Abstract
Individuals with an at-risk mental state (ARMS) have a risk of developing a psychotic disorder significantly greater than the general population. However, it is not currently possible to predict which ARMS individuals will develop psychosis from clinical assessment alone. Comparison of ARMS subjects who do, and do not, develop psychosis can reveal which factors are critical for the onset of illness. In the present study, 37 patients with an ARMS were followed clinically at least 24 months subsequent to initial referral. Functional MRI data were collected at the beginning of the follow-up period during performance of an executive task known to recruit frontal lobe networks and to be impaired in psychosis. Graph theoretical analysis was used to compare the organization of a functional brain network in ARMS patients who developed a psychotic disorder following the scan (ARMS-T) to those who did not become ill during the same follow-up period (ARMS-NT) and aged-matched controls. The global properties of each group's representative network were studied (density, efficiency, global average path length) as well as regionally-specific contributions of network nodes to the organization of the system (degree, farness-centrality, betweenness-centrality). We focused our analysis on the dorsal anterior cingulate cortex (ACC), a region known to support executive function that is structurally and functionally impaired in ARMS patients. In the absence of between-group differences in global network organization, we report a significant reduction in the topological centrality of the ACC in the ARMS-T group relative to both ARMS-NT and controls. These results provide evidence that abnormalities in the functional organization of the brain predate the onset of psychosis, and suggest that loss of ACC topological centrality is a potential biomarker for transition to psychosis.
Keywords: Graph theory, At-risk mental state, Schizophrenia, Cingulate, fMRI
Highlights
► Performed between-groups analysis of graph theoretical data derived from fMRI. ► Compared at-risk mental state patients with and without subsequent transition to psychosis. ► At-risk patients with subsequent disease manifestation show abnormal network topology. ► ACC’s topological centrality in the network is decreased in ARMS patients with disease progression. ► Global network properties are preserved in all at-risk subjects.
1. Introduction
The onset of schizophrenia and other chronic psychotic disorders is usually preceded by a prodromal phase characterized by cognitive impairments (Fusar-Poli et al., 2012c), mood alterations, attenuated psychotic symptoms, and a decline in social and occupational function (Yung and McGorry, 1996). In recent years, operationalized criteria have been developed that identify individuals in the prodrome to the first psychotic episode. These criteria require the recent onset of specific symptoms or clinical features, termed an ‘at risk mental state’ (ARMS). A recent meta-analysis confirmed these individuals have an increased risk of developing psychosis (Fusar-Poli et al., 2012a).
Approximately 30% of ARMS individuals will go on to be diagnosed with schizophrenia or related disorders usually within 24 months (Fusar-Poli et al., 2012a). Currently, it is not possible to predict which ARMS will develop psychosis from clinical impression alone (Nelson and Yung, 2010). There is thus a pressing need to identify biomarkers that can identify those ARMS subjects who are most likely to develop a psychotic disorder, so that appropriate clinical resources can be focused effectively.
Brain imaging techniques offer a promising avenue towards the early and accurate evaluation of at-risk mental state individuals (Fusar-Poli et al., 2012c). Earlier neuroimaging studies have demonstrated that the ARMS is associated with both structural and functional alterations relative to healthy individuals. These deficits are generally intermediate to those seen in first-episode psychosis patients and aged-matched controls. In particular, the ARMS is associated with decreases in gray matter volume in temporal, insular and cingulate cortices relative to controls (Pantelis et al., 2003a; Borgwardt et al., 2007; Borgwardt et al., 2008; Fusar-Poli et al., 2011) and functional imaging studies report altered activation of frontal, cingulate, and temporal regions (Fusar-Poli et al., 2007; Broome et al., 2009; \Fusar-Poli et al., 2010; Allen et al., 2012).
In an earlier fMRI study, to our knowledge the first graph theoretical investigation of ARMS subjects, we characterized the functional connectivity of the anterior cingulate cortex (ACC) in a network of brain regions supporting executive function (Lord et al., 2011). The ACC is known to support information processing across many cognitive domains that are impaired in schizophrenia and the at-risk mental state (Hawkins et al., 2004; Eastvold et al., 2007), and is thus a good target region for analyses seeking to identify brain changes specific to the transition to psychosis. Our findings were broadly consistent with studies using global functional connectivity measures and graph theoretical analyses of MRI data reporting neurofunctional abnormalities in schizophrenia patients (Bassett et al., 2008; Liu et al., 2008; Alexander-Bloch et al., 2010; Lynall et al., 2010; Zalesky et al., 2011), which are thought to reflect impaired functional integration; a characteristic feature of schizophrenia and related disorders (Friston, 2002; Pettersson-Yeo et al., 2010). We reported a significant reduction in the ACC's contribution to information routing in the network under study in ARMS subjects with elevated symptoms relative to healthy controls during performance of a verbal fluency task. In this same investigation, the most dramatic loss of topological centrality (an estimate of the importance of a node in the network organization) of all the nodes included in the analysis was in fact observed in the ACC, thus reflecting functional impairment in this region. Importantly, this initial analysis focused on symptomatology rather than clinical outcome. At the time the study was carried out, we did not have full knowledge of which ARMS subjects would eventually go on to develop a psychotic disorder, as the 24-month clinical follow-up period was incomplete. Thus, four ARMS subjects from the cohort who were known to have developed psychosis were excluded from the study, and the analysis was restricted to ARMS subjects that had yet to receive a formal schizophrenia diagnosis.
The aim of the present study was to re-analyze data from the same cohort at the end of the 24-month clinical observation period. On the basis of our earlier results reflecting an association between ACC impairment and symptomatology in the ARMS (Lord et al., 2011), we employed the same methodology to characterize the role of the ACC in ARMS subjects according to their clinical outcome. The scans of subjects who developed psychosis during clinical follow-up (ARMS-transition group, ARMS-T) were compared to ARMS subjects who did not become ill over the same period (ARMS-NonTransition group, ARMS-NT) and healthy controls. We hypothesized that ARMS subjects who subsequently developed psychosis (ARMS-T) would show a reduction in ACC topological centrality relative to both healthy controls and ARMS-NT subjects, thus reflecting functional impairment in this region during executive function. We also hypothesized that, in the ARMS-NT group, the ACC's topological importance would be intermediate to that of ARMS-T and healthy subjects.
