Abstract
Temporal lobe epilepsy patients with unilateral mesial temporal sclerosis (TLE + uMTS) have been demonstrated to have extensive white matter abnormalities both ipsilateral and contralateral to the seizure onset zone. However, comparatively less is known about the white matter integrity of TLE patients without MTS (non-lesional TLE, nl-TLE). The purpose of the study was to investigate the diffusion properties of thirteen major white matter tracts in patients with TLE + uMTS and nl-TLE. Diffusion tensor imaging (DTI) was performed on 23 TLE + uMTS (15 left MTS and 8 right MTS), 15 nl-TLE and 21 controls. Thirteen tracts were delineated by tractography and their diffusion parameters compared for the two TLE groups relative to controls, with left and right hemispheres combined per tract. A subgroup analysis investigated left and right MTS separately. Compared to controls, reduced anisotropy was detected in ten tracts for TLE + uMTS, but only the parahippocampal cingulum and tapetum for nl-TLE. Right MTS subgroup showed reduced anisotropy in 7 tracts bilaterally (3 limbic, 3 association, 1 projection) and 2 tracts ipsilaterally (1 association, 1 projection) and the body of the corpus callosum whereas the left MTS subgroup showed reduced anisotropy in 4 tracts bilaterally (2 limbic, 1 association, 1 projection) and 2 tracts ipsilaterally (1 limbic, 1 association). Diffusion abnormalities in tracts were observed within and beyond the temporal lobe in TLE + uMTS and were more widespread than in nl-TLE. Patients with right MTS had more extensive, bilateral abnormalities in comparison to left MTS. These findings suggest different dysfunctional networks in TLE patients with and without MTS.
Abbreviations: TLE + uMTS, temporal lobe epilepsy with unilateral mesial temporal sclerosis; nl-TLE, temporal lobe epilepsy without mesial temporal sclerosis, non-lesional
Keywords: Diffusion tensor imaging, Temporal lobe epilepsy, Mesial temporal sclerosis, White matter, Tractography
Highlights
► Tractography of 13 tracts in TLE patients with and without mesial temporal sclerosis (MTS). ► TLE patients with MTS showed more widespread FA reduction than without MTS. ► Patients with right MTS had more extensive bilateral changes than with left MTS.
1. Introduction
Temporal lobe epilepsy (TLE) is the most common localization related epilepsy syndrome. Most TLE cases are associated with mesial temporal sclerosis (MTS) which can be detected by abnormal signal intensity and reduced volume of the hippocampus on magnetic resonance imaging (MRI) (Van Paesschen et al., 1997). Along with mesial temporal abnormalities, TLE patients have also demonstrated extensive gray and white matter abnormalities both ipsilateral and contralateral to the seizure onset zone (Keller and Roberts, 2008; Otte et al., 2012). Patients with TLE and unilateral MTS (TLE + uMTS) often demonstrate gray matter atrophy within and beyond the ipsilateral temporal lobe including inferior-medial and posterior temporal, limbic system, insular, frontal regions, basal ganglia, and thalamus ipsilateral, and not uncommonly contralateral to the seizure onset zone (Keller and Roberts, 2008; Pail et al., 2010; Riederer et al., 2008). White matter abnormalities have been demonstrated with volumetry and voxel-based morphometry (VBM) (Keller and Roberts, 2008; Coste et al., 2002) and diffusion tensor imaging (DTI) in the fornix, parahippocampal cingulum, dorsal cingulum, uncinate fasciculus, inferior/superior longitudinal fasciculus, inferior fronto-occipital fasciculus, corticospinal tracts, anterior thalamic radiation, and splenium of the corpus callosum in TLE patients (Otte et al., 2012; Kim et al., 2008; Shon et al., 2010; Focke et al., 2008; Thivard et al., 2005; Kemmotsu et al., 2011; Gross et al., 2006).
Although MTS is observed in most TLE patients, some patients do not demonstrate evidence of MTS on MRI. While it is assumed that some of these non-lesional TLE patients (nl-TLE) have subtle pathological features of MTS that cannot be detected with MRI, Carne et al. have demonstrated a strong correlation between negative MRI findings and the absence of histological features of MTS in surgical specimens (Carne et al., 2004). Although most reports of imaging findings outside of the mesial temporal regions in TLE have focused on TLE + uMTS or have not clearly separated TLE + uMTS and nl-TLE, we have recently reported more extensive white matter abnormalities in TLE + uMTS as compared with nl-TLE in a small number of white matter tracts (Concha et al., 2009). Whether TLE + uMTS and nl-TLE demonstrate differences in other white matter tracts previously reported as abnormal in TLE remains unknown (Otte et al., 2012; Kim et al., 2008; Shon et al., 2010; Focke et al., 2008; Thivard et al., 2005; Kemmotsu et al., 2011; Gross et al., 2006).
The purpose of the current study is to compare the diffusion properties (i.e., fractional anisotropy and mean, parallel and perpendicular diffusivities) of thirteen major white matter tracts in patients with temporal lobe epilepsy and unilateral mesial temporal sclerosis (TLE + uMTS) versus patients with non-lesional TLE (nl-TLE) using diffusion tensor tractography.
2. Materials and methods
Approval of the research protocol was obtained from the local Health Research Ethics Board, and informed consent was obtained from all participants.
2.1. Subjects
TLE with unilateral MTS (TLE + uMTS, n = 23, age 40 ± 11 years, range 19–58 years, 14F/9M): Electroencephalogram (EEG) video-telemetry demonstrated unilateral temporal lobe ictal onset in all patients, among which 15 were left (n = 15, age 39 ± 13 years, range 19–58 years, 8F/7M) and eight were right (n = 8, age 42 ± 5 years, range 36–48 years, 6F/2M). Hippocampal sclerosis was defined based on T2 relaxometry analysis where the ipsilateral hippocampal T2 values were above two standard deviations of the overall mean of the controls (i.e., 120 ms using the methodology below). Patients with contralateral hippocampal T2 greater than two standard deviations of controls were included if the ipsilateral hippocampal T2 was greater than that of the contralateral value and EEG demonstrated ictal EEG onset solely from the ipsilateral temporal region. No other lesions were identified from the clinical imaging for all subjects.
Non-lesional TLE (nl-TLE, n = 15, age 35 ± 8 years, range 17–45 years, 6F/9M): All patients had temporal lobe epileptic EEG abnormalities. EEG lateralization was left temporal for four patients, right temporal for seven, and bitemporal for four. No lesions were visually identified on the clinical imaging and hippocampal T2 was within two standard deviations of controls bilaterally.
