Abstract
Hydrogen peroxide (H2O2) at moderate steady-state concentrations synergizes with TNF-α, leading to increased nuclear levels of NF-κB p65 subunit and to a cell-type specific up-regulation of a limited number of NF-κB-dependent genes. Here, we address how H2O2 achieves this molecular specificity. HeLa and MCF-7 cells were exposed to steady-state H2O2 and/or TNF-α and levels of c-Rel, p65, IκB-α, IκB-β and IκB-ε were determined. For an extracellular concentration of 25 µM H2O2, the intracellular H2O2 concentration is 3.7 µM and 12.5 µM for respectively HeLa and MCF-7 cells. The higher cytosolic H2O2 concentration present in MCF-7 cells may be a contributing factor for the higher activation of NF-κB caused by H2O2 in this cell line, when compared to HeLa cells. In both cells lines, H2O2 precludes the recovery of TNF-α-dependent IκB-α degradation, which may explain the observed synergism between H2O2 and TNF-α concerning p65 nuclear translocation. In MCF-7 cells, H2O2, in the presence of TNF-α, tripled the induction of c-Rel triggered either by TNF-α or H2O2. Conversely, in HeLa cells, H2O2 had a small antagonistic effect on TNF-α-induced c-Rel nuclear levels, concomitantly with a 50 % induction of IκB-ε, the preferential inhibitor protein of c-Rel dimers. The 6-fold higher c-Rel/IκB-ε ratio found in MCF-7 cells when compared with HeLa cells, may be a contributing factor for the cell-type dependent modulation of c-Rel by H2O2.
Our results suggest that H2O2 might have an important cell-type specific role in the regulation of c-Rel-dependent processes, e.g. cancer or wound healing.
Abbreviations: GPx, glutathione peroxidase; H2O2, hydrogen peroxide; IκB-α, inhibitory protein α of NF-κB; IκB-β, inhibitory protein β of NF-κB; IκB-ε, inhibitory protein ε of NF-κB; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NF-κB, nuclear factor-kappa B; TNF-α, tumor necrosis factor-alpha
Keywords: NF-κB, Steady-state, H2O2 gradient, HeLa cells, MCF-7 cells, Inflammation
Graphical abstract
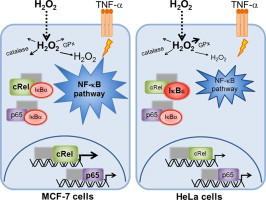
Highlights
-
•
Selective modulation of individual NF-κB-dependent genes expression by H2O2.
-
•
In MCF-7 cells H2O2 tripled the TNF-α 4-fold induction of c-Rel nuclear levels.
-
•
In HeLa cells, H2O2 had an antagonistic effect on TNF-α induced c-Rel translocation.
-
•
c-Rel dimers bind chiefly to IκB-α/IκB-ε in MCF-7 cells and to IκB-ε in HeLa cells.
Introduction
An inflammatory environment is rich in cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1), chemokines, such as IL-8 and monocyte chemotactic protein-1 (MCP-1) and also leukocytes, such as neutrophils and macrophages. The latter produce reactive oxygen species (ROS) for a germicide action; however some ROS, especially hydrogen peroxide (H2O2), which is a small molecule able to diffuse through membranes [1], [2], may leak the phagosome and participate in other cellular processes [3], [4]. Although H2O2 has been linked to oxidative stress, nowadays the mild oxidative properties of H2O2 and the tight control of intracellular H2O2 levels support its role in signal transduction [2], [5]. Since in inflammatory sites the presence of H2O2 is concomitant with that of cytokines, investigating the cellular effects of the simultaneous presence of these agents is biologically relevant. We have previously shown in MCF-7 and HeLa cells [6] that H2O2, at concentrations close to those occurring during an inflammatory situation, i.e. 5–15 µM [4], [7], caused a synergistic effect on the TNF-α-dependent translocation of p65 from the cytosol to the nucleus. p65 belongs to the NF-κB/Rel family of transcription factors, which have key regulatory roles in inflammation, innate and adaptive immune response, proliferation and apoptosis [8], [9]. The increased nuclear translocation of p65 in the presence of H2O2 and TNF-α increases expression levels of a sub-set of NF-κB-dependent genes, including pro-inflammatory genes, e.g. IL-8, MCP-1, TLR2, and TNF-α, and anti-inflammatory genes, e.g. heme oxygenase-1 [6]. This indicates that H2O2 exerts molecular specificity [6] since only a sub-set of NF-κB genes has their expression increased, with most of them being unaffected. Moreover, this molecular specificity is cell-type dependent since NF-κB-dependent genes whose expression is stimulated by H2O2 are not the same in the two epithelial cell lines studied. The mechanism for this selective stimulation by H2O2 is probably multi-factorial and may include alterations in chromatin state, post-translational modifications of p65 whose effects are dependent on the κB promoter sequence, and the combined effects of several transcription factors [10]. In addition, we have shown that H2O2 leads to the preferential expression of genes whose κB promoter sequences have a lower affinity towards NF-κB [10]. Other molecular mechanisms may be relevant for this important question of how the small molecule H2O2 achieves molecular specificity. Here we continue to address this problem by characterizing the modulatory role of H2O2 on other members of the NF-κB family.