2. Methods
2.1. Participant characteristics
The present analyses included 22 healthy volunteers and 37 subjects with an at-risk mental state (ARMS). Thirty-six of the ARMS subjects included in the study were right-handed and one was left handed according to the Lateral Preference Inventory. All control subjects were right-handed. All subjects were native English speakers. ARMS subjects were recruited via OASIS (Outreach and Support in South London), a clinical service for people at high risk of developing psychosis. Diagnosis of an At Risk Mental State was made using the Comprehensive Assessment of At Risk Mental States (CAARMS) (Yung et al., 1998; Yung et al., 2003). One or more of the following criteria had to be met for inclusion in the study 1) attenuated psychotic symptoms, 2) brief limited intermittent psychotic symptoms in the past year, 3) a recent decline in function together with either a family history of psychosis in a first degree relative or personal history of schizotypal personality disorder. The subjects' consent was obtained according to the Declaration of Helsinki.
Psychopathology on the day of scanning was assessed using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) and premorbid IQ for all subjects was estimated using the National Adult Reading Test (NART) (Nelson, 1982). The self-reported ethnicity of the ARMS group was 25 White British, 5 Black, 4 Asian, and 3 of mixed origins. Four of the ARMS subjects were being treated with low doses (less than 1.5 mg haloperidol equivalents per day) of antipsychotic medication. Healthy controls were recruited from the local community through advertisements. The control group consisted of 14 White British, 7 Black, and 1 of Asian origin. Control subjects were excluded if they had a history of neurological or psychiatric disorder, substance abuse, or received prescription medication. Both Controls and ARMS subjects were excluded if they met DSM-IV criteria for a substance misuse or dependence disorder or if there was a history of neurological disorder.
2.2. Clinical follow-up
All ARMS subjects were followed clinically for at least 24 months subsequent to initial referral (mean duration = 24.67 months). During the follow up period, seven ARMS subjects (19%) made a transition to psychosis (5 males, 2 females; mean age = 24.2 ± 4.9). Transition was defined according to the criteria in the CAARMS (Yung et al., 1998; Yung et al., 2003), and required the presence of one or more psychotic symptom (such as delusions, hallucinations or formal thought disorder) continuously present for at least a week and associated with a functional impact and a need for clinical intervention. These same individuals were all initially diagnosed with attenuated psychotic syndrome (APS) prior to conversion. The mean duration between scanning and transition was 8.5 months. None of the transition cases (ARMS-T) were receiving antipsychotics at the time of scanning, although one was taking antidepressant medication. Four of the transition cases received a diagnosis of schizophrenia, one a diagnosis of schizoaffective disorder, and two cases have yet to receive a formal diagnosis but are receiving antipsychotic medication.
2.3. Experimental task
Functional MRI data were acquired while subjects performed a verbal fluency task (Broome et al., 2009). Subjects were instructed to overtly generate a word in response to a visually presented letter. They were asked not to use names, repeat words, or to use grammatical variations of a previously used word. The letters were presented at the rate of one letter every 4 s. There were two conditions which differed in difficulty. In the ‘easy’ condition, the letters C, P, S, T, L were presented, while the ‘hard’ condition consisted of the five-letter set: I, F, O, N, E. Each condition was presented in blocks lasting 28 s, with seven presentations of a given letter per block, and five blocks for each condition. The experimental condition alternated with a control condition, also presented in blocks of 28 s, in which the subjects were presented with the word ‘rest’ at 4 s intervals and were instructed to repeat the word overtly. Verbal responses were recorded with a microphone compatible with MRI apparatus. Responses were considered incorrect when participants passed on a given letter, and when the word recorded was a repetition or grammatical variation of a previous word. To maximize the statistical power of the functional connectivity analysis (see below), data from the ‘easy’, ‘hard’, and ‘rest’ VF conditions were collapsed together.
2.4. Image acquisition and processing
Functional MRI data were acquired at the Institute of Psychiatry at the Maudsley Hospital, London, on a GE Sigma 1.5-T system (General Electric, Milwaukee). Verbal fluency was studied using a T2-weighted echoplanar image sequence (TR = 4000 ms, TE = 40 ms) with each acquisition compressed into the first 2000 ms of the repetition time, in order to create a 2000 ms silent period in which subjects could articulate a response in the absence of scanner noise. Compressed acquisition sequences were also used to reduce motion artifact due to head movement during articulation. One hundred and eight brain volumes were collected from each subject. Each volume contained 22 axial 5 mm slices with a 0.5 mm gap between each slice. This sequence delivered a voxel resolution of 3.75 × 3.75 × 5.5 mm. The University of Oxford's fMRIB software library (FSL) was used for image processing.
Spatial smoothing (5 mm) was performed on the raw images, and a high pass filter was applied (0.01 Hz). Each subject's fMRI data was registered to a standard space by FSL's linear registration algorithm (FLIRT) (Jenkinson et al., 2002). Motion correction was performed using MCFLIRT, FSL's intramodal motion correction tool. In this approach, the time-series is loaded in its entirety and defaults to the middle volume as an initial template image. A coarse 8 mm search for the motion parameters was then carried out using the cost function specified followed by two subsequent searches at 4 mm using increasingly tighter tolerances. All optimizations used trilinear interpolation (Jenkinson et al., 2002). As part of the initial 8 mm search, an identity transformation was assumed between the middle volume and the adjacent volume. The transformation found in this first search was then used as the estimate for the transformation between the middle volume and the volume beyond the adjacent one. The motion parameters extracted using this approach for each study participant were included as co-variates of non-interest in the HamNet partial correlation analysis (Section 2.5) used to define functional connectivity between pairs of nodes. In this fashion, variance due to motion was regressed out of the connectivity analysis in a subject-specific manner.
Task-elicited BOLD time-series were extracted from bilateral regions-of-interest (ROI) defined by the Harvard-Oxford Cortical Structural Atlas. Probabilistic maps were thresholded to 25% of the original atlas value to ensure regional specificity. Regional time-series were calculated by taking the mean of the voxel time-series within each ROI. ROI templates were applied to the standard space used for registration. Nuisance variables (ventricles, white matter noise, motion) were extracted from the raw time-series, and added as co-variates of non-interest. For consistency, the same functional system reported in our earlier study was analyzed (Lord et al., 2011), and consists of an anatomically-widespread network of 19 regions that show changes in activation during executive function, and in most of which functional impairments have been detected in schizophrenia (Hill et al., 2004; Tan et al., 2007). The locations and identities of network nodes on the cortical surface are indicated in Fig. 1. The ROI templates are also shown in their native (2-D) volumetric space in Fig. S7.