Healthy controls (n = 21, age 37 ± 12 years, range 19–58 years, 8F/13M): All control subjects had no history of any neurological or psychiatric disorders and had no evidence of structural lesions on T1 weighted MRI.
There were no significant age differences among the three groups (analysis of variance (ANOVA), p = 0.31). The TLE + uMTS patients had an earlier age of seizure onset than the nl-TLE patients (TLE + uMTS: 14 ± 12 years; nl-TLE: 23 ± 10 years; Mann–Whitney U, p = 0.033) and longer disease duration than the nl-TLE patients (TLE + uMTS: 26 ± 14 years; nl-TLE: 12 ± 9 years, Mann–Whitney U, p = 0.006).
Note that 17 of the 23 patients in the TLE + uMTS group and 10 of the 15 in the nl-TLE have been previously reported in a more restricted analysis of the thalamus, limbic tracts (fornix, cingulum), external capsule, and corpus callosum (Concha et al., 2009; Gong et al., 2008).
2.2. Image acquisition
All imaging was performed on a 1.5 T Siemens Sonata scanner (Siemens Medical Systems, Erlangen, Germany). T2 relaxometry with coverage of the hippocampus used a high-resolution, multi-echo sequence with 32 echoes, 10 coronal slices, 3 mm slice thickness with 3 mm inter-slice gap, TR = 4430 ms, TE1 = 9.1 ms, TE spacing = 9.1 ms, NEX = 1, acquisition matrix = 192 × 176 (interpolated to 384 × 352), FOV = 230 mm × 210 mm, scan time = 8 min 13 s. Both standard DTI and fluid-attenuated inversion recovery (FLAIR) DTI were acquired using the same dual spin-echo, single shot echo planar imaging sequence except FLAIR DTI used an extra inversion pulse and fewer slices. The common parameters include 2 mm slice thickness with no inter-slice gap, TR = 10 s, TE = 88 ms, six diffusion directions with b = 1000 s/mm2, NEX = 8, acquisition matrix = 128 × 128 (interpolated to 256 × 256), FOV = 256 mm × 256 mm. Fifty two axial slices with coverage of whole brain were acquired for standard DTI and 26 axial slices with coverage of the fornix plus an inversion time of 2200 ms were used for FLAIR DTI (Concha et al., 2005a). Scan times for standard DTI and FLAIR DTI were 9:30 min and 8:30 min, respectively.
2.3. Quantitative hippocampal T2 relaxometry
The T2 signal decay was fitted to a mono-exponential curve voxel by voxel across the multi-echo coronal images. T2 values for the left and right hippocampi were calculated by averaging within the regions of interest manually drawn on two consecutive slices (Concha et al., 2005b).
2.4. Diffusion tensor tractography
In total, thirteen major white matter tracts were analyzed in this study. Limbic tracts (i.e. fornix and parahippocampal cingulum) and genu, occipital and temporal callosal tracts were included in order to verify our previous findings (Concha et al., 2009) and the other eight tracts were chosen based on previously reported abnormalities from other groups (Otte et al., 2012; Kim et al., 2008; Shon et al., 2010; Focke et al., 2008; Thivard et al., 2005; Kemmotsu et al., 2011; Gross et al., 2006). The tracts were also selected due to their well-established tracking protocols using deterministic tractography (Wakana et al., 2004). Tensor calculation and tractography of the parahippocampal cingulum (pCg), dorsal cingulum (dCg), uncinate fasciculus (UF), inferior/superior longitudinal fasciculus (ILF/SLF), inferior fronto-occipital fasciculus (IFO), anterior limb of the internal capsule (ALIC), corticospinal tracts (CST) and genu and body of the corpus callosum (gCC/bCC) on the whole brain DTI dataset and the occipital and temporal part of the corpus callosum (oCC/tapetum) on the FLAIR DTI dataset (to be consistent with our previous study) (Concha et al., 2009) were identified using a semiautomatic deterministic tractography method fully described before (Lebel et al., 2008) (Fig. 1). In brief, a non-diffusion weighted image template was created based on six scans of a 33-year-old male control subject coregistered to each other and averaged together for both whole brain DTI and FLAIR DTI datasets. Regions-of-interest (ROIs) for selecting specific tracts were manually drawn on the template subject based on known anatomical landmarks (Wakana et al., 2004). Each subject's non-diffusion weighted images were nonlinearly normalized to the custom template using SPM8 (Wellcome Department of Cognitive Neurology, London, United Kingdom). The estimated deformation map from normalization step was then inverted and applied to transform the template's ROIs into each subject's native space. Fiber tracking was performed in the subject's native space using the deformed ROIs and a deterministic streamline method provided by ExploreDTI (Leemans et al., 2009). All resultant tracts were visually examined and a limited number of spurious tracts which deviated into other distant anatomical locations were excluded manually. The tractography of the crus of the fornix (Fx) was performed manually (due to the requirement of finer ROIs for this small structure) (Malykhin et al., 2008) on the FLAIR DTI dataset to reduce the signal contamination from the adjacent cerebrospinal fluid (Concha et al., 2005a). Fractional anisotropy threshold was set to 0.25 and the angle threshold was set to 60° for all tracts. In order to reduce the effects of crossing fibers and tract variability near the gray matter boundaries, only the portion between the decussation and the body of the corpus callosum axially and the medial central part before the lateral corpus callosum fibers turning up to the primary cortices were analyzed in CST and bCC, respectively (Fig. 1). The oCC and tapetum were also analyzed using the same method as our previous study (Concha et al., 2009). Fractional anisotropy (FA), mean diffusivity (MD), and parallel and perpendicular diffusivities were obtained by averaging over all voxels within the tract.
Fig. 1.
