Abstract
Hypercholesterolemia is a risk factor for the development of hypertrophic cardiomyopathy. Nevertheless, there are few studies aimed at determining the effects of dietary compounds on early or mild cardiac hypertrophy associated with dyslipidemia. Here we describe left ventricular (LV) hypertrophy in 12 week-old Apo E−/− hypercholesterolemic mice. The LV end diastolic posterior wall thickness and overall LV mass were significantly increased in Apo E−/− mice compared with wild type (WT) controls. Fractional shortening, LV end diastolic diameter, and hemodynamic parameters were unchanged from WT mice. Oral low dose quercetin (QCN; 0.1 µmol QCN/kg body weight for 6 weeks) significantly reduced total cholesterol and very low density lipoprotein in the plasma of Apo E−/− mice. QCN treatment also significantly decreased LV posterior wall thickness and LV mass in Apo E−/− mice. Myocardial geometry and function were unaffected in WT mice by QCN treatment. These data suggest that dietary polyphenolic compounds such as QCN may be effective modulators of plasma cholesterol and could prevent maladaptive myocardial remodeling.
Keywords: Atherosclerosis, Hypertrophy, Cholesterol, Quercetin
Graphical abstract
Highlights
-
•
Oral low doses of Quercetin resulted in peak plasma levels of approximately 100 nM.
-
•
Quercetin had no effect on cholesterol profiles in wild type mice but decreased VLDL in ApoE−/− mice.
-
•
Quercetin treatment attenuated the cardiac hypertrophy in ApoE−/− mice but had no effects on heart function in wild type mice.
Introduction
While left ventricular (LV) hypertrophy is generally regarded as an adaptive response to chronic pressure or volume overload, it is also a gateway to pathogenesis and heart failure. Specifically, concentric hypertrophy is an early remodeling event that often precedes myocardial dilatation, progression to eccentric hypertrophy, and global pump failure. Several studies show that hypercholesterolemia itself is independently associated with mild LV concentric hypertrophy and increased LV mass in humans [1,2], underscoring a role for dyslipidemia in myocardial remodeling. Although some of the mechanisms contributing to these events have been elucidated, safe interventions with minimal side effects that prevent or diminish early remodeling events are scant.
Hypercholesterolemic Apo E−/− mice have substantially elevated triglycerides and increased total plasma cholesterol, and spontaneously develop atherosclerosis [3]. It has been shown that these mice exhibit increased LV mass at 6 weeks of age in the absence of hemodynamic stress [4], further suggesting that hyperlipidemia may be an early initiator of cardiac remodeling. Therefore, dietary or other therapeutic interventions that have the potential to decrease plasma lipids are of particular interest for prevention of cardiac hypertrophy. For example, the dietary polyphenol quercetin (QCN) which is present in broccoli, onions, grapes, and wine [5] has been shown to prevent hypertrophy of cultured myocytes in response to angiotensin [6] and to lower blood pressure and attenuate aortic constriction-induced cardiac hypertrophy in rats [7]. Other polyphenolic compounds such as resveratrol have similar effects as QCN on the cardiovascular system [8–10]. Taken together with data showing that dietary intake of polyphenols decreases plasma cholesterol in both humans and animals [11–13], polyphenols appear to have a generally beneficial effect on the cardiovascular system[10]. Nevertheless, there are relatively few reports based on the effects of orally administered polyphenols such as QCN on the heart under conditions of chronic hypercholesterolemia.
In the present study we elected to use QCN as single polyphenolic because of its previously reported beneficial effects on the cardiovascular system and its potential to serve as the basis for the development of more potent and effective therapeutics [14–17]. Therefore, we hypothesized that QCN treatment could diminish the early cardiac remodeling processes that occur during hypercholesterolemia. In this study, we examined the effects of low dose QCN on plasma cholesterol and cardiac geometry and function in Apo E−/− and wild-type (WT) mice. Our findings suggest that dietary intake of polyphenolics such as QCN may be a safe and effective means to prevent or decrease hypercholesterolemia and its associated cardiac structural changes that could lead to maladaptive remodeling and further functional defects.
Materials and methods
Reagents. All chemicals were purchased from Calbiochem (San Diego, CA), BioMol (Plymouth Meeting, PA) or Sigma-Aldrich-Fluca (St.Louis, MO). All primary antibodies were obtained from Abcam Inc (Cambridge, MA).