Apart from p65, the NF-κB/Rel family comprises c-Rel and RelB, which bear transactivation domains, and also the regulatory subunits p50 and p52 [8], [11], [12]. NF-κB/Rel proteins can form homo- and heterodimers and the prototypical NF-κB is a heterodimer composed by the p50 and p65 subunits (p50/p65). In the classical activation pathway, NF-κB dimers remain inactive in the cytosol bound to its inhibitory proteins, the IκBs, which possess the typical ankyrin repeats that mask the nuclear localization signal of NF-κB thus preventing its translocation to the nucleus [8], [9]. The three most common members of the IκBs family are IκB-α, IκB-β and IκB-ε. IκB-α and IκB-β bind preferentially to heterodimers p50/p65 and p50/c-Rel [9], while IκB-ε binds preferentially to p65 homodimers and c-Rel/p65 heterodimers [13]. In this work, the modulatory effect of H2O2 on TNF-α-dependent NF-κB activation is addressed by following the levels of p65, c-Rel and the inhibitory proteins IκB-α, IκB-β and IκB-ε. We have shown that the usual initial bolus addition of a high H2O2 concentration produces opposite results regarding NF-κB activation to those obtained with low steady-state H2O2 levels that simulate the inflammatory situation [5], [6]. Therefore, when studying the biology of H2O2 it is essential not only to control and quantify the effective dose of H2O2 delivered to the cells [5], [14], [15], [16], [17] but also to estimate the actual intracellular concentration that is causing the effects observed, as H2O2 is being reduced in reactions catalyzed by enzymes such as catalase and glutathione peroxidase (GPx).
In this work we delivered a controlled and steady-state dose of H2O2 [6], [18] and compared NF-κB activation in two different cell lines, taking into consideration not only differences in basal levels of NF-κB family members, but also the actual intracellular H2O2 concentration. The results obtained highlight the need of a quantitative approach when working with H2O2.
Materials and methods
Cell culture and reagents
HeLa (American Type Culture Collection, Manassas, VA, USA) and MCF-7 (European Collection of Cell Cultures, Salisbury Wiltshire, UK) cells were grown in RPMI 1640 medium supplemented with 10% of fetal bovine serum, penicillin 100 U mL−1, streptomycin 100 µg mL−1 and l-glutamine 2 mM, all from Cambrex, Verviers, Belgium. Glucose oxidase (Aspergillus Niger), TNF-α (human recombinant), and MTT were obtained from Sigma-Aldrich, Inc. (Saint Louis, MO, USA). H2O2 was obtained from Merck & Co., Inc. (Whitehouse Station, NJ, USA).
Cell incubations
Cells were counted and plated approximately 46 h before the experiment. Fresh medium was added to cells 1 h before the incubations. Exposure to H2O2 was performed using the steady-state titration [18]. The method is extensively described in [6]. Briefly, a steady-state level of H2O2 was maintained during the entire assay by adding simultaneously H2O2 at the concentration under study and a quantity of glucose oxidase enough to counteract H2O2 consumption by cells. H2O2 concentrations were checked during the incubations by using catalase and measuring O2 formation in an oxygen electrode (Hansatech, UK). All experiments were performed with steady-state 25 µM H2O2 and 0.37 ng mL−1 of TNF-α.
Protein extraction and immunoblot analysis
HeLa and MCF-7 cells were plated onto 100-mm dishes to achieve respectively 1.5×106 and 1.8×106 cells per dish at the day of experiment. Preparation of cytosolic and nuclear extracts and immunoblot assays were performed as described previously [6]. Contamination of the nuclear fraction with cytosolic components was ruled out by performing controls as described in [10]. All proteins were analyzed on either 8% or 12.5% polyacrylamide gels, or onto an Amersham ECL gel 4–12%. LMW-SDS protein markers from GE Healthcare Life Sciences (Uppsala, Sweden) or LMW protein markers from NZYTech (Lisboa, Portugal) were used. Antibodies sc-372 (1:1000), sc-70 (1:300), sc-371 (1:800), sc-945 (1:400) and sc-7156 (1:800), all from Santa Cruz Biotechnology, Santa Cruz, California, USA, were used to identify p65, c-Rel, IκB-α, IκB-β and IκB-ε respectively. The corresponding bands for each proteins were quantified by signal intensity analysis, normalized to the protein loading (membrane stained with Ponceau S), using the ImageJ software [19].
Estimation of intracellular H2O2 concentrations in HeLa cells
Intracellular H2O2 concentrations were estimated from the H2O2 gradient across the plasma membrane as described in [1], [6]. The gradient, i.e. the ratio between H2O2 concentration outside and inside (cytosol) the cell, may be inferred from the pseudo-first-order rate constants that characterize the H2O2 consumption by intact cells (kintact cell), catalase (kcatalase) and glutathione peroxidase activities (kGPx), the main enzymes catalyzing H2O2 reduction in disrupted cells (Eq. (1)).
| (1) |
Statistical analysis
All data shown is the mean±standard deviation of at least four independent experiments except where otherwise stated. Independent time points were compared with control levels (1 a.u.) by using the two-tailed t-test or, in the case of multiple comparisons, by using analysis of variance (ANOVA) with the Student–Newman–Keuls post-hoc test. Statistically significant differences found for data represented in Fig. 1 are only referred in the text.