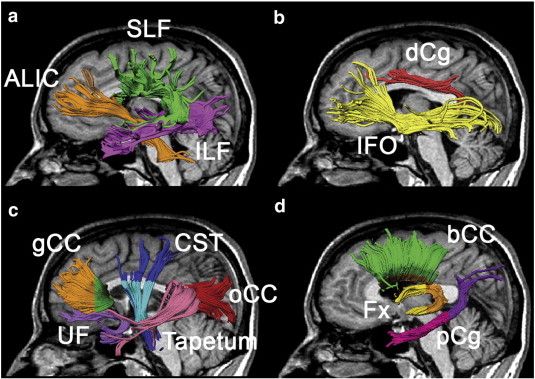
Three-dimensional visualization of thirteen tracts derived from tractography on a mid-sagittal slice in a 36-year-old male TLE + uMTS patient including: a) anterior limb of internal capsule (ALIC) and inferior/superior longitudinal fasciculi (ILF/SLF), b) dorsal cingulum (dCg) and inferior fronto-occipital fasciculus (IFO), c) uncinate fasciculus (UF), frontal, occipital and temporal part of the corpus callosum (gCC/oCC/tapetum) and corticospinal tracts (CST), and d) body of corpus callosum (bCC), fornix (Fx) and parahippocampal cingulum (pCg). For some tracts, only a portion was analyzed and is indicated by a different color, namely the portion between the decussation and the body of the corpus callosum in light blue for CST, the crus of the Fx posterior to the coronal slice placed at the fusion of the two crura in orange for Fx, the portion anterior to the most anterior slice of the splenium of the corpus callosum in pink for pCg, and, the medial central part before the fibers fan out to the cortices (~ 15 mm from the midline) for all callosal fibers (gCC/bCC/oCC/tapetum) are colored differently.
2.5. Statistical analyses
Statistical analyses were performed using SPSS version 18. Paired Student's t test on FA was performed in control group to evaluate the left and right symmetry of the nine paired tracts (except gCC, bCC, oCC and tapetum). A left-greater-than-right asymmetry was observed for FA in the dCg (left = 0.54 ± 0.02; right = 0.52 ± 0.02; p = 0.001) and ILF (left = 0.48 ± 0.02; right = 0.47 ± 0.02; p = 0.03), while a right-greater-than-left asymmetry was observed in the UF (left = 0.44 ± 0.03; right = 0.46 ± 0.02; p = 0.021). The lateralization of nl-TLE patients was complicated due to the presence of independent bilateral seizure onset in four subjects. The analyses of left and right symmetry in our previous study (Concha et al., 2009) further demonstrated low dependence of seizure lateralization on FA of Fx, pCg and external capsule in nl-TLE patients with different onset sides. As our primary interest in the current study was to look for differences between patients with TLE + uMTS and nl-TLE, and given the fact that the right to left differences for the control group were small (all less than 0.02) and to simplify the analysis from a practical perspective, the paired tracts were collapsed by averaging the value from each hemisphere to yield a single value per tract. The between-group differences of FA were tested by multivariate analysis of covariance (MANCOVA) with age included as a covariate for all thirteen tracts together among TLE + uMTS, nl-TLE and controls. To better understand the basis of any FA differences, the mean, parallel and perpendicular diffusivities were also compared between-groups secondarily. Only if Wilks' Lambda was significant at p = 0.05, then univariate analysis of covariance (ANCOVA) was performed for each tract followed by post-hoc t-tests for those tracts that survived ANCOVA at p = 0.05 between groups. All the post-hoc tests for each diffusion measurement were corrected with False Discovery Rate (FDR) at p = 0.05. Additionally, the FA of the white matter tracts with significant between-group differences were evaluated for linear correlations with disease duration (controlling for age) and age of seizure onset (controlling for disease duration) in each patient group. The correlation between the ipsilateral FA of the white matter tracts and ipsilateral hippocampal T2 were also examined in all TLE + uMTS patients (n = 23) and 11 of the 15 nl-TLE patients excluding the four patients with bitemporal seizure onset. Both correlation results with and without FDR correction at p = 0.05 level were reported.
To probe the effect of laterality of MTS, the TLE + uMTS patients were divided into TLE with left MTS (left TLE + uMTS, n = 15) and TLE with right MTS (right TLE + uMTS, n = 8) and compared with controls (n = 21). Statistical tests showed neither age differences among the three groups (left TLE + uMTS: 39 ± 13 years; right TLE + uMTS: 42 ± 5 years; controls: 37 ± 12 years; Student's t test p = 0.5), nor differences of age of seizure onset and duration of epilepsy between the two TLE + uMTS subgroups (left TLE + uMTS: onset 15 ± 12 years, duration: 24 ± 15 years; right TLE + uMTS: onset 14 ± 13 years, duration: 29 ± 12 years; onset Mann–Whitney U, p = 0.87; duration Mann–Whitney U, p = 0.52). For this analysis, each side of the nine paired tracts (except the four commissural tracts, i.e. gCC, bCC, oCC and tapetum) was considered separately to yield an FA value per side. Z-scores of FA (zFA) were calculated for each side of the nine paired fiber tracts in patients based on the mean FA and standard deviation of the corresponding side of controls. MANCOVA and following ANCOVA and post hoc tests were performed for each side of the tracts to compare the zFA between patients and controls separately for left TLE + uMTS and right TLE + uMTS with age included as a covariate. All post hoc tests were corrected by FDR at p = 0.05. To probe tract asymmetry, the left and right zFA for each tract were tested by paired t tests for each patient group. For the other four commissural tracts, i.e. gCC, bCC, oCC and tapetum, the FA of the whole tract was compared among left TLE + uMTS, right TLE + uMTS and controls using ANCOVA and post hoc tests with age included as a covariate.
3. Results
The hippocampal T2 values of controls were 112 ± 4 ms for the left side and 111 ± 4 ms for the right side. The overall mean of both sides was 112 ± 4 ms. All TLE + uMTS patients demonstrated ipsilateral hippocampal T2 more than two standard deviations greater than controls (mean: 135 ms, range: 122–160 ms); these differences were highly significant (Student's t test, p < 0.000001). T2 of the contralateral side was also significantly larger than that of controls (mean: 117 ms, range: 107–129 ms, p = 0.0001). Four TLE + uMTS patients had contralateral T2 equal to or higher than 120 ms; however, in each case ipsilateral hippocampal T2 was greater than contralateral T2 (range difference in T2 ipsilateral–contralateral: 6–10 ms). None of the nl-TLE patients demonstrated elevated T2 on either side by definition. The average hippocampal T2 for the nl-TLE group was 114 ms (range: 106–118 ms) for the left and 112 (range: 103–118 ms) for the right side, both of which were not significantly different from the overall mean of controls (left side p = 0.07, right side p = 0.87).
3.1. Primary analysis
The MANCOVA test on FA showed a significant Wilks' Lambda at p < 0.001. ANCOVA and post-hoc tests revealed that ten tracts had significantly lower FA in TLE + uMTS relative to controls (all tracts except gCC, oCC and tapetum) and seven of these (all except the CST, UF and SLF) had lower FA with respect to nl-TLE group (Fig. 2a). The nl-TLE group demonstrated significantly reduced FA of pCg and tapetum only with respect to controls.