Animal treatments. Six-week old wild-type (C57BL/6, WT) or Apo E−/− (Apoetm1unc) mice were obtained from Jackson Laboratories (Bar Harbor, Maine) and fed a standard mouse pellet diet (Ralston Purina Diet) and water ad libitum for 1 week. For the following 6 weeks, QCN was administered as gelatin pellets prepared by mixing flavored gelatin solution (25% w/v) at 37 °C with QCN solution in DMSO to a final concentration of QCN 0.1% (v/v). Control groups received the pellets without QCN. All mice were maintained at constant humidity (60±5%), temperature (24±1 °C), and light cycle (6AM to 6PM). All protocols were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham and were consistent with The Guide for the Care and Use of Laboratory Animals (USDHHS, NIH publication no 86-23, 1996).
Plasma QCN analyses. After bolus gavage of QCN (equivalent to one feeding), plasma samples were acquired at various time points by retro-orbital bleeding and by cardiac puncture. They were treated with a Helix pomati preparation (Sigma, St, Louis, MO) to hydrolyze glucuronide and sulfate conjugates. In brief, phenolphthalein glucuronide, 4-methylumbelliferyl sulfate, and chrysin were added as internal standards to each plasma sample before hydrolysis with 400 U of ß-glucuronidase and aryl sulfatase in 150 mmol/L ammonium acetate buffer, pH 5, for 16 h at 37 °C. The samples were extracted with ethyl acetate, then evaporated to dryness, and finally reconstituted in 80% aqueous methanol. LC-MS/MS analyses of plasma QCN and methylated-QCN were performed using a system consisting of a model SIL-HT refrigerated Shimadzu auto sampler and HPLC instrument (Shimadzu Scientific Instruments, Inc. Columbia, MD), and an API 4000 mass spectrometer (Applied Biosystems/MDS Sciex, Concord, Ontario, Canada). Chromatography was carried out on a reversed-phase Thermo C18 Gold column (50×2.0 mm i.d.) pre-equilibrated with 0.1% formic acid. The mobile phase consisted of two eluents, solvent A (water containing 0.1% formic acid) and solvent B (acetonitrile containing 0.1% formic acid) with a flow rate of 0.5 ml/min. The total run time was 7 min. Initially, the starting solvent was 30% B and went up to 100% B over the first 4 min. The system was returned to the initial 30% B condition over 6 min. The column effluent was introduced into the mass spectrometer using electrospray ionization (ESI) in the negative ion mode. Nitrogen was used as nebulizer, and curtain gas. The source temperature and collision energy were 300 °C and -30 eV, respectively. For multiple reaction monitoring (MRM) experiments, the mass transitions m/z 301/151 (quercetin), m/z 315/300 (methyl quercetin), m/z 315/151 (methyl quercetin) and m/z 253/143 (chrysin) were used. Chrysin was used as internal standard. The LC-MS-MS system was controlled by BioAnalyst 1.4.1 software. The quantification of quercetin and methyl quercetin was based on a calibration curve over the concentration range 1–10 μmol/L. The assay method for quantification was demonstrated to be specific, with lower limit of quantification of 1 nmol/L.
Analysis of the lipid profiles in mouse plasma. Blood was collected from anesthetized animals during sacrifice by retro-orbital bleeding. Total plasma cholesterol levels were determined colorimetrically using a commercial kit (Infinity cholesterol reagent; Sigma, St. Louis, MO). Plasma lipoprotein cholesterol profiles were analyzed by the chromatographic method described in [18].
Echocardiography and measurement of blood pressure. Animals were anesthetized using isofluorane (1–2%) and their body weight was measured. Analysis of LV parameters by two-dimensional B-mode, M-mode and PW-Doppler mode echocardiography was performed non-invasively using an echocardiography high resolution imaging system VEVO 770 equipped with a 30 MHz transducer (Visual Sonics, Toronto, Canada). To monitor arterial blood pressure (BP), the non-invasive device Coda 2 (Kent Scientific Corp.; Torrington, CT) was used. Five acclimatization cycles were performed prior to measurement of BP followed by 15 cycles of measurements of the systolic and diastolic pressure. Mean arterial pressure (MAP) was calculated using the equation: MAP=2/3 Pdias+1/3 Psys. Measurements were carried out over 3 days until a stable baseline was obtained.
Statistical analysis of data. Results of the echocardiography measurements are presented as the mean±standard error (SEM) for 12 animals per phenotype and treatment group. Significant differences were assessed using one-way analysis of variance (ANOVA). Intergroup comparisons were analyzed using Student-Newman-Kuels post hoc test with statistical significance set at p<0.05.
