, we report that surgical menopause induced an imbalance in the redox status that can be prevented by estrogen replacement therapy.
Very interestingly, here we report that estrogen acts as a positive signal in gene control of antioxidant mRNA expression which in turn modulates redox balance.
Bilateral oophorectomy-induced menopause has been associated with increasing plasma malondialdehyde [16]. Supplementation of E2 after menopause decreases serum lipid peroxides and restores total plasma antioxidant capacity [18,24]. Several studies have reported a protective role of E2 against oxidative damage both in vitro and in vivo, suggesting that E2 could exert a role in the control of the antioxidant signaling pathways [26,27]. The novelty of the present study was in the model applied where the analysis of the change of circulating redox activity and gene expression was performed in the same patients before surgical oophorectomy, during estrogen deprivation after surgical procedure, and after restoring normal E2 level. Our data show, for the first time, that circulating redox state is closely modulated by E2 level and that estrogen depletion exposes the patient to the risk of oxidative stress. The intracellular antioxidant system is based on the glutathione, which plays a key role in cellular detoxification reactions and in regulating the thiol-disulfide cellular status [28]. This tripeptide can exist intracellularly in either an oxidized (GSSG) or reduced (GSH) state. Maintaining optimal GSSG/GSH ratio in the cell is critical to survival, hence, the antioxidant equilibrium is tightly regulated [29]. A recent report showed a higher GSSG/GSH ratio in oophorectomized aged rats [30], and Bednarek-Tupikowska et al. reported a linear relationship between estrogen and GSH-Px in erythrocytes of postmenopausal women [16].
In addition to glutathione, several antioxidant enzymatic systems, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px) and glutathione S-transferase (GST) transform reactive free radicals into less reactive, or inactive species. There are contrasting evidences about the effects of menopause and hormone replacement therapy on the activities of these enzymes [12,31,32]. Studies performed in liver homogenates or femurs of oophorectomized rats evidenced reduced activities of SOD, CAT, GSH-Px and GST [33,34]. A recent study reported the effect of E2 supplementation on lipid oxidation and catalase activity [24]; however, no data concerning the effect of E2 deprivation on the entire antioxidant enzyme system are available in humans.
The regulatory effects of estrogen are mediated by transcriptional activation of estrogen-responsive genes, involving intracellular estrogen receptors [35,36]. As ligand-dependent transcriptional factors, the hormone-bound estrogen receptors interact with estrogen response elements to stimulate several genes in estrogen-responsive tissues and to regulate gene trans-activation [37]. Estrogen in vitro activate MAPK and NFκB, driving the expression of the antioxidant enzymes SOD and GSH-Px [21]. Another evidence provided on skeletal muscle of oophorectomized mice reported that estrogen replacement increased antioxidant gene expression via Estrogen Receptor α [23]. Our data definitively support such observation.
Very interestingly, we did not observe any variation in the expression of GST, which is considered a phase II enzyme, catalyzing conjugation reactions that convert the highly reactive species produced during phase I metabolism to less reactive products. It has been demonstrated that E2 may inhibit phase II enzymes expression via a pathway involving NRF2 and the antioxidant response element [38,39]. Even though a repression of GST has not been reported in the current study, we could speculate that variations in the E2 level did not significantly influence the regulation of GST. In accordance with a recent report [24], we also observed that surgical menopause and ERT did not alter CAT expression. CAT is especially important in the case of limited glutathione content or reduced GSH-Px activity and plays a significant role in the development of tolerance to oxidative stress in the adaptive response of cells [40]. We could hypothesize that the regulation of this enzyme was not influenced by E2 levels because of the variations in glutathione and GSH-Px expression; new studies are needed to address this specific mechanism.