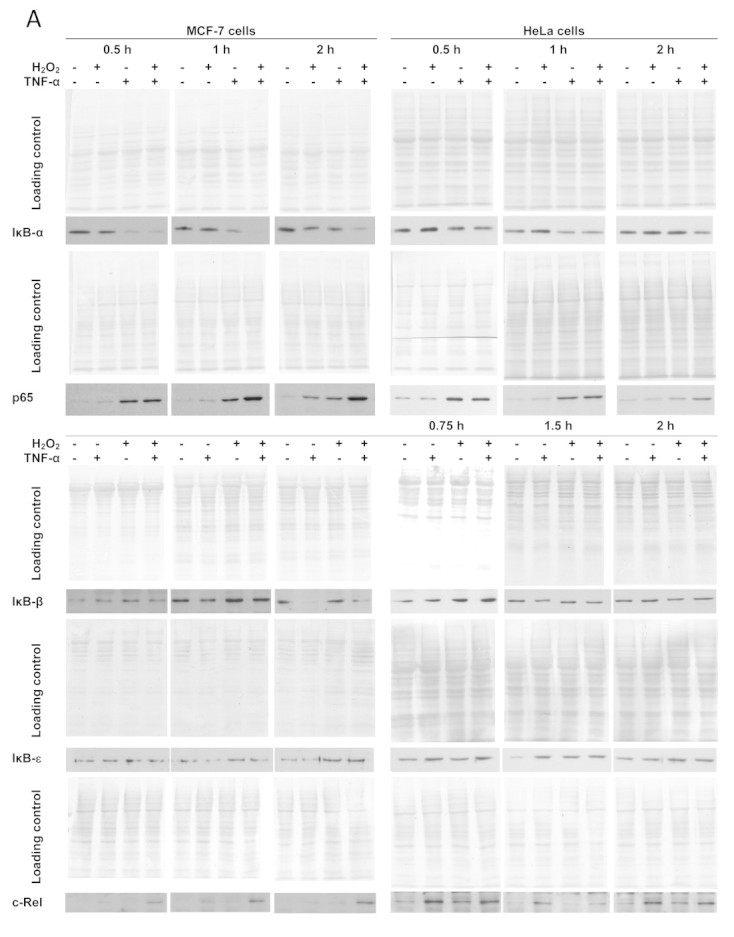
Fig. 1.
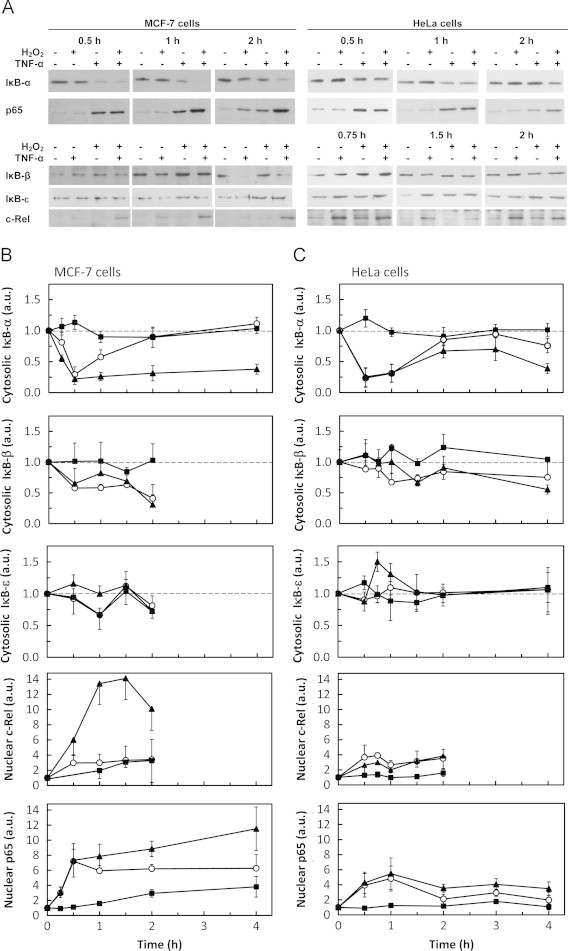
Differential modulation of IκB and Rel proteins by H2O2 and TNF-α. The levels of cytosolic IκB-α, IκB-β and IκB-ε and of nuclear p65 and c-Rel were followed by western blot. (A) Representative immunoblots of n=3–8 independent experiments showing the effect of H2O2 and TNF-α on IκB-α, IκB-β, IκB-ε, p65 and c-Rel. Signal intensity quantification of protein levels expressed as the mean±standard deviation in arbitrary units (a.u.) relative to control for MCF-7 cells (B) and HeLa (C) cells exposed to either steady-state 25 µM H2O2 (■) or 0.37 ng mL−1 TNF-α (○) or both agents simultaneously (▲). Protein levels were normalized to the protein loading (membrane stained with Ponceau S). In order to make the figure easier to analyze, the protein loading controls are only shown as supplementary information (Supplementary Fig. S1). In MCF-7 cells, the values for c-Rel are a minimal value because no band was observed in control cells and normalization was made with the lighter band visualized in the immunoblot. Statistically significant differences found for data represented in Fig. 1 are only referred in the text.