Fig. 2.
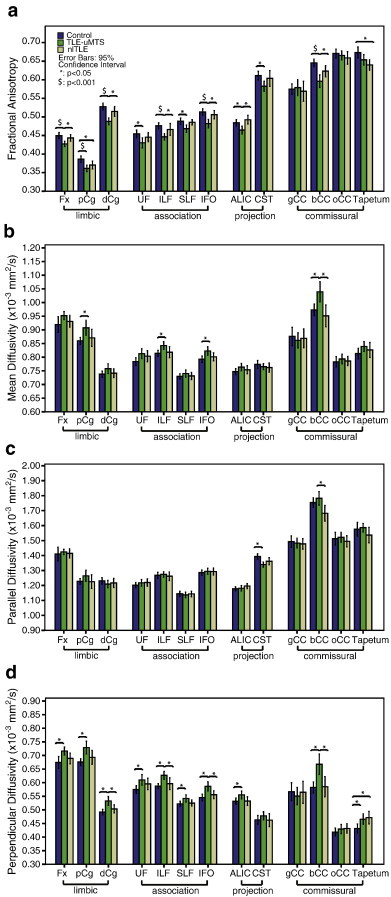
The mean and 95% confidence interval of a) FA, b) MD, c) parallel and d) perpendicular diffusivities of the thirteen white matter tracts (averaged across left and right) in TLE + uMTS (n = 23), nl-TLE (n = 15) and controls (n = 21). The tracts are categorized into limbic, association, projection and commissural fibers. Significant between-group differences after FDR correction are marked. Many tracts show reduction of FA or elevations of perpendicular diffusivity, although this is more prevalent for the TLE + uMTS patients with the reduction in FA being primarily explained by increased perpendicular diffusivity.
3.2. Secondary analysis
The MANCOVA test on MD also demonstrated significant Wilks' Lambda at p < 0.001. The subsequent univariate analyses revealed four tracts (pCg, ILF, IFO and bCC) with significantly higher MD in TLE + uMTS compared to controls, among which, the bCC also showed higher MD relative to nl-TLE group (Fig. 2b). There were no significant MD differences between nl-TLE patients and controls. The MANCOVA test on parallel diffusivity showed significant Wilks' Lambda at p = 0.002. The ANCOVA tests further showed the CST with reduced parallel diffusivity in the TLE + uMTS group versus controls and the bCC with significantly higher parallel diffusivity in TLE + uMTS versus nl-TLE (Fig. 2c). Wilks' Lambda for perpendicular diffusivity was significant at p < 0.001. The elevation of perpendicular diffusivity was observed in ten tracts (all except the CST, gCC and oCC) in TLE + uMTS patients compared to controls, among which the elevation in Fx, pCg, dCg, ILF, IFO and bCC was also significant with respect to nl-TLE patients (Fig. 2d). Only the tapetum showed elevated perpendicular diffusivity in nl-TLE group relative to controls. Uncorrected correlations between disease duration and FA demonstrated no significant findings in 11 of 13 tracts with positive findings being observed in dCg (r = − 0.58, p = 0.029) and bCC (r = − 0.59, p = 0.027) in nl-TLE patients. However, neither results approached significance following FDR correction (FDR corrected dCg p = 0.19, bCC p = 0.35). For hippocampal T2 versus FA, no significant correlation was found before or after FDR correction.
3.3. Analysis of right and left TLE + uMTS
For the nine paired tracts, right TLE + uMTS had FA reductions in all with seven bilateral and two right only whereas left TLE + uMTS had FA reductions in six tracts with four bilateral and two left only (Fig. 3). Specifically, for patients with left TLE + uMTS (n = 15), four tracts (pCg, dCg, ILF and CST) demonstrated bilateral FA reduction and two tracts (Fx and IFO) showed ipsilateral FA reduction. For patients with right TLE + uMTS (n = 8), significant bilateral FA reduction was shown in seven tracts (Fx, pCg, dCg, ILF, SLF, IFO and ALIC) and ipsilateral FA reduction was shown in two tracts (UF and CST). Among the six tracts with significant changes in the left TLE + uMTS group, paired t tests revealed four tracts (dCg, ILF, Fx and IFO) with greater FA reduction on the left side, the UF with greater FA reduction on the right side and two tracts (pCg and CST) without significant asymmetry. Among all the nine paired tracts showing significant change in the right TLE + uMTS group, paired t tests revealed two tracts (pCg and UF) with greater FA reduction on the right side with the other seven tracts not showing any asymmetry. For the four commissural tracts, the only between group difference was shown in bCC (ANOVA, p < 0.001) where the FA of all three groups was significantly different from each other with right TLE + uMTS showing the most FA reduction compared to controls (controls: 0.65 ± 0.02, left TLE + uMTS: 0.61 ± 0.03, right TLE + uMTS: 0.58 ± 0.05).
Fig. 3.
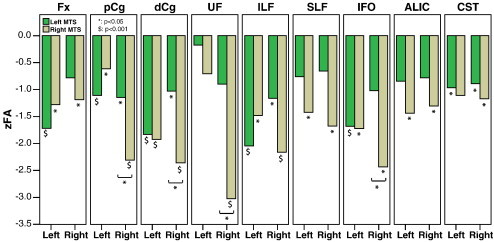
The z scores of FA (zFA, based on the mean and standard deviation of the corresponding side in control subjects) of the nine paired tracts with left and right sides assessed in patients with TLE and left MTS and with TLE and right MTS. Significant differences between patients and controls are marked beneath the individual bars and significant differences between the two patient groups are denoted by brackets. Right MTS shows FA reductions in all 9 tracts with 7 bilateral and 2 right only. Left MTS shows FA reductions in 6 tracts with 4 bilateral and 2 left only.
4. Discussion
In the current study, widespread abnormalities of white matter tracts within and beyond the temporal lobe were demonstrated in TLE + uMTS patients, while nl-TLE patients demonstrated fewer white matter abnormalities. These results suggest disruption of brain networks in TLE patients with and without MTS, although the affected network was more extensive in TLE + uMTS. Interestingly, in our cohort of subjects, TLE patients with right MTS showed more extensive bilateral changes than in TLE patients with left MTS, despite similar ages of onsets or disease durations.