Results
In the course of the study, WT and Apo E−/− mice were fed the control or the QCN-containing diets for 6 weeks. Daily consumption of QCN by animals during the 6 week treatment period was approximately 0.1 µmol QCN/kg body weight. For the validation of QCN absorption into the blood, solubilized gelatin pellets were fed to WT mice (N=5) by gavage and the concentration of QCN derivatives was measured in the mouse plasma by mass-spectrometry at various times (Table 1). Thirty min after the consumption of solubilized QCN-containing pellets, plasma QCN levels reached readily detectable levels that decreased linearly with time (R2=0.99; Table 1). QCN was not detectable (<0.1 nmol/L) in untreated animals. The methylated form of QCN, Met-QCN, which is a metabolite of free QCN, had similar pharmacokinetics, decreasing with time in the plasma (Table 1). These data confirm that oral intake of QCN increases systemic QCN levels for a significant period of time.
Table 1.
Accumulation of quercetin (QCN) and its derivative, methyl-QCN (Met-QCN) in WT mouse plasma after oral consumption of gelatin pellets containing QCN.
| Time after gavage, (min) | QCN (nmol/L) | Met-QCN (nmol/L) |
|---|---|---|
| 30 | 111.47±12.03 | 23.58±5.61 |
| 60 | 65.90±6.81 | 10.33±2.35 |
| 90 | 22.86±1.25 | 2.69±0.42 |
Liquefied quercetin pellets were administered by gavage, and the mouse plasma was collected at different time points after feeding. Concentrations of the compounds in the plasma were measured by reverse-phase liquid chromatography-mass spectrometry. Data are presented as the mean±SEM, N=5.
Separation of the total cholesterol from the plasma of WT and Apo E−/− mice, fed the control or QCN-diet, by fractions, was performed using FPLC. The resulting chromatograms are presented as Fig. 1A. In agreement with previous reports and as expected [3], Apo E−/− mice displayed severe hypercholesterolemia: the amount of total cholesterol in Apo E−/− control mice was 6-fold higher than WT. A QCN-dependent decrease in VLDL (peak a) was observed in the total plasma cholesterol of Apo E−/− mice. The difference between the chromatograms of WT and Apo E−/− was attributed to intermediate lipoprotein fractions: IDL-LDL fraction (peak b) in Apo E−/− animals and LDL-HDL1 fraction (peak c) in WT mice. Specifically, VLDL and IDL-LDL fractions were significantly increased in ApoE−/− animals with respect to WT controls. Moreover, high density lipoprotein (HDL) was decreased in Apo E−/− mice compared with WT mice. Dietary QCN treatment for six weeks decreased circulating VLDL in ApoE−/− animals by 30% but did not affect levels of IDL-LDL and HDL. (Fig. 1A and B). Treatment with QCN had no effect on plasma cholesterol fractions in WT mice.
Fig. 1.
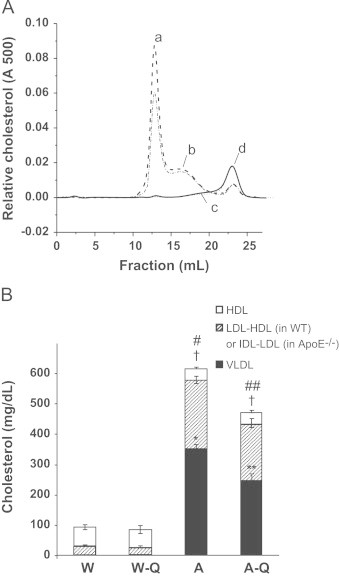
Plasma cholesterol profiles of wild type and ApoE−/− mice fed control and QCN-supplemented diets: Lipoprotein fractions of total plasma cholesterol were separated using fast performance liquid chromatography (FPLC). Panel A: Representative plasma cholesterol lipoprotein profiles for the experimental mouse groups: WT control (solid line), WT+QCN (dotted line), ApoE−/− control (dashed line) and ApoE−/−+ QCN (dash-dot line). Peak a: VLDL; peak b: IDL/LDL; peak c: LDL/HDL1; peak d: HDL. Panel B: Quantitative analysis of individual lipoprotein peaks from the plasma cholesterol profile. Solid bars indicate the amount of VLDL, hatched bars represent LDL-HDL1 in WT or IDL-LDL in ApoE−/−, and empty bars correspond to the HDL fraction. N=6 per group. Data are presented as the mean±SEM. ⁎,⁎⁎p<0.05 for the VLDL fraction when compared to WT. †p<0.05 for the LDL-HDL1/IDL-LDL and #.##p<0.05 for the total plasma cholesterol when compared to WT.
Apo E−/− mouse echocardiographic measurements showed no significant changes in LV end diastolic diameter (LVEDD), but demonstrated significant increases in LV posterior wall diameter (LVPWd) and decreased LVEDD/LVPWd ratios when compared with WT controls (Figs. 2 and 3). Moreover, overall LV masses were also increased in Apo E−/− mice (Table 2). LV fractional shortening and systolic, diastolic, and mean arterial blood pressures were not significantly different when compared with WT controls (Table 2). Therefore, unlike Apo E−/− mice at 24 weeks of age and older which have relatively high arterial blood pressures [19,20], the 12 week-old Apo E−/− mice showed no indication of derangements of vascular tone or blood pressure. Taken together, these data suggest that hypercholesterolemia alone could promote a mild myocardial concentric hypertrophy in the absence of hypertension.