Fig. 1.
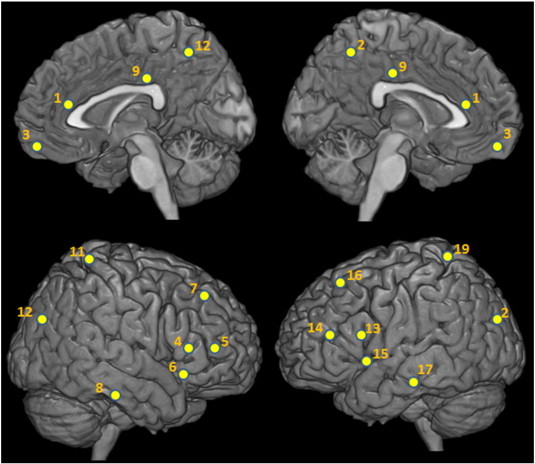
Regions of interest for the graph analysis. Anatomical locations of the centroids for the 19 regions of interest comprising the network considered in the functional connectivity analysis. 1 — anterior cingulate cortex (ACC); 2,12 — cuneate cortex; 3 — ventromedial prefrontal cortex; 4,13 — inferior frontal gyrus, pars-opercularis; 5,14 — inferior frontal gyrus, pars-triangularis; 6,15 — insula; 7,16 — middle frontal gyrus; 8,17 — parahippocampal area; 9 — posterior cingulate cortex; 10,18 — precuneus; 11,19 — superior parietal cortex. ROI templates are shown in their native space in Fig. S7.
Regions of interest for the graph analysis. Anatomical locations of the centroids for the 19 regions of interest comprising the network considered in the functional connectivity analysis. 1 — anterior cingulate cortex (ACC); 2,12 — cuneate cortex; 3 — ventromedial prefrontal cortex; 4,13 — inferior frontal gyrus, pars-opercularis; 5,14 — inferior frontal gyrus, pars-triangularis; 6,15 — insula; 7,16 — middle frontal gyrus; 8,17 — parahippocampal area; 9 — posterior cingulate cortex; 10,18 — precuneus; 11,19 — superior parietal cortex. ROI templates are shown in their native space in Fig. S7.
Fig. S7.
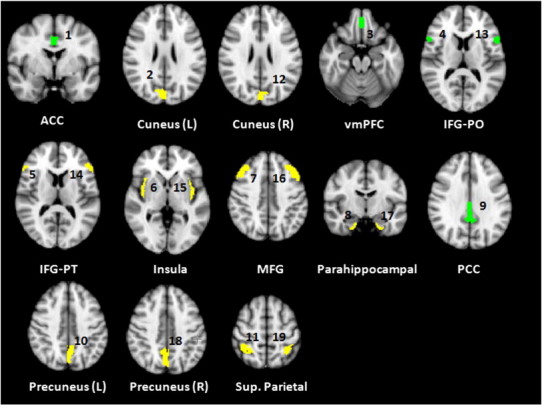
Regions of interest displayed in native volumetric space anatomical templates (ROI) from which bold time-series were extracted showed in their native volumetric space. These regions were defined according to the Harvard-Oxford Cortical Atlas and subsequently thresholded to 25% of the atlas value to ensure regional specificity.
Task-elicited BOLD time-series were extracted from bilateral regions-of-interest (ROI) defined by the Harvard-Oxford Cortical Structural Atlas. Probabilistic maps were thresholded to 25% of the original atlas value to ensure regional specificity. Regional time-series were calculated by taking the mean of the voxel time-series within each ROI. ROI templates were applied to the standard space used for registration. Nuisance variables (ventricles, white matter noise, motion) were extracted from the raw time-series, and added as co-variates of non-interest. For consistency, the same functional system reported in our earlier study was analyzed (Lord et al., 2011), and consists of an anatomically-widespread network of 19 regions that show changes in activation during executive function, and in most of which functional impairments have been detected in schizophrenia (Hill et al., 2004; Tan et al., 2007). The locations and identities of network nodes on the cortical surface are indicated in Fig. 1. The ROI templates are also shown in their native (2-D) volumetric space in Fig. S7.
2.5. Functional connectivity analysis
Subjects were organized into three groups: Control subjects (N = 22), ARMS subjects who did not convert to psychosis during the 2 year follow-up period (ARMS-NT; N = 30), and ARMS subjects who subsequently developed psychosis during the follow-up period (ARMS-T; N = 7).
Functional connectivity was estimated following the same procedure described in Lord et al., (2011). For each subject, functional connectivity was determined by performing partial correlations between fMRI time-series for all pair of nodes. Partial correlations between the activity time-series of pairs of nodes were preferred over bivariate correlations as, by regressing the variance associated with the other (N-2) regions contained within the system, third-party and global contributions to pairwise correlations are limited, thus yielding specific estimates of the BOLD signal synchrony between regions. (Hampson et al., 2002; .Salvador et al., 2005; Lord et al., 2011).
Random effect analysis was performed by transforming partial correlation coefficients derived for each node pair for each subject into normalized Z-scores via a Fisher transform.
For each group, the Z-scores obtained by Fisher transform were averaged and then transformed into p-values for testing. Family-wise error rate at the α = 0.05 level was taken into account using the Hochberg procedure (Turkheimer et al., 2001). This multiple comparison correction procedure is the most powerful to date to control for the False Detection Rate when the proportion of non-null hypotheses is large, as is expected when analyzing functional brain networks (Hwang, 2010). All functional connectivity methods described above are contained in the HamNet software (Imperial College London) that is coded as a toolbox for Matlab (The Mathworks, Natick, MA).
2.6. Network analysis
An adjacency matrix A representing the functional connectivity of each group was constructed, an edge being present between two nodes iand j when the corresponding partial-correlation was found significant, and the corresponding matrix entry is equal to 1 (Ai,j = 1).
To investigate the structural properties of the constructed networks and quantify the importance of each region, we used three centrality measures defined in (Freeman, 1977, 1979), which pinpoint different notions of importance.