These findings on a larger sample (an additional 6 TLE + uMTS and 5 nl-TLE patients) confirm our previous findings of FA reduction in the Fx and pCg in TLE + uMTS patients (Concha et al., 2005b, 2009). Furthermore, the nl-TLE patients here showed abnormalities of the pCg and tapetum, in agreement with our previous report (Concha et al., 2009). However, the gCC and oCC did not show any changes in either the TLE + uMTS or the nl-TLE groups, as was demonstrated before (Gross et al., 2006; Concha et al., 2009). Along with the increased sample size, the different methodologies used for gCC in the previous studies (ROI drawing on a single slice) and here (three-dimensional tractography) may contribute to the discrepancy of results.
The current observation of the abnormal frontal lobe (ALIC) and temporal lobe connected tracts (IFO, ILF, SLF and UF) as well as limbic system involvement (Fx, pCg and dCg) in TLE + uMTS are in agreement with several former DTI reports on cohorts of purely or largely TLE + uMTS patients (Thivard et al., 2005; Ahmadi et al., 2009; McDonald et al., 2008; Rodrigo et al., 2007; Kim et al., 2011). The diffusion abnormalities identified in the central part of the bCC are in accordance with a previous voxel-based study (Knake et al., 2009). Interestingly, the CST has not been measured in many previous studies in TLE or has been used as a ‘control’ tract (no significant FA or MD changes in 17 unilateral TLE patients, 14 of whom had MTS, in a recent paper) (McDonald et al., 2008); this is in contrast to our study where the CST had reduced anisotropy in adult TLE + uMTS patients. Our results are however consistent with findings of bilaterally decreased FA of CST in 13 children with left TLE (Govindan et al., 2008). Discrepancy between our CST findings and (McDonald et al., 2008) could relate to differences in methodology. For example, our CST measurement was restricted to the central part of the tract (as shown by the blue highlight in Fig. 1) where the fiber orientation is expected to be most uniform while they measured the full extent of the tracts. Our findings are also compatible with a number of earlier MRI-based volumetric and VBM studies in which white matter volume in total (Hermann et al., 2003), ipsilateral temporal lobe and body of the corpus callosum (Bernasconi et al., 2004), temporal pole (Coste et al., 2002), and ipsilateral frontal and parietal lobes and cerebellum (Mueller et al., 2006) was reduced in TLE + uMTS.
The reduction of FA observed here was mainly driven by an increase of perpendicular diffusivity, which is consistent with previous reports (Gross et al., 2006; Concha et al., 2009; Arfanakis et al., 2002; Lin et al., 2008). A few histopathological studies based on animal models proposed parallel and perpendicular diffusivities as surrogate indices of axonal membrane and myelination status, respectively (Budde et al., 2009; Song et al., 2002, 2003, 2005; Wu et al., 2007; Sun et al., 2006). Previous human in vivo DTI studies lent support to the notion by demonstrating increased perpendicular diffusivity with unchanged parallel diffusivity at the chronic stage of Wallerian degeneration when the myelin degradation is expected (Pierpaoli et al., 2001; Concha et al., 2006). These observations would suggest that the observed increase in perpendicular diffusion reflects reduced myelin in the chronically degenerated fibers; however, the expected disruption of axonal membranes associated with degeneration could also impact the perpendicular diffusivity (Concha et al., 2010).
The white matter findings in the pCg and tapetum in nl-TLE patients are in line with a previous VBM study demonstrating white matter concentration/volume reduction in the ipsilateral temporal lobe of nl-TLE patients (McMillan et al., 2004). In contrast to our more limited findings in nl-TLE, a recent DTI study detected widespread and bilateral reduction of white matter FA in the temporal lobes, the entire corpus callosum and the thalamus in a group of ten nl-TLE patients using voxel-based statistics (Keller et al., 2011). A possible explanation for the more extensive findings in the Keller et al. study is the fact that qualitative as opposed to quantitative methods were used to classify subjects as non-lesional and therefore, it is possible that their patient group included some TLE + uMTS patients. One other recent voxel-based DTI study reported significant MD elevation in the ipsilateral posterior fornix and posterior cingulum in ten left nl-TLE patients without changes in seven right nl-TLE patients (Shon et al., 2010). We did not look at left and right nl-TLE patients separately due to a small sample size. This, together with the different patient selection criteria and methodologies used (voxel-based statistics for them and tractography here), may account for the discrepancy among the aforementioned DTI studies.
Overall, we found that the white matter abnormalities were extensive and dramatic in TLE + uMTS and more limited in nl-TLE patients. Given the differences in age of seizure onset and disease duration between our TLE + uMTS and nl-TLE patients, where the MTS patient's onset was 9 years earlier and had 14 years longer disease duration, a possible explanation for the distinct white matter abnormalities observed is that these changes are secondary to seizure related white matter injury; however, the lack of correlations between FA and age of seizure onset/disease duration would argue against this hypothesis. That said, given the known changes of diffusion parameters in white matter during neurodevelopment (Lebel et al., 2008), it is possible that seizure onset during critical neurodevelopment periods of childhood and adolescence in the TLE + uMTS patients may have caused aberrant white matter development that is not evident if the seizures occur after the wiring is mostly laid down by adulthood (Kaaden et al., 2011; Hermann et al., 2002). The distinct white matter differences between TLE + uMTS and nl-TLE agree with previous reports on structural and functional MRI and EEG. A more widespread gray matter volume reduction or white matter diffusivity elevation was seen in TLE + uMTS while nl-TLE patients showed either no change (Mueller et al., 2006) or a less extensive pattern (Riederer et al., 2008; Shon et al., 2010). Evidence from EEG recording analysis (Zaveri et al., 2001), multi-slice proton magnetic resonance spectroscopic imaging (Mueller et al., 2004) and structure-function correlation (Mueller et al., 2012) indicate differences between the two TLE sub-syndromes. Our findings further support the idea that TLE + uMTS and nl-TLE are associated with different epileptic networks. Along with more extensive structural changes, TLE + uMTS have also demonstrated more extensive cognitive dysfunction including measures not only of temporal lobe dysfunction (memory and language) but also executive function (Ahmadi et al., 2009; Mueller et al., 2004, 2012; Diehl et al., 2008; Corcoran and Upton, 1993). Further study is required to assess a possible relationship between white matter integrity and cognitive function in TLE. It has been hypothesized that extratemporal structural abnormalities may predict poor surgical outcome in TLE (Sisodiya et al., 1997). Our observation of more extensive white matter abnormalities in TLE + uMTS (who are expected to have a better surgical outcome Sisodiya et al., 1997) however contradicts this hypothesis.