Fig. 2.
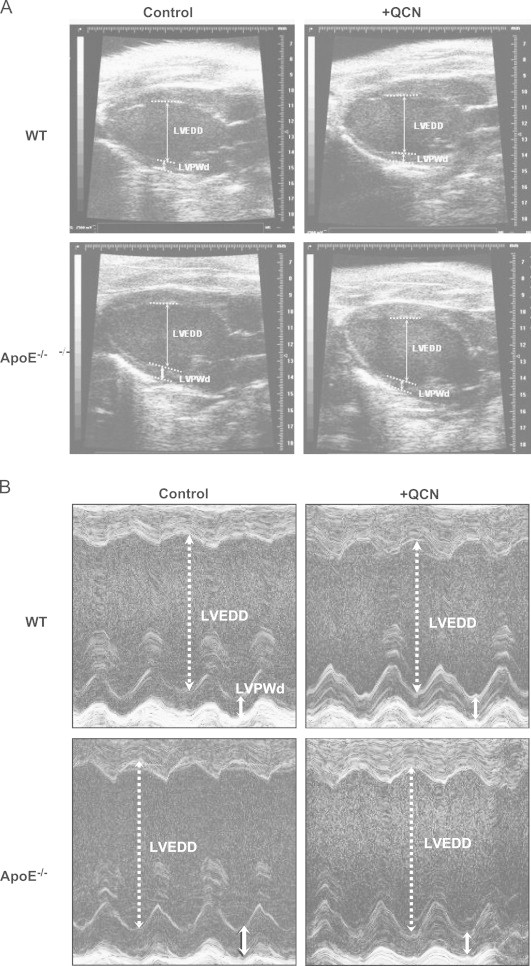
Two-dimensional echocardiographic images of the left ventricle in WT and Apo E−/− mice fed control and QCN-supplemented diets: Panel A: Representative B-mode images of the LV in the experimental animal groups. Panel B: Representative M-mode images of the LV. Arrows indicate LV diameters and LV posterior wall dimensions in diastole (LVEDD and LVPWd, respectively).
Fig. 3.
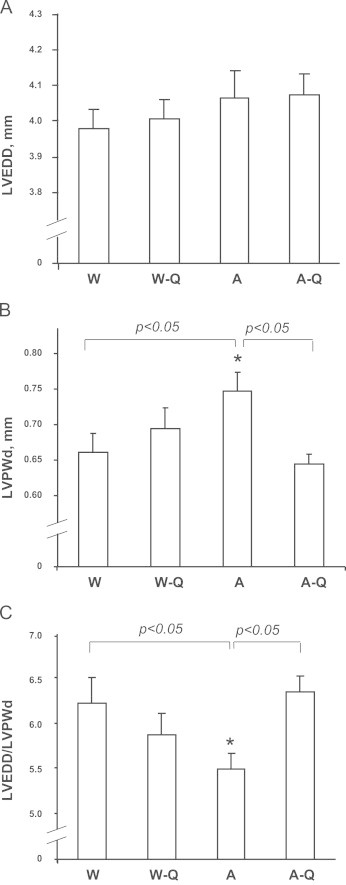
Group data of echocardiographic measurements taken from hearts of WT and ApoE−/− mice fed control and QCN-supplemented diets: M-mode images of the LV of WT and ApoE−/− mice on control and QCN diet were obtained using echocardiography. Parameters of LV geometry were measured directly using the resulting images. Panel A: LVEDD. Panel B: LVPWd. Panel C: ratio between LVEDD and LVPWd. N=12 in each group. Data are presented as the mean±SEM. ⁎p<0.05 compared to WT-controls (one-way ANOVA).
Table 2.
Parameters of cardiac function measured in WT and ApoE−/− mice fed control diets or diets with QCN supplementation.
| Animal group | Wild Type | Wild Type+QCN | Apo E−/− | Apo E−/−+QCN |
|---|---|---|---|---|
| Body weight, (g) | 22.35±0.31 | 23.12±0.37 | 25.44±0.64⁎ | 25.31±0.63⁎ |
| Heart rate, BPM | 439±13 | 442±14 | 449±13 | 453±9 |
| Systolic BP | 146±3 | 152±3 | 160±6 | 150±7 |
| Diastolic BP | 115±3 | 116±3 | 126±6 | 114±7 |
| Mean arterial BP | 125±3 | 128±3 | 137±6 | 126±7 |
| LV mass (corrected) | 81.41±3.72 | 87.0±3.74 | 92.42±3.95⁎ | 88.14±3.42 |
| LV FS | 0.30±0.02 | 0.28±0.02 | 0.28±0.01 | 0.28±0.01 |
p<0.05 (n=12) compared to WT (W; one-way ANOVA).