In summary, the present study showed that circulating redox status is closely correlated to estrogen levels. In addition, surgical menopause associated with increased risk to develop oxidative stress via downregulation of antioxidant genes expression. Estrogen replacement therapy is able to restore antioxidant status and may be effective in the prevention of morbidity related to the oxidative stress after oophorectomy, even though wider long-term studies are required in order to better understand the influence of the acute E2 deprivation and of the antioxidant system activity on the pathogenesis of menopause-related morbidity. These findings strongly suggest that sex hormones may control, almost in part, antioxidant gene expression. Moreover, this study support that gender differences in the pathophysiology of diseases may depend on redox biology.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Al-Azzawi F., Palacios S. Hormonal changes during menopause. Maturitas. 2009;63:135–137. doi: 10.1016/j.maturitas.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Mercuro G., Zoncu S., Saiu F., Mascia M., Melis G.B., Rosano G.M. Menopause induced by oophorectomy reveals a role of ovarian estrogen on the maintenance of pressure homeostasis. Maturitas. 2004;47:131–138. doi: 10.1016/s0378-5122(03)00252-4. [DOI] [PubMed] [Google Scholar]
- 3.Ozdemir S., Celik C., Gorkemli H., Kiyici A., Kaya B. Compared effects of surgical and natural menopause on climacteric symptoms, osteoporosis, and metabolic syndrome. International Journal of Gynaecology and Obstetrics. 2009;106:57–61. doi: 10.1016/j.ijgo.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Parker W.H., Jacoby V., Shoupe D., Rocca W. Effect of bilateral oophorectomy on women's long-term health. Womens Health (London, England ) 2009;5:565–576. doi: 10.2217/whe.09.42. [DOI] [PubMed] [Google Scholar]
- 5.Judd H.L., Lucas W.E., Yen S.S. Effect of oophorectomy on circulating testosterone and androstenedione levels in patients with endometrial cancer. American Journal of Obstetrics and Gynecology. 1974;118:793–798. doi: 10.1016/0002-9378(74)90490-6. [DOI] [PubMed] [Google Scholar]
- 6.Fogle R.H., Stanczyk F.Z., Zhang X., Paulson R.J. Ovarian androgen production in postmenopausal women. Journal of Clinical Endocrinology and Metabolism. 2007;92:3040–3043. doi: 10.1210/jc.2007-0581. [DOI] [PubMed] [Google Scholar]
- 7.Atsma F., Bartelink M.L., Grobbee D.E., van der Schouw Y.T. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13:265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 8.Rocca W.A., Bower J.H., Maraganore D.M. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 9.Rocca W.A., Bower J.H., Maraganore D.M. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200–209. doi: 10.1212/01.wnl.0000280573.30975.6a. [DOI] [PubMed] [Google Scholar]
- 10.Miquel J., Ramirez-Bosca A., Ramirez-Bosca J.V., Alperi J.D. Menopause: a review on the role of oxygen stress and favorable effects of dietary antioxidants. Archives of Gerontology and Geriatrics. 2006;42:289–306. doi: 10.1016/j.archger.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Sies H. Oxidative stress: oxidants and antioxidants. Experimental Physiology. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 12.Signorelli S.S., Neri S., Sciacchitano S. Behaviour of some indicators of oxidative stress in postmenopausal and fertile women. Maturitas. 2006;53:77–82. doi: 10.1016/j.maturitas.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Rodriguez M.A., Zacarias-Flores M., Arronte-Rosales A., Correa-Munoz E., Mendoza-Nunez V.M. Menopause as risk factor for oxidative stress. Menopause. 2011 doi: 10.1097/gme.0b013e318229977d. [DOI] [PubMed] [Google Scholar]
- 14.Michos C., Kiortsis D.N., Evangelou A., Karkabounas S. Antioxidant protection during the menstrual cycle: the effects of estradiol on ascorbic-dehydroascorbic acid plasma levels and total antioxidant plasma status in eumenorrhoic women during the menstrual cycle. Acta Obstetricia et Gynecologica Scandinavica. 2006;85:960–965. doi: 10.1080/00016340500432812. [DOI] [PubMed] [Google Scholar]
- 15.Massafra C., Gioia D., De F.C. Effects of estrogens and androgens on erythrocyte antioxidant superoxide dismutase, catalase and glutathione peroxidase activities during the menstrual cycle. Journal of Endocrinology. 2000;167:447–452. doi: 10.1677/joe.0.1670447. [DOI] [PubMed] [Google Scholar]
- 16.Bednarek-Tupikowska G., Bohdanowicz-Pawlak A., Bidzinska B., Milewicz A., Antonowicz-Juchniewicz J., Andrzejak R. Serum lipid peroxide levels and erythrocyte glutathione peroxidase and superoxide dismutase activity in premenopausal and postmenopausal women. Gynecological Endocrinology. 2001;15:298–303. [PubMed] [Google Scholar]
- 17.Serviddio G., Loverro G., Vicino M. Modulation of endometrial redox balance during the menstrual cycle: relation with sex hormones. Journal of Clinical Endocrinology and Metabolism. 2002;87:2843–2848. doi: 10.1210/jcem.87.6.8543. [DOI] [PubMed] [Google Scholar]
- 18.Chang S.P., Yang W.S., Lee S.K., Min W.K., Park J.S., Kim S.B. Effects of hormonal replacement therapy on oxidative stress and total antioxidant capacity in postmenopausal hemodialysis patients. Renal Failure. 2002;24:49–57. doi: 10.1081/jdi-120002660. [DOI] [PubMed] [Google Scholar]