An increasing number of studies suggest that patients with left TLE have greater impairment of learning and memory (Bell and Davies, 1998) and more distributed gray and white matter changes than patients with right TLE (Riederer et al., 2008; Ahmadi et al., 2009; Bonilha et al., 2007; Coan et al., 2009). One recent DTI study focusing on TLE patients with unilateral MTS reported more widespread diffusion changes in the ipsilateral temporal lobe and bilateral limbic system in 21 patients with left TLE + uMTS than in 12 patients with right TLE + uMTS, while more prominent contralateral changes were observed in the temporal lobe and the inferior frontal gyrus in the right TLE + uMTS group (Focke et al., 2008). Another DTI tractography study reported overall smaller FA in Fx, pCg, UF, ILF, IFO and arcuate fasciculus in 18 patients with left TLE, among which 14 had left MTS, relative to controls and 18 right TLE patients, among which 11 had right MTS (Kemmotsu et al., 2011). The exact mechanism of this asymmetry remains unknown. Focke et al. (2008) suggested that the seizure propagation may be more widespread in the language dominant hemisphere, commonly the left side, on the basis of a pre-existing better connectivity (Powell et al., 2007) and Kemmotsu et al. (2011) speculated that the left hemisphere undergoes a more prolonged maturation process than the right and is therefore more vulnerable to early brain insults (Corballis and Morgan, 1978). Interestingly, despite the comparable age, disease duration, age of seizure onset and ipsilateral T2 relaxometry time (left TLE + uMTS: 135 ± 10 ms, right TLE + uMTS: 132 ± 6 ms, p = 0.45) of the two TLE + uMTS subgroups in our study, more extensive white matter abnormalities were observed in patients with right TLE + uMTS in comparison with left TLE + uMTS. The discrepancy between our study and previous reports may be partly attributed to the tighter age range of our right TLE + uMTS patients (mean 42 ± 5 years, range 36–48 years) compared to the other two studies with a wider age range (22–54 years for the right TLE + uMTS group in (Focke et al., 2008) and 38 ± 11 years for right TLE group in (Kemmotsu et al., 2011). While many reports suggest more extensive structural changes in left TLE + uMTS, two recent studies using VBM of T1-weighted images or SPECT have demonstrated more extensive abnormalities in right TLE + uMTS (Pail et al., 2010; Tae et al., 2005). More frequent interictal hypoperfusion of the contralateral hippocampus on SPECT was seen in right TLE + uMTS as compared with left TLE + uMTS (Tae et al., 2005) and gray matter volume reduction was significantly more extensive in right TLE + uMTS than left TLE + uMTS (Pail et al., 2010). Given the conflicting observations in the literature more work is needed to better characterize the differences in the extent of structural changes associated with right and left TLE + uMTS.
5. Conclusions
In summary, the current study demonstrated widespread diffusion abnormalities of white matter tracts within and beyond the temporal lobe in patients with TLE and unilateral MTS while, in contrast, patients with non-lesional TLE showed fewer changes. Specifically, patients with TLE and right MTS showed a more extensive bilateral white matter disruption than patients with left MTS. In conjunction with previous findings of white and gray matter abnormalities reported by other independent investigators, there is considerable evidence to suggest different dysfunctional networks in TLE patients with and without MTS.
Acknowledgment
Operating support was provided by Canadian Institutes of Health Research (D.W.G. and C.B.). Salary support was provided by Alberta Innovates — Health Solutions (C.B.) and scholarship by China Scholarship Council (M.L.). The authors would like to thank all patients and volunteers who participated in the study.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Ahmadi M.E., Hagler D.J., McDonald C.R., Tecoma E.S., Iragui V.J., Dale A.M., Halgren E. Side matters: diffusion tensor imaging tractography in left and right temporal lobe epilepsy. AJNR. American Journal of Neuroradiology. 2009;30:1740–1747. doi: 10.3174/ajnr.A1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfanakis K., Hermann B.P., Rogers B.P., Carew J.D., Seidenberg M., Meyerand M.E. Diffusion tensor MRI in temporal lobe epilepsy. Magnetic Resonance Imaging. 2002;20:511–519. doi: 10.1016/s0730-725x(02)00509-x. [DOI] [PubMed] [Google Scholar]
- Bell B.D., Davies K.G. Anterior temporal lobectomy, hippocampal sclerosis, and memory: recent neuropsychological findings. Neuropsychology Review. 1998;8:25–41. doi: 10.1023/a:1025679122911. [DOI] [PubMed] [Google Scholar]
- Bernasconi N., Duchesne S., Janke A., Lerch J., Collins D.L., Bernasconi A. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. NeuroImage. 2004;23:717–723. doi: 10.1016/j.neuroimage.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Bonilha L., Rorden C., Halford J.J., Eckert M., Appenzeller S., Cendes F., Li L.M. Asymmetrical extra-hippocampal grey matter loss related to hippocampal atrophy in patients with medial temporal lobe epilepsy. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78:286–294. doi: 10.1136/jnnp.2006.103994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde M.D., Xie M., Cross A.H., Song S.K. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. The Journal of Neuroscience. 2009;29:2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carne R.P., O'Brien T.J., Kilpatrick C.J., MacGregor L.R., Hicks R.J., Murphy M.A., Bowden S.C., Kaye A.H., Cook M.J. MRI-negative PET-positive temporal lobe epilepsy: a distinct surgically remediable syndrome. Brain. 2004;127:2276–2285. doi: 10.1093/brain/awh257. [DOI] [PubMed] [Google Scholar]
- Coan A.C., Appenzeller S., Bonilha L., Li L.M., Cendes F. Seizure frequency and lateralization affect progression of atrophy in temporal lobe epilepsy. Neurology. 2009;73:834–842. doi: 10.1212/WNL.0b013e3181b783dd. [DOI] [PubMed] [Google Scholar]