Body weight was increased in the Apo E−/− group relative to controls and was not altered by QCN treatment (Table 2). Treatment with QCN, however, significantly decreased LVPWd in Apo E−/− mice and restored LVEDD/LVPWd ratios to control values (Figs. 2 and 3). Furthermore, QCN treatment prevented the increases in LV mass that were observed in Apo E−/− mice fed vehicle alone. QCN did not affect heart rate or fractional shortening in Apo E−/− mice or WT controls (Table 2). Taken together, these data demonstrate that Apo E−/− mice display an early, mild concentric hypertrophy that is independent of hemodynamic stress and is ameliorated by QCN treatment.
Discussion
Polyphenolic compounds have several purported beneficial cardiovascular effects and have been shown to decrease oxidative stress, reduce or prevent growth of atherosclerotic plaques, inhibit platelet aggregation, decrease blood pressure, improve vascular reactivity, and decrease plasma lipids and lipoproteins [21]. In general, QCN has a beneficial effect on the cardiovascular system, and has been shown to prevent cardiac remodeling in severe models of cardiac hypertrophy [7]. Although other studies have shown that Apo E−/− mice even at 6 weeks of age demonstrate increased myocardial mass [4], the study described herein is the first to demonstrate a concentric hypertrophy that develops seemingly due to hypercholesterolemia alone. This finding is particularly important because hypercholesterolemia is a major contributor to the mild concentric hypertrophy that occurs in humans [1,2], emphasizing a need for therapeutic interventions to prevent or diminish the early myocardial structural changes that could potentially lead to hypertrophic cardiomyopathy. The development of left ventricular hypertrophy is progressive and one of the commonest risk factors for cardiovascular mortality. Importantly, the risk for developing cardiac hypertrophy is associated with the metabolic syndrome, increased LDL to HDL ratio and unfavorable fatty acid profile suggesting that intervention with low dose QCN could be potentially beneficial [2,22]. Recently, it has been suggested that polyphenolics may have benefit in lessening the pathological effects of the metabolic syndrome [23].
Interestingly, we found that low dose dietary QCN treatment prevents hypertrophy of the LV posterior wall in Apo E−/− mice. The molecular mechanisms by which QCN prevents hypertrophy in this mouse model are unknown. Since hypercholesterolemia alone has been suggested to play a causative role in the etiology of cardiac hypertrophy [1,2], we suggest that the QCN-dependent decrease in cardiac hypertrophy in this study can be ascribed to the decrease in plasma lipids. Recent studies suggest that QCN may modulate the inflammatory response of pro-atherogenic macrophages and decreased atherosclerotic lesions [14,16]. Several polyphenolic compounds, including those found in grapes [12], wine [24], chocolate [25], and olive oil [26], decrease blood lipids and seem to have a general beneficial effect on the cardiovascular system. Indeed, we observed a 25% decrease in total plasma cholesterol and a 30% decrease in plasma VLDL in Apo E−/− mice fed QCN (Fig. 1). As the most pro-atherogenic lipoprotein, VLDL specifically has been shown to promote transformation of macrophages to foam cells [27] and to induce synthesis of the pro-inflammatory cytokines TNF-α and interleukin-1β [28,29]. The VLDL in the Apo E−/− mouse model appears to be aggressively pro-atherogenic, possibly because of preferential existence of Apo B48 over Apo B100 in its structure [30,31] and, subsequently an 8-fold higher cholesterol to triglyceride ratio compared to human VLDL [32]. Therefore, the decrease in circulating VLDL in Apo E−/− mice after QCN intake may reduce inflammation and decrease vascular stress which could play a role in the prevention of cardiac hypertrophy. It is important to note that the amount of the orally-given QCN in the present study was >300-fold smaller compared to other previously reported in vivo studies on vascular dysfunction [7,33–35] and is consistent with the average amount of this polyphenol consumed by humans on the Mediterranean diet [36]. The molecular mechanisms underlying both hypercholesterolemia-induced cardiac hypertrophy and QCN-mediated improvements in plasma lipids and preclusion from cardiac remodeling are not known at present.