- 19.Bednarek-Tupikowska G., Tupikowski K., Bidzinska B. Serum lipid peroxides and total antioxidant status in postmenopausal women on hormone replacement therapy. Gynecological Endocrinology. 2004;19:57–63. doi: 10.1080/09513590412331272328. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Larrea M.B., Leal A.M., Martin C., Martinez R., Lacort M. Antioxidant action of estrogens in rat hepatocytes. Revista Española de Fisiología. 1997;53:225–229. [PubMed] [Google Scholar]
- 21.Borras C., Gambini J., Gomez-Cabrera M.C. 17beta-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NFkappaB cascade. Aging Cell. 2005;4:113–118. doi: 10.1111/j.1474-9726.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 22.Strehlow K., Rotter S., Wassmann S. Modulation of antioxidant enzyme expression and function by estrogen. Circulation Research. 2003;93:170–177. doi: 10.1161/01.RES.0000082334.17947.11. [DOI] [PubMed] [Google Scholar]
- 23.Baltgalvis K.A., Greising S.M., Warren G.L., Lowe D.A. Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. PLoS One. 2010;5:e10164. doi: 10.1371/journal.pone.0010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escalante G.C., Quesada M.S. HRT decreases DNA and lipid oxidation in postmenopausal women. Climacteric. 2012 doi: 10.3109/13697137.2012.660711. [DOI] [PubMed] [Google Scholar]
- 25.Serviddio G., Romano A.D., Greco A. Frailty syndrome is associated with altered circulating redox balance and increased markers of oxidative stress. International Journal of Immunopathology and Pharmacology. 2009;22:819–827. doi: 10.1177/039463200902200328. [DOI] [PubMed] [Google Scholar]
- 26.Ejima K., Nanri H., Araki M., Uchida K., Kashimura M., Ikeda M. 17beta-estradiol induces protein thiol/disulfide oxidoreductases and protects cultured bovine aortic endothelial cells from oxidative stress. European Journal of Endocrinology. 1999;140:608–613. doi: 10.1530/eje.0.1400608. [DOI] [PubMed] [Google Scholar]
- 27.Bokov A.F., Ko D., Richardson A. The effect of gonadectomy and estradiol on sensitivity to oxidative stress. Endocrine Research. 2009;34:43–58. doi: 10.1080/07435800902913600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplowitz N. Physiological significance of glutathione S-transferases. American Journal of Physiology. 1980;239:G439–G444. doi: 10.1152/ajpgi.1980.239.6.G439. [DOI] [PubMed] [Google Scholar]
- 29.Townsend D.M., Tew K.D., Tapiero H. The importance of glutathione in human disease. Biomedicine and Pharmacotherapy. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baeza I., Fdez-Tresguerres J., Ariznavarreta C., De la Fuente M. Effects of growth hormone, melatonin, oestrogens and phytoestrogens on the oxidized glutathione (GSSG)/reduced glutathione (GSH) ratio and lipid peroxidation in aged ovariectomized rats. Biogerontology. 2010;11:687–701. doi: 10.1007/s10522-010-9282-7. [DOI] [PubMed] [Google Scholar]
- 31.Gurdol F., Oner-Yyidothan Y., Yalcyn O., Genc S., Buyru F. Changes in enzymatic antioxidant defense system in blood and endometrial tissues of women after menopause. Research Communications in Molecular Pathology and Pharmacology. 1997;97:38–46. [PubMed] [Google Scholar]
- 32.Unfer T.C., Conterato G.M., da Silva J.C., Duarte M.M., Emanuelli T. Influence of hormone replacement therapy on blood antioxidant enzymes in menopausal women. Clinica Chimica Acta. 2006;369:73–77. doi: 10.1016/j.cca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Ha B.J. Oxidative stress in ovariectomy menopause and role of chondroitin sulfate. Archives of Pharmacal Research. 2004;27:867–872. doi: 10.1007/BF02980181. [DOI] [PubMed] [Google Scholar]
- 34.Muthusami S., Ramachandran I., Muthusamy B. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clinica Chimica Acta. 2005;360:81–86. doi: 10.1016/j.cccn.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Katzenellenbogen B.S. Estrogen receptors: bioactivities and interactions with cell signaling pathways. Biology of Reproduction. 1996;54:287–293. doi: 10.1095/biolreprod54.2.287. [DOI] [PubMed] [Google Scholar]
- 36.McDonnell D.P., Wijayaratne A., Chang C.Y., Norris J.D. Elucidation of the molecular mechanism of action of selective estrogen receptor modulators. American Journal of Cardiology. 2002;90:35F–43F. doi: 10.1016/s0002-9149(01)02221-4. [DOI] [PubMed] [Google Scholar]
- 37.Green S., Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends in Genetics. 1988;4:309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- 38.Ansell P.J., Espinosa-Nicholas C., Curran E.M. In vitro and in vivo regulation of antioxidant response element-dependent gene expression by estrogens. Endocrinology. 2004;145:311–317. doi: 10.1210/en.2003-0817. [DOI] [PubMed] [Google Scholar]
- 39.Ansell P.J., Lo S.C., Newton L.G. Repression of cancer protective genes by 17beta-estradiol: ligand-dependent interaction between human Nrf2 and estrogen receptor alpha. Molecular and Cellular Endocrinology. 2005;243:27–34. doi: 10.1016/j.mce.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Goyal M.M., Basak A. Human catalase: looking for complete identity. Protein & Cell. 2010;1:888–897. doi: 10.1007/s13238-010-0113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