All three metrics are defined both locally and have a global counterpart. The global measures were used to verify that the global organization of the groups did not significantly differ, while the local measures enabled the identification of regions with statistically different importance between the groups. The statistical significance of the differences in local and global measures between each pair of groups was assessed by performing a permutation test. For global measures, a two-sided test was used, while a one-sided test was used for local measures as we were looking for a reduction in centrality in the latter and for variation in the former.
The first metric we considered is the degree-centrality (DC). The degree of a node is the number of connections that a node has. This metric measures the direct influence a node has and it is therefore the most local of the metrics we considered. For a node k, it is computed by summing the rows of the adjacency matrix:
Averaging all the entries of the adjacency matrix gives the density (ρ) of the network as the percentage of significant edges that are present out of the total number of edges possible:
The second quantity we measured is the farness-centrality (FC). It is defined as the average shortest path length between a node i and all the other nodes constituting the network. It is thus related to the speed at which information emitted from i can reach other nodes in the network. For node i, it is defined as:
where σi,j is the length of the shortest path between i and j. The global farness-centrality () is a measure of the diameter of the system and is the average of the nodal farness:
Thus, the smaller is, the faster information can be transferred in the system on average.
The last measure we considered is betweenness-centrality (BC). It expresses the frequency at which a node is visited when information is transferred along the shortest routes between any given pair of nodes in the system, thus giving the importance of a node as a relay for information in the network. For a node k, it is defined as:
where is the total number of shortest paths going from node i to node j, and the fraction of those shortest paths passing through k. The global betweenness centrality is computed as the average of the nodal betweenness centrality:
The higher the global betweenness‐centrality, the more navigable the system is, in that many nodes can serve to mediate information transfer on the graph.
All graphs were plotted with Visone software (http://visone.info/index). The values for all metrics were computed with Visone and the free Matlab toolbox BCT (Bullmore and Sporns, 2009). To facilitate comparison between groups whose total connectivities may be slightly different, the values of the BC, FC and DC metrics for each node are given as percentage of the total metric value for each graph.
3. Results
3.1. Demographic and clinical data
The control, ARMS-NT, and ARMS-T groups did not differ on measures of age [F(2,56) = 0.24, p = 0.78] or premorbid-IQ [F(2,56) = 1.35, p = 0.27]. The proportion of male:female subjects also did not differ across experimental groups [Χ2(2) = .03, p = 0.98]. ARMS-T and ARMS-NT subjects did not differ on measures of positive symptoms [t(35) = 0.77, p = 0.44], negative symptoms [t(35) = 0.17, p = 0.87], or general psychopathology symptoms [t(35) = 0.36, p = 0.72] assessed with the PANSS (Table 1).
Table 1.
Demographic, behavioral and clinical data.
Mean age, premorbid IQ, PANSS sub-scores and total VF task errors for each experimental group. Corresponding standard deviations are also reported.
| Control | ARMS-NT | ARMS-T | |
|---|---|---|---|
| Age | 25.5 ± 4.6 | 24.5 ± 5.4 | 26.2 ± 5.0 |
| Premorbid IQ | 107 ± 8 | 102 ± 12 | 101 ± 13 |
| PANSS-POS | N/A | 12.3 ± 4.2 | 11.9 ± 2.7 |
| PANSS-NEG | N/A | 10.4 ± 4.5 | 11.7 ± 4.7 |
| PANSS-GEN | N/A | 25.2 ± 16.8 | 25.5 ± 6.5 |
| VF-task errors | 13 ± 11 | 15 ± 9 | 16 ± 16 |
3.2. Behavioral data
All subjects performed the verbal fluency task with a high degree of accuracy (see Table 1). There was a main effect for load, with all subjects making more errors in response to Hard compared to Easy letters [F(1,112) = 13.84, p < .001]. There was no significant main effect for group [F(2,112) = 1.69, p = 0.19], and no significant group by load interaction [F(2,112) = 0.08, p = 0.92].
3.3. Comparisons of global network measures: network density, global betweenness-centrality and global average path length
Table 2 contains the network density, global betweenness-centrality and global farness-centrality values for each network under study. The comparisons did not reveal any significant differences between the groups on these global network measures.
Table 2.
Global network metrics. Network density (ρ), compactness/global betweenness-centrality (BC) and global farness-centrality (F) for each experimental group's representative network.
| Global metric | Control | ARMS-NT | ARMS-T |
|---|---|---|---|
| ρ | 0.39 | 0.40 | 0.33 |
| BC | 11.9 | 11.8 | 15.1 |
| F | 1.66 | 1.65 | 1.84 |
3.4. Between-groups comparisons of regional metrics in the ACC: degree centrality, betweenness-centrality and farness-centrality
-
i
Controls and ARMS-NT subjects:
-
Figs. 2a, b and c respectively show the DC, BC and FC values for each node in each experimental group, expressed as a percentage of the total metric value of the network (these values are also provided in supplementary Tables S1–3). Fig. 3 provides a graphical representation of the node-specific values for BC across the three experimental groups. Results (p-values) from the permutation test applied for ACC metric differences can be found in Tables S4–6 for the ARMS-NT vs. Control, ARMS-T vs. Control and ARMS-T vs. ARMS-NT comparisons, respectively. In the control network, the ACC had, by a considerable margin, the highest topological centrality among all network nodes. In the ARMS-NT group, the ACC also occupied a relatively central role in the network topological centrality during verbal fluency, although other nodes including the left and right MFG, right IFG-pO and right precuneus had greater topological importance than the ACC. The reduction in the topological centrality of the ACC in the ARMS-NT group relative to controls was reflected by a statistically significant difference in betweenness-centrality (p-val = 0.04), but not in DC (p-val = 0.17) nor FC (p-val = 0.23).