Collective anecdotal and epidemiological evidence suggest that a diet rich in QCN promotes cardiovascular health. The translational potential of polyphenolics is remains an active area of research with impact on both dietary recommendations and as a route to more selective and potent therapeutics [37]. Since QCN is rapidly metabolized it has limited utility as cardiovascular therapeutic but the pharmacophore can serve as a basis for the development of more efficacious agents. Interestingly, some more metabolically stable QCN derivatives have been reported to have benefit in the more severe form of pressure overload-dependent cardiac failure induced by aortic banding [15,38]. To conclude, in our study, we demonstrated that dietary supplementation with the polyphenol QCN decreases plasma cholesterol and diminishes LV hypertrophy in young Apo E−/− mice. These beneficial effects were achieved with QCN doses that are comparable to the range attainable by the Mediterranean diet. These findings may in part underlie the dietary anomaly known as the “French Paradox,” and support the concept that supplementation of the diet with foods or drinks rich in polyphenols such as QCN could offer cardioprotective benefits.
Acknowledgments
The authors gratefully acknowledge a support from the National Institutes of Health HL070610 and SCCOR in Cardiac Dysfunction HL077100. The mass spectrometer used in these studies was purchased from funds provided by a Shared Instrumentation grant from the National Center for Research Resources (S10 RR19231, SB, PI). Operation of the Comprehensive Cancer Center Proteomics-Mass Spectrometry Shared Facility came from funds from a P30 Core support grant (CA13148-35, E. Partridge, PI) to the UAB Comprehensive Cancer Center. Dr. Ali Arabshahi is thanked for his assistance for the LC-MS assays.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Celentano A., Crivaro M., Roman M.J., Pietropaolo I., Greco R., Pauciullo P., Lirato C., Devereux R.B., de Simone G. Left ventricular geometry and arterial function in hypercholesterolemia. Nutrition, Metabolism and Cardiovascular Diseases. 2001;11:312–319. [PubMed] [Google Scholar]
- 2.Sundstrom J., Lind L., Vessby B., Andren B., Aro A., Lithell H. Dyslipidemia and an unfavorable fatty acid profile predict left ventricular hypertrophy 20 years later. Circulation. 2001;103:836–841. doi: 10.1161/01.cir.103.6.836. [DOI] [PubMed] [Google Scholar]
- 3.Plump A.S., Smith J.D., Hayek T., Aalto-Setala K., Walsh A., Verstuyft J.G., Rubin E.M., Breslow J.L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 4.Yang R., Powell-Braxton L., Ogaoawara A.K., Dybdal N., Bunting S., Ohneda O., Jin H. Hypertension and endothelial dysfunction in apolipoprotein E knockout mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:2762–2768. doi: 10.1161/01.atv.19.11.2762. [DOI] [PubMed] [Google Scholar]
- 5.Dadakova E., Prochazkova E., Krizek M. Application of micellar electrokinetic capillary chromatography for quantitative analysis of quercetin in plant materials. Electrophoresis. 2001;22:1573–1578. doi: 10.1002/1522-2683(200105)22:8<1573::AID-ELPS1573>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 6.Qin T.C., Chen L., Yu L.X., Gu Z.L. Inhibitory effect of quercetin on cultured neonatal rat cardiomyocytes hypertrophy induced by angiotensin. Acta Pharmacologica Sinica. 2001;22:1103–1106. [PubMed] [Google Scholar]
- 7.Jalili T., Carlstrom J., Kim S., Freeman D., Jin H., Wu T.C., Litwin S.E., David Symons J. Quercetin-supplemented diets lower blood pressure and attenuate cardiac hypertrophy in rats with aortic constriction. Journal of Cardiovascular Pharmacology. 2006;47:531–541. doi: 10.1097/01.fjc.0000211746.78454.50. [DOI] [PubMed] [Google Scholar]
- 8.Juric D., Wojciechowski P., Das D.K., Netticadan T. Prevention of concentric hypertrophy and diastolic impairment in aortic-banded rats treated with resveratrol. American Journal of Physiology—Heart and Circulatory Physiology. 2007;292:H2138–2143. doi: 10.1152/ajpheart.00852.2006. [DOI] [PubMed] [Google Scholar]
- 9.Cheng T.H., Liu J.C., Lin H., Shih N.L., Chen Y.L., Huang M.T., Chan P., Cheng C.F., Chen J.J. Inhibitory effect of resveratrol on angiotensin II-induced cardiomyocyte hypertrophy. Naunyn-Schmiedeberg's Archives of Pharmacology. 2004;369:239–244. doi: 10.1007/s00210-003-0849-6. [DOI] [PubMed] [Google Scholar]