Results
H2O2 effect on IκB and Rel proteins
To characterize NF-κB activation by a moderate dose of steady-state H2O2 the cytosolic levels of the three more abundant IκBs, IκB-α, IκB-β and IκB-ε, as well as p65 and c-Rel were analyzed in MCF-7 (Fig. 1A, B) and HeLa cells (Fig. 1A, C).
In general, MCF-7 cells showed a more responsive activation of NF-κB by H2O2 than HeLa cells, both when H2O2 effects are considered by itself and when H2O2 modulation of TNF-α-dependent NF-κB activation is considered. Modulation occurs when the effects observed by adding simultaneously H2O2 and TNF-α to cells are not just the sum of the effects observed by adding individually each one of these agents. NF-κB activation by H2O2 alone measured as c-Rel or p65 nuclear translocation occurs in MCF-7 cells (Fig. 1B), while it is absent in HeLa cells (Fig. 1C). However, in MCF-7 cells, activation of NF-κB by H2O2 has slower kinetics than activation by TNF-α. The most dramatic effect observed was the synergism between TNF-α and H2O2 in c-Rel translocation in MCF-7 cells, where the 4-fold activation caused by these agents individually was tripled (Fig. 1B). This synergism is cell-type dependent because in HeLa cells an antagonism was observed under the same conditions (Fig. 1C).
Concerning the inhibitory κB proteins, H2O2 by itself had minor effects in all IκB proteins in both cell lines. TNF-α induced a rapid and large decrease of IκB-α levels followed by a recovery to control levels, as expected. However, when in the presence of TNF-α, H2O2 completely thwarted the recovery of IκB-α levels after 1-h of stimulus in MCF-7 cells (Fig. 1B), while in HeLa cells the recovery of IκB-α levels was only partially inhibited (Fig. 1C). IκB-β levels also showed a fast decrease in the presence of TNF-α in MCF-7 cells, although the degradation was incomplete and more sustained over time, when compared with IκB-α. This agrees with data from the literature because IκB-β expression, unlike IκB-α, is not dependent on NF-κB [20], [21]. However, for IκB-β no modulatory effect by H2O2 was observed for both cell types. For IκB-ε, a modulatory role of H2O2 in the presence of TNF-α was observed. This effect was particularly evident in HeLa cells (Fig. 1C), where a near 50 % increase in IκB-ε levels occurred at 45 min (P=0.002).
Constitutive levels of IκB and Rel proteins
Since our results showed a differential regulation of NF-κB by H2O2 in MCF-7 and HeLa cells, we put forward the hypothesis that such behavior could be related to differences in the levels of Rel and IκB proteins in MCF-7 and HeLa cells. Therefore, we characterized the relative constitutive levels of both p65/c-Rel and IκB protein levels in the cytosol of MCF-7 and HeLa cells. In MCF-7 cells c-Rel levels were about 3-fold higher (Fig. 2C and D) while those of IκB-ε (Fig. 2B and D) were about half of those in HeLa cells, a result that is expected because c-Rel is overexpressed in breast cancers [22], [23]. The levels of p65, IκB-α (Fig. 2A and D) and IκB-β (Fig. 2B and D) were about 25 % higher in MCF-7 cells than in HeLa cells. Thus, the six fold-difference in the c-Rel/IκB-ε ratio found between the two cell lines may explain the opposite regulation of c-Rel translocation caused by H2O2 in the presence of TNF-α.
Fig. 2.
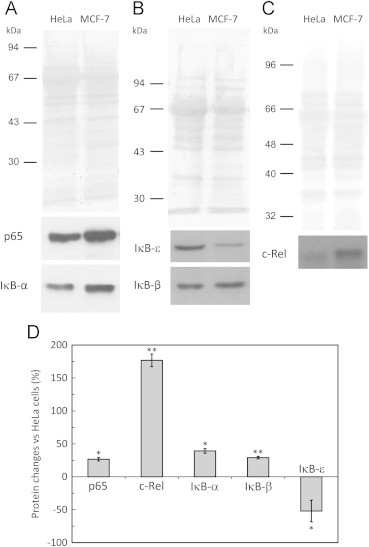
Constitutive levels of IκB and Rel proteins in MCF-7- and HeLa cells. The levels of constitutive cytosolic IκB-α, IκB-β, IκB-ε and of p65 and c-Rel were determined in cytosolic extracts (35 µg) by western blot in MCF-7 and HeLa cells. Representative immunoblots and loading controls of n=4–7 independent experiments for p65 and IκB-α (A), IκB-β and IκB-ε (B), and c-Rel (C). Signal intensity quantification (D) expressed as the mean±standard deviation of the relative difference in MCF-7 protein levels relative to HeLa protein levels. Protein levels were normalized to the protein loading (membrane stained with Ponceau S). * P<0.01; ** P<0.001.