- Concha L., Gross D.W., Beaulieu C. Diffusion tensor tractography of the limbic system. AJNR. American Journal of Neuroradiology. 2005;26:2267–2274. [PMC free article] [PubMed] [Google Scholar]
- Concha L., Beaulieu C., Gross D.W. Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Annals of Neurology. 2005;57:188–196. doi: 10.1002/ana.20334. [DOI] [PubMed] [Google Scholar]
- Concha L., Gross D.W., Wheatley B.M., Beaulieu C. Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. NeuroImage. 2006;32:1090–1099. doi: 10.1016/j.neuroimage.2006.04.187. [DOI] [PubMed] [Google Scholar]
- Concha L., Beaulieu C., Collins D.L., Gross D.W. White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry. 2009;80:312–319. doi: 10.1136/jnnp.2007.139287. [DOI] [PubMed] [Google Scholar]
- Concha L., Livy D.J., Beaulieu C., Wheatley B.M., Gross D.W. In vivo diffusion tensor imaging and histopathology of the fimbria-fornix in temporal lobe epilepsy. The Journal of Neuroscience. 2010;30:996–1002. doi: 10.1523/JNEUROSCI.1619-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corballis M., Morgan M. On the biological basis of human laterality: I. Evidence for a maturational left–right gradient. The Behavioral and Brain Sciences. 1978;2:261–269. [Google Scholar]
- Corcoran R., Upton D. A role for the hippocampus in card sorting? Cortex. 1993;29:293–304. doi: 10.1016/s0010-9452(13)80182-7. [DOI] [PubMed] [Google Scholar]
- Coste S., Ryvlin P., Hermier M., Ostrowsky K., Adeleine P., Froment J.C., Mauguière F. Temporopolar changes in temporal lobe epilepsy: a quantitative MRI-based study. Neurology. 2002;59:855–861. doi: 10.1212/wnl.59.6.855. [DOI] [PubMed] [Google Scholar]
- Diehl B., Busch R.M., Duncan J.S., Piao Z., Tkach J., Luders H.O. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008;49:1409–1418. doi: 10.1111/j.1528-1167.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- Focke N.K., Yogarajah M., Bonelli S.B., Bartlett P.A., Symms M.R., Duncan J.S. Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. NeuroImage. 2008;40:728–737. doi: 10.1016/j.neuroimage.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Gong G., Concha L., Beaulieu C., Gross D.W. Thalamic diffusion and volumetry in temporal lobe epilepsy with and without mesial temporal sclerosis. Epilepsy Research. 2008;80:184–193. doi: 10.1016/j.eplepsyres.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Govindan R.M., Makki M.I., Sundaram S.K., Juhász C., Chugani H.T. Diffusion tensor analysis of temporal and extra-temporal lobe tracts in temporal lobe epilepsy. Epilepsy Research. 2008;80:30–41. doi: 10.1016/j.eplepsyres.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D.W., Concha L., Beaulieu C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia. 2006;47:1360–1363. doi: 10.1111/j.1528-1167.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- Hermann B., Seidenberg M., Bell B., Rutecki P., Sheth R., Ruggles K., Wendt G., O'Leary D., Magnotta V. The neurodevelopmental impact of childhood-onset temporal lobe epilepsy on brain structure and function. Epilepsia. 2002;43:1062–1071. doi: 10.1046/j.1528-1157.2002.49901.x. [DOI] [PubMed] [Google Scholar]
- Hermann B., Seidenberg M., Bell B., Rutecki P., Sheth R.D., Wendt G., O'Leary D., Magnotta V. Extratemporal quantitative MR volumetrics and neuropsychological status in temporal lobe epilepsy. Journal of the International Neuropsychological Society. 2003;9:353–362. doi: 10.1017/S1355617703930013. [DOI] [PubMed] [Google Scholar]
- Kaaden S., Quesada C.M., Urbach H., Koenig R., Weber B., Schramm J., Rudinger G., Helmstaedter C. Neurodevelopmental disruption in early-onset temporal lobe epilepsy: evidence from a voxel-based morphometry study. Epilepsy & Behavior. 2011;20:694–699. doi: 10.1016/j.yebeh.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Keller S.S., Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia. 2008;49:741–757. doi: 10.1111/j.1528-1167.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- Keller S.S., Ahrens T., Mohammadi S., Gerdes J.S., Möddel G., Kellinghaus C., Kugel H., Weber B., Ringelstein E.B., Deppe M. Voxel-based statistical analysis of fractional anisotropy and mean diffusivity in patients with unilateral temporal lobe epilepsy of unknown cause. Journal of Neuroimaging. 2011:1–8. doi: 10.1111/j.1552-6569.2011.00673.x. [DOI] [PubMed] [Google Scholar]
- Kemmotsu N., Girard H.M., Bernhardt B.C., Bonilha L., Lin J.J., Tecoma E.S., Iragui V.J., Hagler D.J., Halgren E., McDonald C.R. MRI analysis in temporal lobe epilepsy: cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia. 2011:1–10. doi: 10.1111/j.1528-1167.2011.03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Piao Z., Liu P., Bingaman W., Diehl B. Secondary white matter degeneration of the corpus callosum in patients with intractable temporal lobe epilepsy: a diffusion tensor imaging study. Epilepsy Research. 2008;81:136–142. doi: 10.1016/j.eplepsyres.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Kim C.H., Chung C.K., Koo B.B., Lee J.M., Kim J.S., Lee S.K. Changes in language pathways in patients with temporal lobe epilepsy: diffusion tensor imaging analysis of the uncinate and arcuate fasciculi. World Neurosurgery. 2011;75:509–516. doi: 10.1016/j.wneu.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Knake S., Salat D.H., Halgren E., Halko M.A., Greve D.N., Grant P.E. Changes in white matter microstructure in patients with TLE and hippocampal sclerosis. Epileptic Disorders. 2009;11:244–250. doi: 10.1684/epd.2009.0272. [DOI] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Leemans A., Jeurissen B., Sijbers J., Jones D. 17th Annual Meeting of Intl. Soc. Mag. Reson. Med., Hawaii, USA. 2009. ExploreDTI: a graphical toolbox for processing, analyzing , and visualizing diffusion MR data; p. 3537. [Google Scholar]
- Lin J.J., Riley J.D., Juranek J., Cramer S.C. Vulnerability of the frontal-temporal connections in temporal lobe epilepsy. Epilepsy Research. 2008;82:162–170. doi: 10.1016/j.eplepsyres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Malykhin N., Concha L., Seres P., Beaulieu C., Coupland N.J. Diffusion tensor imaging tractography and reliability analysis for limbic and paralimbic white matter tracts. Psychiatry Research. 2008;164:132–142. doi: 10.1016/j.pscychresns.2007.11.007. [DOI] [PubMed] [Google Scholar]