Figs. 2A, B and C respectively show the DC, BC and FC values for each node in each experimental group, expressed as a percentage of the total metric value of the network (these values are also provided in supplementary Tables S1–3). Fig. 3 provides a graphical representation of the node-specific values for BC across the three experimental groups. Results (p-values) from the permutation test applied for ACC metric differences can be found in Tables S4–6 for the ARMS-NT vs. Control, ARMS-T vs. Control and ARMS-T vs. ARMS-NT comparisons, respectively. In the control network, the ACC had, by a considerable margin, the highest topological centrality among all network nodes. In the ARMS-NT group, the ACC also occupied a relatively central role in the network topology during verbal fluency, although other nodes including the left and right MFG, right IFG-pO and right precuneus had greater topological importance than the ACC. The reduction in the topological centrality of the ACC in the ARMS-NT group relative to controls was reflected by a statistically significant difference in betweenness-centrality (p-val = 0.04), but not in DC (p-val = 0.17) nor FC (p-val = 0.23).
-
ii
ARMS-T vs. ARMS-NT and ARMS-T vs. Controls:
As hypothesized, ARMS-T individuals showed the most pronounced loss of topological centrality in the ACC of all groups under study. In the ARMS-T network, most of the other network nodes have greater topological centrality than the ACC. Statistical comparisons between ARMS-T and controls revealed significant reductions in degree-centrality (p-val = 0.01) and betweenness-centrality (p-val = 0.02) of the ACC, and a trend for increased farness-centrality (p-val = 0.09), all of which reflect a loss of topological importance. Similarly, when compared to the ARMS-NT group, the ACC of ARMS-T subjects showed significantly decreased DC (p-val = 0.04), and significantly increased FC (p-val = 0.05). Taken together these results indicate that, in ARMS-T subjects, topological centrality reductions in the ACC are observed not only relative to healthy controls, but to ARMS-NT subjects without subsequent disease progression.
Fig. 2.
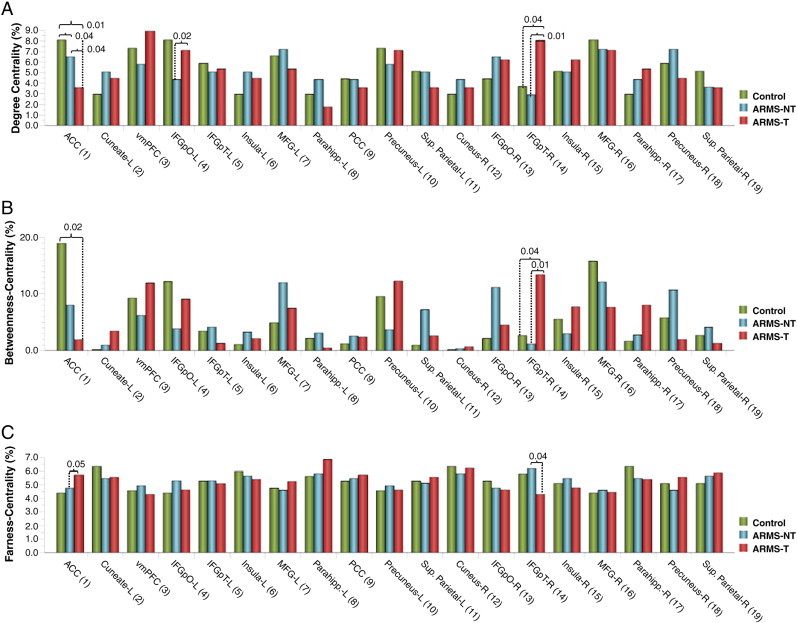
Regional metric values. Degree-centrality (A), betweenness-centrality (B) and farness-centrality (C) values for each node in each network under study, expressed as a percentage of the total metric value for the corresponding graph. Significant statistical effects (p-values) revealed by permutation testing are indicated where found.
Fig. 3.
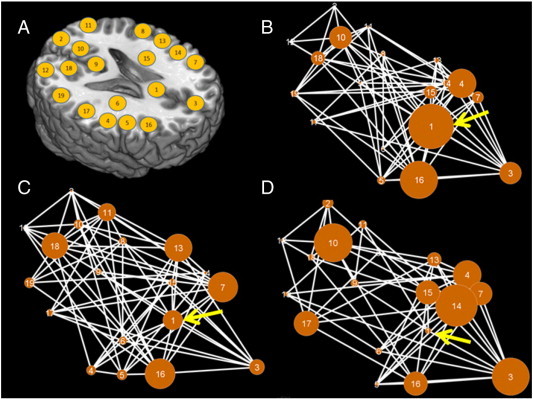
Visual representation of the networks and BC values. A: Node positions superimposed onto an axial plane to facilitate network visualization. B–D: Graphical representation of the networks for the control (B), ARMS-NT (C) and ARMS-T (D) groups. Each edge represents a significant functional connection between a pair of nodes. The surface area of each node is directly proportional to its betweenness-centrality value, expressed as a proportion of the total metric value of the corresponding network. Arrows point to the ACC node.
4. Discussion
The present results indicate that, in the functional network recruited during verbal fluency, ARMS subjects who subsequently developed psychosis (ARMS-T) show a more pronounced decrease in ACC topological centrality than ARMS subjects who do not develop psychosis (ARMS-NT) and healthy controls. In healthy individuals, the ACC plays a major role in the organization of the functional brain network under study. The ACC's importance is reflected by the region's elevated regional BC and DC values, and low FC value. According to these measures, in healthy controls, the ACC has the highest topological importance of all the nodes comprising this network. In ARMS-NT subjects, the ACC's topological importance is reduced, yet it remains among the most central network nodes. However, major reductions in topological centrality were observed in the ACC of ARMS-T subjects and this region no longer occupies a central role in the functional network organization.
Importantly, ARMS-T subjects show a significant reduction in ACC centrality not only relative to controls, but to ARMS-NT subjects. This suggests that the ARMS is associated with a diminished role in the routing of information in executive brain networks via the ACC and that this network alteration is particularly apparent in those ARMS subjects who subsequently develop psychosis.
In a previous graph theoretical analysis of the same executive network, we reported decreased ACC topological centrality in ARMS individuals with elevated symptoms relative to ARMS subjects with less pronounced symptoms and healthy controls (Lord et al., 2011). Although imperfect, this exploratory approach aimed to identify individuals in the early and the late prodrome. Upon completing the two-year clinical follow-up of the same ARMS cohort, we have identified ARMS individuals that have converted to psychosis (ARMS-T) and used a cross-sectional analysis of the fMRI data to show that more profound ACC deficits are present in this group. We note that only one ARMS-T subject was in the ARMS group with elevated symptoms previously reported; the other ARMS-T subjects were either excluded from the original study (n = 4), or belonged to the low-symptoms group (n = 3).