Estimation of cytosolic H2O2 concentrations
The differential regulation of NF-κB in MCF-7 and HeLa cells induced by H2O2 (Fig. 1) may also be caused by different H2O2 gradients across the plasma membrane. When H2O2 is added exogenously to cells it is able to cross the plasma membrane, but this diffusion is not a completely “free” process [1]. In fact, since H2O2 diffusion through the plasma membrane is rate-limiting of H2O2 consumption, the formation of a gradient across the membrane occurs when an external source is added [1]. In Table 1, the H2O2 consumption activities of catalase and GPx and the resulting H2O2 gradient across the plasma membrane for HeLa cells are shown and compared with the results previously obtained by us for MCF-7 cells [6]. The consumption rate by intact cells is approximately the same for both cell lines. However, for the same extracellular H2O2 concentration the estimated intracellular H2O2 concentration for HeLa cells will be lower than that of MCF-7 cells because HeLa cells have higher (about seven-fold) GPx activity. The higher catalase activity found in MCF-7 cells is not enough to compensate the differences in GPx activity and, consequently, MCF-7 cells have a lower H2O2 gradient than HeLa cells. This indicates that, for an extracellular concentration of 25 µM H2O2, the intracellular H2O2 concentration for HeLa cells is expected to be about 3.7 µM, whereas for MCF-7 cells this value increases to approximately 12.5 µM.
Table 1.
Estimation of the H2O2 gradient across the plasma membrane in HeLa cells after exposure to extracellular H2O2. kintact cell, H2O2 consumption by intact cells; kcatalase, catalase activity; kGPx, glutathione peroxidase activity. The H2O2 gradient is calculated from Eq. (1).
| Parameter | HeLa cells | MCF-7 cellsa |
|---|---|---|
| kintact cell (min−1×10–6cells×mL) | 0.50±0.017 | 0.43±0.015 |
| kcatalase (min−1×10–6cells×mL) | 0.21±0.042 | 0.42±0.061 |
| kGPx (min−1×10–6cells×mL) | 3.18±0.450 | 0.41±0.063 |
| [H2O2]out/[H2O2]in | 6.8±2.3 | 1.9±0.6 |
Adapted from [6].
Discussion
The role of H2O2 in the NF-κB pathway has been widely studied. It was first described as the common messenger of any NF-κB-inducer, but nowadays a modulatory role is the most accepted paradigm. During inflammation H2O2 is produced and, together with pro-inflammatory cytokines, may reach neighboring cells where it exerts signaling roles. Using moderate doses of steady-state H2O2 (12.5–25 µM), delivered to cells in a way that mimics in vivo H2O2 formation, we previously described an increased translocation of the subunit p65, which consequently lead to a specific up-regulation of NF-κB-dependent genes [6]. Here we extended these results and found in MCF-7 cells a significant synergism between H2O2 and TNF-α stimulating translocation of c-Rel to the nucleus. H2O2 and TNF-α alone had similar effects on c-Rel translocation, but when these two agents were added together c-Rel translocation near tripled the 4-fold induction triggered by either TNF-α or H2O2 alone in MCF-7 cells. On the other hand, in HeLa cells there was a small antagonism at short incubation times, since H2O2 inhibited the near 3-fold induction in c-Rel nuclear levels induced by TNF-α. What is the cause for this opposite behavior in the two cell lines studied? According to our data, the ratio c-Rel/IκB-ε is six-fold higher in MCF-7 cells than in HeLa cells. Taking in account that IκB-α binds preferentially to the heterodimers p50/p65 and p50/c-Rel [9], while IκB-ε binds preferentially to p65 homodimers and c-Rel/p65 heterodimers [13], in HeLa cells most c-Rel is probably bound to IκB-ε (Fig. 3). So an up-regulation of IκB-ε by H2O2 in TNF-α-treated cells may explain the decreased c-Rel translocation in these cells when compared with the effect of TNF-α alone. By opposition, in MCF-7 cells, where the ratio c-Rel/IκB-ε is 6-fold higher, a significant part of c-Rel is probably bound to IκB-α, explaining why in these cells the sustained low IκB-α levels caused by H2O2 in the presence of TNF-α triggered a very large translocation of c-Rel. According to our data, another factor that should be taken in consideration to explain the cell-specific effects of H2O2 is the actual intracellular concentration of H2O2 achieved in MCF-7 and HeLa cells. The addition of an exogenous dose of H2O2 to cells results in a H2O2 gradient across the plasma membrane of about two in MCF-7 cells [6] and seven in HeLa cells, in great part because of the high GPx activity in HeLa cells (Fig. 3). Therefore, when exposed to an extracellular concentration of steady-state 25 µM H2O2, MCF-7 cells are estimated to have a higher intracellular H2O2 concentration (approximately 12.5 µM) than HeLa cells (approximately 3.7 µM) (Fig. 3). Taking in account that cellular effects triggered by H2O2 are strongly dependent of small changes in its concentration [5], [15], [18], this could explain: (a) the higher general response regarding NF-κB activation and modulation by H2O2 in MCF-7 cells; and (b) the different effects caused by H2O2 in IκB-ε cytosolic concentration observed in HeLa and MCF-7 cells. Exposing HeLa cells to a higher extracellular concentration of H2O2 in an attempt to obtain a similar intracellular concentration in both cell types used is not feasible because it will damage HeLa cells (not shown). In fact, an increase in the rate of H2O2 production of approximately 3.4 times would be needed, which will exert an increase of 3.4 times in the intracellular consumption of H2O2. A concomitant increase in the formation of oxidized glutathione (GSSG) would occur because in HeLa cells H2O2 consumption is mostly done by GPx (Table 1), affecting the intracellular redox balance and thus inducing oxidative stress.