- McDonald C.R., Ahmadi M.E., Hagler D.J., Tecoma E.S., Iragui V.J., Gharapetian L., Dale a.M., Halgren E. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71:1869–1876. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan A.B., Hermann B.P., Johnson S.C., Hansen R.R., Seidenberg M., Meyerand M.E. Voxel-based morphometry of unilateral temporal lobe epilepsy reveals abnormalities in cerebral white matter. NeuroImage. 2004;23:167–174. doi: 10.1016/j.neuroimage.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Mueller S.G., Laxer K.D., Cashdollar N., Flenniken D.L., Matson G.B., Weiner M.W. Identification of abnormal neuronal metabolism outside the seizure focus in temporal lobe epilepsy. Epilepsia. 2004;45:355–366. doi: 10.1111/j.0013-9580.2004.27603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S.G., Laxer K.D., Cashdollar N., Buckley S., Paul C., Weiner M.W. Voxel-based optimized morphometry (VBM) of gray and white matter in temporal lobe epilepsy (TLE) with and without mesial temporal sclerosis. Epilepsia. 2006;47:900–907. doi: 10.1111/j.1528-1167.2006.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S.G., Laxer K.D., Scanlon C., Garcia P., McMullen W.J., Loring D.W., Meador K.J., Weiner M.W. Different structural correlates for verbal memory impairment in temporal lobe epilepsy with and without mesial temporal lobe sclerosis. Human Brain Mapping. 2012;33:489–499. doi: 10.1002/hbm.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte W.M., van Eijsden P., Sander J.W., Duncan J.S., Dijkhuizen R.M., Braun K.P.J. A meta-analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging. Epilepsia. 2012;53:659–667. doi: 10.1111/j.1528-1167.2012.03426.x. [DOI] [PubMed] [Google Scholar]
- Pail M., Brázdil M., Marecek R., Mikl M. An optimized voxel-based morphometric study of gray matter changes in patients with left-sided and right-sided mesial temporal lobe epilepsy and hippocampal sclerosis (MTLE/HS) Epilepsia. 2010;51:511–518. doi: 10.1111/j.1528-1167.2009.02324.x. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C., Barnett A., Pajevic S., Chen R., Penix L.R., Virta A., Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. NeuroImage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- Powell H.W.R., Richardson M.P., Symms M.R., Boulby P.a., Thompson P.J., Duncan J.S., Koepp M.J. Reorganization of verbal and nonverbal memory in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsia. 2007;48:1512–1525. doi: 10.1111/j.1528-1167.2007.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer F., Lanzenberger R., Kaya M., Prayer D., Serles W., Baumgartner C. Network atrophy in temporal lobe epilepsy: a voxel-based morphometry study. Neurology. 2008;71:419–425. doi: 10.1212/01.wnl.0000324264.96100.e0. [DOI] [PubMed] [Google Scholar]
- Rodrigo S., Oppenheim C., Chassoux F., Golestani N., Cointepas Y., Poupon C., Semah F., Mangin J.F., Le Bihan D., Meder J.F. Uncinate fasciculus fiber tracking in mesial temporal lobe epilepsy. Initial findings. European Radiology. 2007;17:1663–1668. doi: 10.1007/s00330-006-0558-x. [DOI] [PubMed] [Google Scholar]
- Shon Y.M., Kim Y.I., Koo B.B., Lee J.M., Kim H.J., Kim W.J., Ahn K.J., Yang D.W. Group-specific regional white matter abnormality revealed in diffusion tensor imaging of medial temporal lobe epilepsy without hippocampal sclerosis. Epilepsia. 2010;51:529–535. doi: 10.1111/j.1528-1167.2009.02327.x. [DOI] [PubMed] [Google Scholar]
- Sisodiya S.M., Moran N., Free S.L., Kitchen N.D., Stevens J.M., Harkness W.F., Fish D.R., Shorvon S.D. Correlation of widespread preoperative magnetic resonance imaging changes with unsuccessful surgery for hippocampal sclerosis. Annals of Neurology. 1997;41:490–496. doi: 10.1002/ana.410410412. [DOI] [PubMed] [Google Scholar]
- Song S.K., Sun S.W., Ramsbottom M.J., Chang C., Russell J., Cross A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song S.K., Sun S.W., Ju W.K., Lin S.J., Cross A.H., Neufeld A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song S.K., Yoshino J., Le T.Q., Lin S.J., Sun S.W., Cross A.H., Armstrong R.C. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sun S.W., Liang H.F., Trinkaus K., Cross A.H., Armstrong R.C., Song S.K. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magnetic Resonance in Medicine. 2006;55:302–308. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- Tae W.S., Joo E.Y., Kim J.H., Han S.J., Suh Y.L., Kim B.T., Hong S.C., Hong S.B. Cerebral perfusion changes in mesial temporal lobe epilepsy: SPM analysis of ictal and interictal SPECT. NeuroImage. 2005;24:101–110. doi: 10.1016/j.neuroimage.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Thivard L., Lehericy S., Krainik A., Adam C., Dormont D., Chiras J., Baulac M., Dupont S. Diffusion tensor imaging in medial temporal lobe epilepsy with hippocampal sclerosis. NeuroImage. 2005;28:682–690. doi: 10.1016/j.neuroimage.2005.06.045. [DOI] [PubMed] [Google Scholar]
- Van Paesschen W., Connelly A., King M.D., Jackson G.D., Duncan J.S. The spectrum of hippocampal sclerosis: a quantitative magnetic resonance imaging study. Annals of Neurology. 1997;41:41–51. doi: 10.1002/ana.410410109. [DOI] [PubMed] [Google Scholar]
- Wakana S., Jiang H., Nagae-Poetscher L.M., van Zijl P.C., Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wu Q., Butzkueven H., Gresle M., Kirchhoff F., Friedhuber A., Yang Q., Wang H., Fang K., Lei H., Egan G.F., Kilpatrick T.J. MR diffusion changes correlate with ultra-structurally defined axonal degeneration in murine optic nerve. NeuroImage. 2007;37:1138–1147. doi: 10.1016/j.neuroimage.2007.06.029. [DOI] [PubMed] [Google Scholar]
- Zaveri H.P., Duckrow R.B., de Lanerolle N.C., Spencer S.S. Distinguishing subtypes of temporal lobe epilepsy with background hippocampal activity. Epilepsia. 2001;42:725–730. doi: 10.1046/j.1528-1157.2001.00500.x. [DOI] [PubMed] [Google Scholar]