That the ACC showed the most important decrease in topological centrality in ARMS subjects who subsequently developed psychosis is consistent with previous studies of the ACC in the schizophrenia prodrome (Rothlisberger et al., 2012). Significant decreases in ACC gray matter volumes (Pantelis et al., 2003a; Pantelis et al., 2003b; Koutsouleris et al., 2009), as well as regional thinning of the ACC (Fornito et al., 2008) have notably been reported when comparing ARMS subjects who develop psychosis to those who do not. The cross-sectional design employed in the present study does not enable us to determine if the functional differences observed in the ACC of ARMS-T subjects reflect stable differences in the organization of brain networks in these individuals, possibly of a neurodevelopmental origin, or whether they are the result of progressive changes in brain function leading up to the first psychotic episode. Longitudinal changes in gray matter volume in the ACC have indeed been observed in ARMS subjects with subsequent disease progression over a ~ 200 day follow-up period (Pantelis et al., 2003a), which suggests that a critical period of neuroanatomical changes may precede the onset of frank psychosis. Future studies should examine the possible association between reductions in the topological centrality of the ACC in functional executive networks and the structural properties of this region.
How a loss of ACC topological centrality could impact the rest of the ARMS-T network is unclear. Computational studies have shown that lesions to highly central nodes have the greatest potential to disrupt the integrative aspects of neocortical function by eliciting widespread changes in brain networks that extend far beyond the affected site (Honey and Sporns, 2008). Thus, some of the topological changes in regions other than the ACC could be a consequence of a loss of function in this highly central node. We also note that, despite a major loss of ACC topological centrality, the ARMS-T network retains its global betweenness-centrality and global average path length in relation to the other experimental groups. This involves the creation of new connections within the rest of the network, and resulting changes in the topological centrality of other nodes, possibly of a compensatory nature.
In this work, the statistical methodology used for graph thresholding considered the partial correlation coefficient between the activity timeseries for each pair-permutation of nodes in the network of interest. This approach conferred the advantage of systematically evaluating the significance of every possible association contained within the system, and their potential physiological relevance. The main drawback of this approach is that networks of differing densities may be generated (Bullmore and Sporns, 2009), which can complicate the interpretation of between-group differences in regional metrics. Alternatively, it is possible to generate networks of a set density for each group (Alexander-Bloch et al., 2012; Nicol et al., 2012). While this approach eliminates a possible density induced bias, it may lead to the inclusion of spurious links in the network by enforcing non-significant connections or ignoring significant connections (van Wijk et al., 2010). Although we opted for the former approach, we did not find between-group differences in network density or global measures of network compactness. Moreover, statistical comparisons were run on the normalized regional metric value for a given network, thus accounting for potentially slight variations in global network architecture. We therefore believe it is unlikely that the pronounced decreases in regional ACC metrics reported in the ARMS-T group relative to ARMS-NT and Controls were due to differences in global network properties. Moreover, the methodology we followed in this work has the advantage of giving a statistical assessment at each stage of the process, from link inference to metric differences.
Despite the small number of ARMS-T subjects available, we note that the use of a random effects approach with small sample sizes typically results in reduced sensitivity rather than false positive results in neuroimaging experiments (Friston et al., 1999). Although metrics could be calculated and tested for each node, the construction of the network impinged on a point-wise hypothesis on the impaired functionality of the ACC and its association to clinical outcome at the end of the observation period, which does not imply any multiplicity to correct for. Exploratory analyses on the role of other nodes in the brain will require larger sample-sizes which are unfortunately difficult to obtain in these cohorts. Furthermore, by comparing the selected clustering of the data for a given metric to a large number of randomly organized clusters, the statistical approach conferred strong protection against false-positive results by accounting for the relative magnitudes of within and inter-group variability in distributions directly estimated from the data. In doing so, potentially inaccurate assumptions about population distributions were avoided. Finally, ACC BOLD activations during the verbal fluency task did not significantly differ between the groups according to a recent analysis of the same cohort (Allen et al., 2012), which suggests that the loss of topological centrality observed in the ACC of ARMS-T subjects is not a secondary consequence of reduced activity in this region.
In the present analyses, both “rest” and “task” experimental blocks were collapsed for each scan. This approach was used to maximize the statistical power of the correlations between regionally-specific time-series which defined the graphs, and for consistency with our earlier investigation of the same cohort (Lord et al., 2011). It is however not possible to distinguish task-dependent from task-independent contributions to network topology from these analyses. Relatively few studies have attempted to isolate task-specific functional connectivity in graph analysis (Wang et al., 2010; Fornito et al., 2011; Minati et al., 2012). Notably, a recent fMRI study of cognitive control in schizophrenia patients found that specific cognitive control deficits in connectivity were superimposed against generalized, pervasive functional connectivity alterations in these patients (Fornito et al., 2011). Further analyses will thus be needed to determine whether abnormalities in the network topology of ARMS subjects are specifically driven by task-related deficits in executive function.
Even upon applying standard fMRI approaches to motion correction (i.e. compensatory registration and regression of motion estimates) as in the present study, movement of the head has recently been shown to produce substantial changes in the fMRI timeseries throughout the brain that can influence functional connectivity measurements (Power et al., 2012; Van Dijk et al., 2012). This is of potential concern for studies comparing connectivity measures in patients and controls, as patients are often assumed to move more during the scan. It is unclear how subject motion may have affected the present results. However, we note that in a recent analysis of the same cohort , Allen et al. (2012) reported no significant differences in head motion between ARMS-T, ARMS-NT and control subjects. Moreover, the effect of motion on connectivity measures is not systematic and depends on the network being analyzed; functional correlations among the default-mode and fronto-parietal regions decrease linearly with increasing head motion, while connectivity between the left and right motor cortices increases with added movement (Van Dijk et al., 2012). Thus, the directionality of the potential effect of motion on ACC connectivity and topological centrality in the network under study cannot be predicted with certainty.