- 10.Kay C.D., Hooper L., Kroon P.A., Rimm E.B., Cassidy A. Relative impact of flavonoid composition, dose and structure on vascular function: a systematic review of randomised controlled trials of flavonoid-rich food products. Molecular Nutrition and Food Research. 2012;56:1605–1616. doi: 10.1002/mnfr.201200363. [DOI] [PubMed] [Google Scholar]
- 11.Arai Y., Watanabe S., Kimira M., Shimoi K., Mochizuki R., Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. Journal of Nutrition. 2000;130:2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 12.Zern T.L., West K.L., Fernandez M.L. Grape polyphenols decrease plasma triglycerides and cholesterol accumulation in the aorta of ovariectomized guinea pigs. Journal of Nutrition. 2003;133:2268–2272. doi: 10.1093/jn/133.7.2268. [DOI] [PubMed] [Google Scholar]
- 13.Kamada C., da Silva E.L., Ohnishi-Kameyama M., Moon J.H., Terao J. Attenuation of lipid peroxidation and hyperlipidemia by quercetin glucoside in the aorta of high cholesterol-fed rabbit. Free Radical Research. 2005;39:185–194. doi: 10.1080/10715760400019638. [DOI] [PubMed] [Google Scholar]
- 14.Lara-Guzman O.J., Tabares-Guevara J.H., Leon-Varela Y.M., Alvarez R.M., Roldan M., Sierra J.A., Londono-Londono J.A., Ramirez-Pineda J.R. Proatherogenic macrophage activities are targeted by the flavonoid quercetin. The Journal of Pharmacology and Experimental Therapeutics. 2012;343:296–306. doi: 10.1124/jpet.112.196147. [DOI] [PubMed] [Google Scholar]
- 15.He T., Chen L., Chen Y., Han Y., Yang W.Q., Jin M.W. In vivo and in vitro protective effects of pentamethylquercetin on cardiac hypertrophy. Cardiovascular Drugs and Therapy. 2012;26:109–120. doi: 10.1007/s10557-011-6363-z. [DOI] [PubMed] [Google Scholar]
- 16.Loke W.M., Proudfoot J.M., Hodgson J.M., McKinley A.J., Hime N., Magat M., Stocker R., Croft K.D. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arteriosclerosis, Thrombosis and Vascular Biology. 2010;30:749–757. doi: 10.1161/ATVBAHA.109.199687. [DOI] [PubMed] [Google Scholar]
- 17.Brookes P.S., Digerness S.B., Parks D.A., Darley-Usmar V. Mitochondrial function in response to cardiac ischemia-reperfusion after oral treatment with quercetin. Free Radical Biology and Medicine. 2002;32:1220–1228. doi: 10.1016/s0891-5849(02)00839-0. [DOI] [PubMed] [Google Scholar]
- 18.Garber D.W., Kulkarni K.R., Anantharamaiah G.M. A sensitive and convenient method for lipoprotein profile analysis of individual mouse plasma samples. Journal of Lipid Research. 2000;41:1020–1026. [PubMed] [Google Scholar]
- 19.Wang Y.X., Halks-Miller M., Vergona R., Sullivan M.E., Fitch R., Mallari C., Martin-McNulty B., da Cunha V., Freay A., Rubanyi G.M., Kauser K. Increased aortic stiffness assessed by pulse wave velocity in apolipoprotein E-deficient mice. American Journal of Physiology Heart and Circulatory Physiology. 2000;278:H428–434. doi: 10.1152/ajpheart.2000.278.2.H428. [DOI] [PubMed] [Google Scholar]
- 20.Wolfle S.E., de Wit C. Intact endothelium-dependent dilation and conducted responses in resistance vessels of hypercholesterolemic mice in vivo. Journal of Vascular Research. 2005;42:475–482. doi: 10.1159/000088101. [DOI] [PubMed] [Google Scholar]
- 21.Manach C., Mazur A., Scalbert A. Polyphenols and prevention of cardiovascular diseases. Current Opinion in Lipidology. 2005;16:77–84. doi: 10.1097/00041433-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Sundstrom J., Riserus U., Byberg L., Zethelius B., Lithell H., Lind L. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. British Medical Journal. 2006;332:878–882. doi: 10.1136/bmj.38766.624097.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherniack E.P. Polyphenols: planting the seeds of treatment for the metabolic syndrome. Nutrition. 2011;27:617–623. doi: 10.1016/j.nut.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Fremont L., Gozzelino M.T., Linard A. Response of plasma lipids to dietary cholesterol and wine polyphenols in rats fed polyunsaturated fat diets. Lipids. 2000;35:991–999. doi: 10.1007/s11745-000-0610-2. [DOI] [PubMed] [Google Scholar]