Fig. 3.
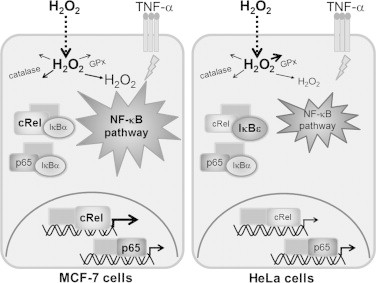
Possible model for the differential c-Rel activation by H2O2 and TNF-α in MCF-7- and HeLa cells. For the same extracellular H2O2 concentration the estimated intracellular H2O2 concentration for HeLa cells will be lower than that of MCF-7 cells because HeLa cells have higher GPx activity. Moreover, MCF-7 cells have a 3-fold higher concentration of c-Rel and 2-fold lower IκB-ε levels than HeLa cells. Therefore in HeLa cells most c-Rel is probably bound to IκB-ε. The differences described between MCF-7 and HeLa cells justify the specific outcome in NF-κB-dependent gene expression. Higher concentrations are shown as bigger lettering.
What is the relevance of the synergism versus the antagonism observed in MCF-7 and HeLa cells concerning c-Rel translocation? The synergism is probably more relevant than the antagonism because it has been found that overexpression of c-Rel has much more dramatic effects on gene expression than deletion of c-Rel [24]. Reasons for these are the redundancy within NF-κB family since many NF-κB genes have promoter regions that bind more than one NF-κB member, and some genes bind to all members of the family. This differential regulation of c-Rel may have consequences in c-Rel-dependent gene expression. c-Rel can complex with p65, p50 and with itself, and binds preferentially to κB sites different than those that p65 binds to. For example, the canonical κB consensus sequence for p50/p65 is GGGRNNYYCC (R for purine, Y for pyrimidine and N for any base), while the consensus sequence for the heterodimer c-Rel/p65 is HGGARNYYCC (H for not G) [25]. The human chromosome 22 contains 35% of p65 canonical κB sites, but only 6% c-Rel/p65 κB sequences [26]. Thus, the higher number of NF-κB-dependent genes up-regulated by H2O2 in MCF-7 cells, when compared with HeLa cells [6], is probably a consequence of the increased translocation of c-Rel and p65 observed in MCF-7 cells when compared with HeLa cells (Fig. 3). Genes that are up-regulated by H2O2 in MCF-7 cells and that are known to be regulated by c-Rel include the intracellular adhesion molecule 1 (ICAM-1) [24], IL-8 [27], and monocyte chemotactic protein 1 (MCP-1) [28].
c-Rel is mainly expressed in hematopoietic cells [29] and plays a role in lymphoid cell growth and survival [30]. It is also involved in mammalian B and T cell function and has been associated with malignancies in these cells [31], [32]. In addition, c-Rel has been also associated with solid tumors, particularly breast cancer, where 85 % of tissue samples show elevated nuclear levels of c-Rel [22], [23], as further supported by our data that show a 3-fold higher abundance of c-Rel in the breast cancer MCF-7 cell line when compared with the cervix cancer HeLa cell line. The normal breast epithelial phenotype is maintained through the repression of c-Rel transcriptional activity by inhibiting both DNA binding and transactivation, which is accomplished via estrogen receptor α signaling [33]. An epigenetic mechanism involving the activation of a PKCθ-Akt pathway leads to downregulation of estrogen receptor α synthesis and, consequently, derepression of c-Rel. The activation of c-Rel induces expression of genes encoding cyclin D1, c-Myc, and Bcl-xL, RelB, which may lead to cancerization. This may explain why we observed in MCF-7 cells, strong stimulatory effects by H2O2. In fact, H2O2 is a known activator of the Akt pathway, which inactivates forkhead box O protein 3a (FOXO3a) leading to decreased synthesis of its target genes, including ERα [33]. Therefore, both through the already known Akt activation, and by the strong synergism with TNF-α causing c-rel nuclear translocation found in this work, H2O2 may play a role in chronic inflammation-induced cancerization, raising the hypothesis that antioxidant therapy could be preventive of breast cancer.
A recent study [34] showed that c-Rel may be considered an important regulator of hepatic wound-healing response. In c-Rel null (c-rel−/−) mice, the wound-healing response to bile duct ligation induced injury was impaired and this was associated with deficiencies in the expression of fibrogenic genes, collagen I and α-smooth muscle actin, by hepatic stellate cells. H2O2 has an important role in the rapid recruitment of leukocytes to the wound during the early events of wound responses [35]. The use of a genetically encoded H2O2 sensor in zebrafish larvae revealed that dual oxidase (Duox) creates a sustained rise in H2O2 concentration at the wound margin which extends into the tail-fin epithelium as a decreasing concentration gradient. Our results show that another possible function of H2O2 in the wound healing response may involve its synergism with TNF-α to increase c-Rel translocation to the nucleus.
Conclusions
Our data show that H2O2 is an important physiological modulator of the NF-κB pathway. By itself, depending on the cell type, H2O2 activates c-Rel at the expense of IκB-ε degradation. More relevant is, however, the modulatory effects observed in the presence of cytokines, like TNF-α. In this case an activation of p65 translocation that is caused by a sustained degradation of IκB-α is observed. The most dramatic effect triggered by H2O2, in the presence of TNF-α, was a large increase in the nuclear translocation of c-Rel in the MCF-7 cell line. Thus, H2O2 may have an important role in the regulation c-Rel-dependent processes. A better knowledge of H2O2 metabolism in each cell type present in different organs is probably a necessary approach for specific therapies involving NF-κB-targeting.
Acknowledgments
This work was supported by Fundação para a Ciência e a Tecnologia (FCT), Portugal (Grants PTDC/QUI/69466/2006, PEst-OE/QUI/UI0612/2013). VOM was a recipient of a FCT-PhD Fellowship (SFRH/BD/16681/2004) and GC was a recipient of a BI fellowship (PTDC/QUI/69466/2006).
Footnotes
Supplementary data associated with this article can be found in the online version at 10.1016/j.redox.2013.05.004.
Appendix A. Supplementary materials
Fig. S1.
Differential modulation of IκB and Rel proteins by H2O2and TNF-α. This figure contains the protein loading controls for the Western blot results shown in Figure 1 of the main manuscript.
References
- 1.Antunes F., Cadenas E. Estimation of H2O2 gradients across biomembranes. FEBS Letters. 2000;475:121–126. doi: 10.1016/s0014-5793(00)01638-0. [DOI] [PubMed] [Google Scholar]
- 2.Bienert G.P., Schjoerring J.K., Jahn T.P. Membrane transport of hydrogen peroxide. Biochimica et Biophysica Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Karlsson A., Dahlgren C. Assembly and activation of the neutrophil NADPH oxidase in granule membranes. Antioxidants and Redox Signaling. 2002;4:49–60. doi: 10.1089/152308602753625852. [DOI] [PubMed] [Google Scholar]
- 4.Test S.T., Weiss S.J. Quantitative and temporal characterization of the extracellular H2O2 pool generated by human neutrophils. Journal of Biological Chemistry. 1984;259:399–405. [PubMed] [Google Scholar]
- 5.Oliveira-Marques V., Marinho H.S., Cyrne L., Antunes F. Role of hydrogen peroxide in NF-kappaB activation: from inducer to modulator. Antioxidants and Redox Signaling. 2009;11:2223–2243. doi: 10.1089/ars.2009.2601. [DOI] [PubMed] [Google Scholar]
- 6.de Oliveira-Marques V., Cyrne L., Marinho H.S., Antunes F. A quantitative study of NF-kappaB activation by H2O2: relevance in inflammation and synergy with TNF-alpha. Journal of Immunology. 2007;178:3893–3902. doi: 10.4049/jimmunol.178.6.3893. [DOI] [PubMed] [Google Scholar]
- 7.Liu X., Zweier J.L. A real-time electrochemical technique for measurement of cellular hydrogen peroxide generation and consumption: evaluation in human polymorphonuclear leukocytes. Free Radical Biology and Medicine. 2001;31:894–901. doi: 10.1016/s0891-5849(01)00665-7. [DOI] [PubMed] [Google Scholar]
- 8.Chen L.F., Greene W.C. Shaping the nuclear action of NF-kappaB. Nature Reviews Molecular Cell Biology. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh S., May M.J., Kopp E.B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annual Review of Immunology. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira-Marques V., Marinho H.S., Cyrne L., Antunes F. Modulation of NF-kappaB-dependent gene expression by H2O2: a major role for a simple chemical process in a complex biological response. Antioxidants and Redox Signaling. 2009;11:2043–2053. doi: 10.1089/ars.2008.2279. [DOI] [PubMed] [Google Scholar]
- 11.Gilmore T.D. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 12.Li Q., Verma I.M. NF-kappaB regulation in the immune system. Nature Reviews Immunology. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 13.Whiteside S.T., Israel A. I kappa B proteins: structure, function and regulation. Seminars in Cancer Biology. 1997;8:75–82. doi: 10.1006/scbi.1997.0058. [DOI] [PubMed] [Google Scholar]
- 14.Brigelius-Flohé R., Flohé L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxidants and Redox Signaling. 2011;15:2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matias A.C., Marinho H.S., Cyrne L., Herrero E., Antunes F. Biphasic modulation of fatty acid synthase by hydrogen peroxide in Saccharomyces cerevisiae. Archives of Biochemistry and Biophysics. 2011;515:107–111. doi: 10.1016/j.abb.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Sobotta M.C., Barata A.G., Schmidt U., Mueller S., Millonig G., Dick T.P. Exposing cells to H2O2: a quantitative comparison between continuous low-dose and one-time high-dose treatments. Free Radical Biology and Medicine. 2013;60:325–335. doi: 10.1016/j.freeradbiomed.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Wagner B.A., Witmer J.R., van’t Erve T.J., Buettner G.R. An assay for the rate of removal of extracellular hydrogen peroxide by cells. Redox Biology. 2013;1:210–217. doi: 10.1016/j.redox.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antunes F., Cadenas E. Cellular titration of apoptosis with steady state concentrations of H(2)O(2): submicromolar levels of H(2)O(2) induce apoptosis through Fenton chemistry independent of the cellular thiol state. Free Radical Biology and Medicine. 2001;30:1008–1018. doi: 10.1016/s0891-5849(01)00493-2. [DOI] [PubMed] [Google Scholar]
- 19.W.S. Rasband, ImageJ. U.S. National Institutes of Health, Bethesda, Maryland, USA, 1997.
- 20.Weil R., Laurent-Winter C., Israel A. Regulation of IkappaBbeta degradation. Similarities to and differences from IkappaBalpha. Journal of Biological Chemistry. 1997;272:9942–9949. doi: 10.1074/jbc.272.15.9942. [DOI] [PubMed] [Google Scholar]
- 21.Griffin B.D., Moynagh P.N. In vivo binding of NF-kappaB to the IkappaBbeta promoter is insufficient for transcriptional activation. Biochemical Journal. 2006;400:115–125. doi: 10.1042/BJ20060786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sovak M.A., Bellas R.E., Kim D.W., Zanieski G.J., Rogers A.E., Traish A.M., Sonenshein G.E. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. Journal of Clinical Investigation. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cogswell P.C., Guttridge D.C., Funkhouser W.K., Baldwin A.S., Jr. Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene. 2000;19:1123–1131. doi: 10.1038/sj.onc.1203412. [DOI] [PubMed] [Google Scholar]
- 24.Bunting K., Rao S., Hardy K., Woltring D., Denyer G.S., Wang J., Gerondakis S., Shannon M.F. Genome-wide analysis of gene expression in T cells to identify targets of the NF-kappa B transcription factor c-Rel. Journal of Immunology. 2007;178:7097–7109. doi: 10.4049/jimmunol.178.11.7097. [DOI] [PubMed] [Google Scholar]
- 25.Parry G.C., Mackman N. A set of inducible genes expressed by activated human monocytic and endothelial cells contain kappa B-like sites that specifically bind c-Rel-p65 heterodimers. Journal of Biological Chemistry. 1994;269:20823–20825. [PubMed] [Google Scholar]
- 26.Martone R., Euskirchen G., Bertone P., Hartman S., Royce T.E., Luscombe N.M., Rinn J.L., Nelson F.K., Miller P., Gerstein M., Weissman S., Snyder M. Distribution of NF-kappaB-binding sites across human chromosome 22. Proceedings of the National Academy of Sciences of USA. 2003;100:12247–12252. doi: 10.1073/pnas.2135255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunsch C., Rosen C.A. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Molecular and Cellular Biology. 1993;13:6137–6146. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann A., Leung T.H., Baltimore D. Genetic analysis of NF-kappaB/Rel transcription factors defines functional specificities. EMBO Journal. 2003;22:5530–5539. doi: 10.1093/emboj/cdg534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrasco D., Weih F., Bravo R. Developmental expression of the mouse c-rel proto-oncogene in hematopoietic organs. Development. 1994;120:2991–3004. doi: 10.1242/dev.120.10.2991. [DOI] [PubMed] [Google Scholar]
- 30.Kontgen F., Grumont R.J., Strasser A., Metcalf D., Li R., Tarlinton D., Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes and Development. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 31.Gilmore T.D., Gerondakis S. The c-Rel transcription factor in development and disease. Genetics and Cancer. 2011;2:695–711. doi: 10.1177/1947601911421925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fullard N., Wilson C.L., Oakley F. Roles of c-Rel signalling in inflammation and disease. International Journal of Biochemistry and Cell Biology. 2012;44:851–860. doi: 10.1016/j.biocel.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Belguise K., Sonenshein G.E. PKCtheta promotes c-Rel-driven mammary tumorigenesis in mice and humans by repressing estrogen receptor alpha synthesis. Journal of Clinical Investigation. 2007;117:4009–4021. doi: 10.1172/JCI32424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gieling R.G., Elsharkawy A.M., Caamano J.H., Cowie D.E., Wright M.C., Ebrahimkhani M.R., Burt A.D., Mann J., Raychaudhuri P., Liou H.C., Oakley F., Mann D.A. The c-Rel subunit of nuclear factor-kappaB regulates murine liver inflammation, wound-healing, and hepatocyte proliferation. Hepatology. 2010;51:922–931. doi: 10.1002/hep.23385. [DOI] [PubMed] [Google Scholar]
- 35.Niethammer P., Grabher C., Look A.T., Mitchison T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]