It is important to note that, in the months following the acquisition of imaging data, ARMS subjects were offered active treatment in a naturalistic and uncontrolled design through the OASIS clinic. These included need-based interventions (psychosocial, psychoeducational, vocational support and crisis management), psychological (cognitive behavioral therapy) and/or psychopharmacological treatment (antidepressants, anxyiolitic or low dosage second generation antipsychotics). The clinical management of the OASIS patients is fully detailed in a recent publication (Fusar-Poli et al., 2012b). However, these interventions had not been initiated prior to the imaging data acquisition, and all but 4 ARMS-NT subjects and 1 ARMS-T were medication-naive when baseline scans were collected. It would eventually be important to address the potential effects of treatment on the present findings, but this is not currently possible given that they were not administered in a controlled setting.
To our knowledge, only one previous functional MRI study has provided a comparison of the data from ARMS subjects who did and did not develop psychosis (Sabb et al., 2010; Allen et al., 2012). In a re-analysis of the data previously reported (Allen et al., 2012), we provide novel evidence that measures of regional topological centrality can be applied to functional brain networks derived from fMRI data. This analysis has revealed differences in network organization in ARMS subjects with subsequent transition to psychosis relative to both healthy controls and ARMS patients without disease progression. The present work focused on topological changes in the ACC during executive function, but related approaches could be used to study various network parameters under a wide range of experimental conditions.
In conclusion, this study provides evidence that abnormalities in the functional organization of the brain predate the onset of psychosis, and suggests that loss of ACC topological centrality is a potential biomarker for transition to psychosis.
The following are the supplementary data related to this article.
Supplementary Tables
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2012.09.008.
Footnotes
Funding: The project has been funded in part by the EPSRC Grant EP/E049451/1 and MRC Grant U.1200.04.007.00001.01.
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Alexander-Bloch A.F., Gogtay N., Meunier D., Birn R., Clasen L., Lalonde F., Lenroot R., Giedd J., Bullmore E.T. Disrupted modularity and local connectivity of brain functional networks in childhood-onset schizophrenia. Frontiers in Systems Neuroscience. 2010;4:147. doi: 10.3389/fnsys.2010.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch A.F., Vertes P.E., Stidd R., Lalonde F., Clasen L., Rapoport J., Giedd J., Bullmore E.T., Gogtay N. The Anatomical Distance of Functional Connections Predicts Brain Network Topology in Health and Schizophrenia. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhr388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P., Howes O., Egerton A., Kazuyuki H., Valli I., Kambeitz J., Fusar-Poli P., Broome M., McGuire P. Transition to psychosis associated with prefrontal and subcortical dysfunction in ultra high-risk individuals. Schizophrenia Bulletin. 2012 doi: 10.1093/schbul/sbr194. http://dx.doi.org/10.1093/schbul/sbr194. ([Epub ahead of print] PubMed PMID: 22290265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D.S., Bullmore E., Verchinski B.A., Mattay V.S., Weinberger D.R., Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. Journal of Neuroscience. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgwardt S.J., Riecher-Rossler A., Dazzan P., Chitnis X., Aston J., Drewe M., Gschwandtner U., Haller S., Pfluger M., Rechsteiner E. Regional gray matter volume abnormalities in the at risk mental state. Biological Psychiatry. 2007;61:1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Borgwardt S.J., McGuire P.K., Aston J., Gschwandtner U., Pfluger M.O., Stieglitz R.D., Radue E.W., Riecher-Rossler A. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophrenia Research. 2008;106:108–114. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Broome M.R., Matthiasson P., Fusar-Poli P., Woolley J.B., Johns L.C., Tabraham P., Bramon E., Valmaggia L., Williams S.C., Brammer M.J. Neural correlates of executive function and working memory in the ‘at-risk mental state’. The British Journal of Psychiatry. 2009;194:25–33. doi: 10.1192/bjp.bp.107.046789. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Eastvold A.D., Heaton R.K., Cadenhead K.S. Neurocognitive deficits in the (putative) prodrome and first episode of psychosis. Schizophrenia Research. 2007;93:266–277. doi: 10.1016/j.schres.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Yung A.R., Wood S.J., Phillips L.J., Nelson B., Cotton S., Velakoulis D., McGorry P.D., Pantelis C., Yucel M. Anatomic abnormalities of the anterior cingulate cortex before psychosis onset: an MRI study of ultra-high-risk individuals. Biological Psychiatry. 2008;64:758–765. doi: 10.1016/j.biopsych.2008.05.032. [DOI] [PubMed] [Google Scholar]
- Fornito A., Yoon J., Zalesky A., Bullmore E.T., Carter C.S. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biological Psychiatry. 2011;70:64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman L. A set of measures of centrality based on betweenness. Sociometry. 1977;40:35–41. [Google Scholar]
- Freeman L. Centrality in social networks: conceptual clarification. Social Networks. 1979;1:215–239. [Google Scholar]
- Friston K.J. Dysfunctional connectivity in schizophrenia. World Psychiatry. 2002;1:66–71. [PMC free article] [PubMed] [Google Scholar]
- Friston K., Holmes A., Worsley K. How many subjects constitute a study? NeuroImage. 1999;10(1):1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Perez J., Broome M., Borgwardt S., Placentino A., Caverzasi E., Cortesi M., Veggiotti P., Politi P., Barale F. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews. 2007;31:465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Howes O.D., Allen P., Broome M., Valli I., Asselin M.C., Grasby P.M., McGuire P.K. Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Archives of General Psychiatry. 2010;67:683–691. doi: 10.1001/archgenpsychiatry.2010.77. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Borgwardt S., Crescini A., Deste G., Kempton M.J., Lawrie S., Mc Guire P., Sacchetti E. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neuroscience and Biobehavioral Reviews. 2011;35:1175–1185. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Bonoldi I., Yung A.R., Borgwardt S., Kempton M.J., Valmaggia L., Barale F., Caverzasi E., McGuire P. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Archives of General Psychiatry. 2012;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., McGuire P., Borgwardt S. Mapping prodromal psychosis: a critical review of neuroimaging studies. European Psychiatry. 