- 25.Allen R.R., Carson L., Kwik-Uribe C., Evans E.M., Erdman J.W., Jr. Daily consumption of a dark chocolate containing flavanols and added sterol esters affects cardiovascular risk factors in a normotensive population with elevated cholesterol. Journal of Nutrition. 2008;138:725–731. doi: 10.1093/jn/138.4.725. [DOI] [PubMed] [Google Scholar]
- 26.Covas M.I., Fito M., Lamuela-Raventos R.M., Sebastia N., de la Torre-Boronat C., Marrugat J. Virgin olive oil phenolic compounds: binding to human low density lipoprotein (LDL) and effect on LDL oxidation. International Journal of Clinical Pharmacology Research. 2000;20:49–54. [PubMed] [Google Scholar]
- 27.Hakamata H., Sakaguchi H., Zhang C., Sakashita N., Suzuki H., Miyazaki A., Takeya M., Takahashi K., Kitamura N., Horiuchi S. The very low- and intermediate-density lipoprotein fraction isolated from apolipoprotein E-knockout mice transforms macrophages to foam cells through an apolipoprotein E-independent pathway. Biochemistry. 1998;37:13720–13727. doi: 10.1021/bi980762v. [DOI] [PubMed] [Google Scholar]
- 28.Stollenwerk M.M., Lindholm M.W., Porn-Ares M.I., Larsson A., Nilsson J., Ares M.P. Very low-density lipoprotein induces interleukin-1beta expression in macrophages. Biochemical and Biophysical Research Communications. 2005;335:603–608. doi: 10.1016/j.bbrc.2005.07.123. [DOI] [PubMed] [Google Scholar]
- 29.Stollenwerk M.M., Schiopu A., Fredrikson G.N., Dichtl W., Nilsson J., Ares M.P. Very low density lipoprotein potentiates tumor necrosis factor-alpha expression in macrophages. Atherosclerosis. 2005;179:247–254. doi: 10.1016/j.atherosclerosis.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Mensenkamp A.R., Jong M.C., van Goor H., van Luyn M.J., Bloks V., Havinga R., Voshol P.J., Hofker M.H., van Dijk K.W., Havekes L.M., Kuipers F. Apolipoprotein E participates in the regulation of very low density lipoprotein-triglyceride secretion by the liver. Journal of Biological Chemistry. 1999;274:35711–35718. doi: 10.1074/jbc.274.50.35711. [DOI] [PubMed] [Google Scholar]
- 31.Maugeais C., Tietge U.J., Tsukamoto K., Glick J.M., Rader D.J. Hepatic apolipoprotein E expression promotes very low density lipoprotein-apolipoprotein B production in vivo in mice. Journal of Lipid Research. 2000;41:1673–1679. [PubMed] [Google Scholar]
- 32.Medh J.D., Fry G.L., Bowen S.L., Ruben S., Wong H., Chappell D.A. Lipoprotein lipase- and hepatic triglyceride lipase- promoted very low density lipoprotein degradation proceeds via an apolipoprotein E-dependent mechanism. Journal of Lipid Research. 2000;41:1858–1871. [PMC free article] [PubMed] [Google Scholar]
- 33.Duarte J., Perez-Palencia R., Vargas F., Ocete M.A., Perez-Vizcaino F., Zarzuelo A., Tamargo J. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. British Journal of Pharmacology. 2001;133:117–124. doi: 10.1038/sj.bjp.0704064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Saura M.F., Galisteo M., Villar I.C., Bermejo A., Zarzuelo A., Vargas F., Duarte J. Effects of chronic quercetin treatment in experimental renovascular hypertension. Molecular and Cellular Biochemistry. 2005;270:147–155. doi: 10.1007/s11010-005-4503-0. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez M., Galisteo M., Vera R., Villar I.C., Zarzuelo A., Tamargo J., Perez-Vizcaino F., Duarte J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. Journal of Hypertension. 2006;24:75–84. doi: 10.1097/01.hjh.0000198029.22472.d9. [DOI] [PubMed] [Google Scholar]
- 36.Manach C., Morand C., Crespy V., Demigne C., Texier O., Regerat F., Remesy C. Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Letters. 1998;426:331–336. doi: 10.1016/s0014-5793(98)00367-6. [DOI] [PubMed] [Google Scholar]
- 37.Russo M., Spagnuolo C., Tedesco I., Bilotto S., Russo G.L. The flavonoid quercetin in disease prevention and therapy: facts and fancies. Biochemical Pharmacology. 2012;83:6–15. doi: 10.1016/j.bcp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Mao Z., Liang Y., Du X., Sun Z. 3,3′,4′,5,7-Pentamethylquercetin reduces angiotensin II-induced cardiac hypertrophy and apoptosis in rats. Canadian Journal of Physiology and Pharmacology. 2009;87:720–728. doi: 10.1139/y09-069. [DOI] [PubMed] [Google Scholar]