2012;27:181–191. doi: 10.1016/j.eurpsy.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Deste G., Smieskova R., Barlati G., Yung A.R., Howes O., Stieglitz R., Vita A., Mc Guire P., Borgwardt S. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69(6):562–571. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- Hampson M., Peterson B.S., Skudlarski P., Gatenby J.C., Gore J.C. Detection of functional connectivity using temporal correlations in MR images. Human Brain Mapping. 2002;15(4):247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins K.A., Addington J., Keefe R.S., Christensen B., Perkins D.O., Zipurksy R., Woods S.W., Miller T.J., Marquez E., Breier A. Neuropsychological status of subjects at high risk for a first episode of psychosis. Schizophrenia Research. 2004;67:115–122. doi: 10.1016/j.schres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Hill K., Mann L., Laws K.R., Stephenson C.M., Nimmo-Smith I., McKenna P.J. Hypofrontality in schizophrenia: a meta-analysis of functional imaging studies. Acta Psychiatrica Scandinavica. 2004;110:243–256. doi: 10.1111/j.1600-0447.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- Honey C.J., Sporns O. Dynamical consequences of lesions in cortical networks. Human Brain Mapping. 2008;29:802–809. doi: 10.1002/hbm.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y. Comparisons of estimators of the number of true null hypotheses and adaptive FDR procedures on multiplicity testing. Journal of Statistical Computation and Simulation. 2010:1563–5163. [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N., Schmitt G.J., Gaser C., Bottlender R., Scheuerecker J., McGuire P., Burgermeister B., Born C., Reiser M., Moller H.J. Neuroanatomical correlates of different vulnerability states for psychosis and their clinical outcomes. The British Journal of Psychiatry. 2009;195:218–226. doi: 10.1192/bjp.bp.108.052068. [DOI] [PubMed] [Google Scholar]
- Liu Y., Liang M., Zhou Y., He Y., Hao Y., Song M., Yu C., Liu H., Liu Z., Jiang T. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- Lord L.D., Allen P., Expert P., Howes O., Lambiotte R., McGuire P., Bose S.K., Hyde S., Turkheimer F.E. Characterization of the anterior cingulate's role in the at-risk mental state using graph theory. NeuroImage. 2011;56:1531–1539. doi: 10.1016/j.neuroimage.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Lynall M.E., Bassett D.S., Kerwin R., McKenna P.J., Kitzbichler M., Muller U., Bullmore E. Functional connectivity and brain networks in schizophrenia. Journal of Neuroscience. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minati L., Grisoli M., Seth A.K., Critchley H.D. Decision-making under risk: a graph-based network analysis using functional MRI. NeuroImage. 2012;60:2191–2205. doi: 10.1016/j.neuroimage.2012.02.048. [DOI] [PubMed] [Google Scholar]
- Nelson H. NERT; Windsor: 1982. National Adult Reading Test (NART) [Google Scholar]
- Nelson B., Yung A.R. Can clinicians predict psychosis in an ultra high risk group? The Australian and New Zealand Journal of Psychiatry. 2010;44:625–630. doi: 10.3109/00048671003620210. [DOI] [PubMed] [Google Scholar]
- Nicol R.M., Chapman S.C., Vertes P.E., Nathan P.J., Smith M.L., Shtyrov Y., Bullmore E.T. Fast reconfiguration of high-frequency brain networks in response to surprising changes in auditory input. Journal of Neurophysiology. 2012;107:1421–1430. doi: 10.1152/jn.00817.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelis C., Velakoulis D., McGorry P.D., Wood S.J., Suckling J., Phillips L.J., Yung A.R., Bullmore E.T., Brewer W., Soulsby B. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Pantelis C., Yucel M., Wood S.J., McGorry P.D., Velakoulis D. Early and late neurodevelopmental disturbances in schizophrenia and their functional consequences. The Australian and New Zealand Journal of Psychiatry. 2003;37:399–406. doi: 10.1046/j.1440-1614.2003.01193.x. [DOI] [PubMed] [Google Scholar]
- Pettersson-Yeo W., Allen P., Benetti S., McGuire P., Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neuroscience and Biobehavioral Reviews. 2010;35:1110–1124. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlisberger M., Riecher-Rossler A., Aston J., Fusar-Poli P., Radu E.W., Borgwardt S. Cingulate volume abnormalities in emerging psychosis. Current Pharmaceutical Design. 2012;18:495–504. doi: 10.2174/138161212799316316. [DOI] [PubMed] [Google Scholar]
- Sabb F.W., van Erp T.G., Hardt M.E., Dapretto M., Caplan R., Cannon T.D., Bearden C.E. Language network dysfunction as a predictor of outcome in youth at clinical high risk for psychosis. Schizophrenia Research. 2010;116:173–183. doi: 10.1016/j.schres.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R., Suckling J., Coleman M.R., Pickard J.D., Menon D., Bullmore E. Neurophysiological architecture of functional magnetic resonance images of human brain. Cerebral Cortex. 2005;15(9):1332–1342. doi: 10.1093/cercor/bhi016. [DOI] [PubMed] [Google Scholar]
- Tan H.Y., Callicott J.H., Weinberger D.R. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cerebral Cortex. 2007;17(Suppl 1):i171–181. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- Turkheimer F.E., Smith C.B., Schmidt K. Estimation of the number of "true" null hypotheses in multivariate analysis of neuroimaging data. NeuroImage. 2001;13(5):920–930. doi: 10.1006/nimg.2001.0764. [DOI] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk B.C., Stam C.J., Daffertshofer A. Comparing brain networks of different size and connectivity density using graph theory. PloS One. 2010;5:e13701. doi: 10.1371/journal.pone.0013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Metzak P.D., Honer W.G., Woodward T.S. Impaired efficiency of functional networks underlying episodic memory-for-context in schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:13171–13179. doi: 10.1523/JNEUROSCI.3514-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung A.R., McGorry P.D. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophrenia Bulletin. 1996;22:353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- Yung A.R., Phillips L.J., McGorry P.D., McFarlane C.A., Francey S., Harrigan S., Patton G.C., Jackson H.J. Prediction of psychosis. A step towards indicated prevention of schizophrenia. The British journal of psychiatry. Supplement. 1998;172:14–20. [PubMed] [Google Scholar]
- Yung A.R., Phillips L.J., Yuen H.P., Francey S.M., McFarlane C.A., Hallgren M., McGorry P.D. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophrenia Research. 2003;60:21–32. doi: 10.1016/s0920-9964(02)00167-6. [DOI] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Egan G.F., Pantelis C., Bullmore E.T. The relationship between regional and inter-regional functional connectivity deficits in schizophrenia. Human Brain Mapping. 2011 doi: 10.1002/hbm.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables


