Abstract
An objective biomarker is a compelling need for the early diagnosis of attention deficit hyperactivity disorder (ADHD), as well as for the monitoring of pharmacological treatment effectiveness. The advent of fNIRS, which is relatively robust to the body movements of ADHD children, raised the possibility of introducing functional neuroimaging diagnosis in younger ADHD children. Using fNIRS, we monitored the oxy-hemoglobin signal changes of 16 ADHD children (6 to 13 years old) performing a go/no-go task before and 1.5 h after MPH or placebo administration, in a randomized, double-blind, placebo-controlled, crossover design. 16 age- and gender-matched normal controls without MPH administration were also monitored. Relative to control subjects, unmedicated ADHD children exhibited reduced activation in the right inferior frontal gyrus (IFG) and middle frontal gyrus (MFG) during go/no-go tasks. The reduced right IFG/MFG activation was acutely normalized after MPH administration, but not after placebo administration. The MPH-induced right IFG/MFG activation was significantly larger than the placebo-induced activation. Post-scan exclusion rate was 0% among 16 right-handed ADHD children with IQ > 70. We revealed that the right IFG/MFG activation could serve as a neuro-functional biomarker for monitoring the acute effects of methylphenidate in ADHD children. fNIRS-based examinations were applicable to ADHD children as young as 6 years old, and thus would contribute to early clinical diagnosis and treatment of ADHD children.
Keywords: Cortical hemodynamics, Developmental disorder, Dorsolateral prefrontal cortex, Optical topography, Ventrolateral prefrontal cortex
Highlights
► We assessed the effects of MPH administration to ADHD children using fNIRS. ► Normal healthy control subjects recruited the right IFG/MFG during go/no-go task. ► Unmedicated ADHD children exhibited reduced right IFG/MFG activation. ► The activation was acutely normalized by MPH administration, but not by placebo. ► The right IFG/MFG activation may serve as an objective neuro-functional biomarker.
1. Introduction
Attention deficit hyperactivity disorder (ADHD) is characterized by a behavioral phenotype of inattention, hyperactivity and impulsivity, affecting between 3 and 7% of school-aged children in the U.S. (Dittmann et al., 2009; Pietrzak et al., 2006). The symptoms of ADHD can usually be identified during their early elementary school years (Drechsler et al., 2005). Diagnosis of ADHD typically refers to the degree of the symptoms listed in the diagnostic criteria from the DSM-IV (American Psychiatric Association, 1994), which requires rating by the parents or teachers of the children, and thus often entails subjective evaluation. Thus, more objective approaches, preferably based on biomarkers, are a compelling need (Wehmeier et al., 2011; Zhu et al., 2008).
One promising approach is noninvasive functional neuroimaging in combination with neuropsychological tests. A wealth of functional neuroimaging research has started to explore the neural substrates of ADHD. The majority of such research performed thus far has used functional magnetic resonance imaging (fMRI), and many studies have reported less prefrontal activation with ADHD during performance of various cognitive tasks (e.g., Booth et al., 2005; Rubia et al., 1999, 2005; Smith et al., 2006). However, most of the fMRI studies are on adult ADHD patients with only limited implications for children. Among approximately one hundred ADHD-related fMRI studies, twenty-six included patients at the age of eight, eleven at the age of seven (Anderson et al., 2002; Bedard et al., 2010; Booth et al., 2005; Chabernaud et al., 2012; Durston et al., 2007; Fair et al., 2010; Peterson et al., 2009; Slifer et al., 2002; Solanto et al., 2009; Teicher et al., 2000; Vaidya et al., 2005) and only two at the age of six (Durston et al., 2003; Teicher et al., 2000).
The scarcity of child ADHD studies is due to technical obstacles. Severely hyperactive children could not be included in the studies because motion artifacts would have prevented successful fMRI measurement (Vaidya et al., 1998). This leads to a high elimination rate due to excessive motion artifacts: One study enrolling a relatively young childhood sample (6 years and older) rejected 50% of ADHD subjects and 30% of normal control subjects (Durston et al., 2003). This further leads to a skewed distribution of the sample: Since motion problems are generally more severe for patients with hyperactivity, the resulting subject pool might be enriched with mildly and predominantly inattentive type patients (Epstein et al., 2007). Moreover, ADHD children may be withdrawn for various reasons related to their symptoms including refusal to enter the MRI scanner, refusal to begin or finish a run after entering the MRI scanner, inattention such as forgetting task rules, and falling asleep (Yerys et al., 2009).
Alternatively, functional near-infrared spectroscopy (fNIRS) is relatively immune to these problems, and has been successfully adopted in tasks involving body movement (Herrmann et al., 2004, 2005; Hock et al., 1997; Matsuo et al., 2000; Moriai-Izawa et al., 2012; Okamoto et al., 2004b; Shinba et al., 2004; Suto et al., 2004). Moreover, fNIRS offers other advantages including its compact size (useful in confined experimental settings), affordable price, unrestrictiveness and accessibility, serving as a suitable choice for clinically assessing ADHD children. A growing body of fNIRS studies has started to investigate the cortical hemodynamics of ADHD patients (Ehlis et al., 2008; Inoue et al., 2012; Negoro et al., 2010; Schecklmann et al., 2008, 2010; Weber et al., 2005). Most typically, a recent study focusing on Stroop interference revealed that the right prefrontal cortex oxy-Hb increase due to Stroop interference was reduced in ADHD children, suggesting a dysfunction of the area (Jourdan Moser et al., 2009).
The advent of fNIRS raised the possibility of introducing neuroimaging diagnosis in younger ADHD children, and this might further lead to its application in early clinical treatment. The most common treatment for ADHD children is the administration of methylphenidate (MPH), a psychostimulant drug that has been shown to be effective in improving attention and behavior as well as cognition and social function (Spencer, 2004). The behavioral and cognitive characteristics of ADHD are considered to be partly due to dopamine and noradrenaline dysfunction (Wilens, 2008). MPH is considered to inhibit reuptake of catecholamines, including dopamine, by blocking their transporters and to act as a dopamine agonist in the basal ganglia and cerebral cortices (Arnsten, 2006). A recent longitudinal study reported that the use of MPH to treat children and adolescents with ADHD may be conducive to enhancing educational outcomes by reducing the likelihood of disruptive behavior (Biederman et al., 2009). To confer long-term positive effects of treatment and thereby increase the quality of life of ADHD children, early identification of ADHD and appropriate treatment are important. This led us to postulate that fNIRS would be effective in monitoring the effect of MPH in ADHD children, especially for younger children who are difficult to assess in an fMRI environment.
Response inhibition as measured by go/no-go tasks has emerged as one of the principal paradigms for studying ADHD (Aron and Poldrack, 2005). Using this task, it has been clearly demonstrated that children (Beauregard and Levesque, 2006; Derefinko et al., 2008; Durston et al., 2003; Inoue et al., 2012; Ma et al., 2012; Monden et al., 2012; Siniatchkin et al., 2012; Smith et al., 2006; Solanto et al., 2009; Vaidya et al., 1998), adolescents (Schulz et al., 2004; Tamm et al., 2004) and adults (Dibbets et al., 2009; Karch et al., 2010; Mulligan et al., 2011; Sebastian et al., 2012; Vasic et al., in press) with ADHD have response inhibition deficits. An extensive review of functional neuroimaging in healthy adults indicates that widespread regions of the frontal cortex, especially the right inferior frontal gyrus (IFG), are associated with response inhibition (Aron and Poldrack, 2005). Structural neuroimaging in ADHD has fairly consistently indicated gray matter density reductions in the striatum and right IFG (Durston et al., 2004). A former fMRI study on ADHD children with an MPH history reported that MPH increased the activation of the frontal cortices and striatum in go/no-go tasks (Vaidya et al., 1998). The specificity of the implicated brain regions in healthy subjects, as well as functional and structural changes to those regions in ADHD patients, suggests that response inhibition is a good neuro-functional biomarker candidate for ADHD (Aron and Poldrack, 2005).
Thus, using the decrease of IFG activation during a response inhibition task as a potential neuro-functional biomarker for ADHD, we aimed to establish a robust procedure for detecting its recovery with MPH administration. Our initial effort (Monden et al., 2012) was to test whether fNIRS-based diagnosis could be introduced in actual clinical situations. We demonstrated that fNIRS could monitor the cortical hemodynamics of ADHD children (7 to 14 years old) performing a go/no-go task before and 1.5 h after MPH administration, allowing the observation of the acute effect of MPH as a significant increase in the oxy-Hb signal in the right lateral prefrontal cortex. As the monitoring takes only a few minutes, we further showed that the entire process can be implemented within a single-day hospital visit.
However, since that study was optimized for assessing the feasibility of introducing fNIRS as an actual clinical tool that allows the pre- and post-medication comparison to be performed in a single-day hospital visit, a neuro-pharmacological examination of the effects of MPH on ADHD children has yet to be performed. Thus, in the current study, enrolling sixteen ADHD children and age/sex-matched healthy control children, we examined the pharmacological effects of MPH on the cortical hemodynamics of ADHD during a go/no-go task. Subjects received either MPH or a placebo in a randomized, double-blind, placebo-controlled, crossover design. We hypothesized that MPH would modulate hemodynamic responses in the right prefrontal cortex during a go/no-go task while a placebo would not, and assessed this hypothesis using fNIRS. Moreover, we desire to validate the feasibility of introducing fNIRS-based diagnosis of the effects of MPH administration to ADHD children of 6 years old, the earliest age at which the FDA recommends starting MPH administration.
2. Material and methods
2.1. Subjects
Sixteen clinically referred, right-handed Japanese children with a mean age of 8.8 (SD 2.2, range 6–13 years) who met the DSM-IV criteria for ADHD participated in the study (Table 1). The subject group differed from the previous study (Monden et al., 2012). The Wechsler Intelligence Scale of Children—Third Edition (WISC-III) full IQ scores of subjects were all over 70 (mean 90.3, SD 10.0, range 74–110). Sixteen right-handed control subjects were matched with the ADHD subjects according to age (mean 8.9, SD 2.4, range 6–13 years) and gender (10 boys and 6 girls). IQs of controls (mean 111.8, SD 8.7, range 99–135) were significantly (t = 6.40, p < 0.0001) higher than those of ADHD subjects. Written consent was obtained from the parents of all subjects. The study was approved by the Ethics Committees of Jichi Medical University Hospital, and the International University of Health and Welfare. The study was in accordance with the latest version of the Declaration of Helsinki. This study was registered to UMIN-CTR Clinical Trial (UMIN000006277) as “Neurophysiological analysis in developmental disorders: an exploratory neuroimaging study using functional near-infrared spectroscopy (fNIRS)”.
Table 1.
Demographic and clinical profiles for ADHD subjects.
| ID | Age (years) |
Sex | ADHD subtype | Complication | WISC-III full IQ | MPH dose (mg) |
Duration of MPH exposure (years) |
Other medications |
|---|---|---|---|---|---|---|---|---|
| 1 | 7 | M | Inattentive | None | 110 | 18 | 1.0 | None |
| 2 | 8 | M | Combined | None | 95 | 27 | 1.0 | None |
| 3 | 12 | M | Combined | PDD | 96 | 27 | 1.8 | None |
| 4 | 11 | M | Combined | None | 82 | 27 | 3.4 | None |
| 5 | 6 | F | Hyperactive | None | 98 | 18 | 0.6 | None |
| 6 | 7 | M | Combined | PDD | 79 | 18 | Naïve | None |
| 7 | 13 | M | Combined | None | 82 | 45 | 2.1 | Carbamazepine, risperidone |
| 8 | 8 | F | Combined | PDD | 85 | 18 | Naïve | None |
| 9 | 8 | F | Combined | Epilepsy | 85 | 18 | Naïve | Valproic acid |
| 10 | 8 | M | Combined | PDD | 101 | 18 | 2.0 | None |
| 11 | 8 | M | Combined | None | 90 | 27 | 2.2 | None |
| 12 | 7 | M | Combined | None | 95 | 18 | Naïve | None |
| 13 | 6 | F | Inattentive | None | 74 | 18 | Naïve | None |
| 14 | 10 | M | Combined | None | 105 | 18 | Naïve | None |
| 15 | 9 | M | Combined | None | 85 | 18 | Naïve | None |
| 16 | 12 | M | Combined | None | 90 | 18 | 0.1 | None |
| Mean | 8.8 | 90.8 | ||||||
| SD | 2.2 | 9.9 |
Abbreviations: SD, standard deviation; PDD: pervasive developmental disorders.
2.2. Experimental design
The effects of MPH were examined in a randomized, double-blind, placebo-controlled, crossover study while the subjects performed a go/no-go task. Experimental procedure is summarized in Fig. 1. ADHD subjects were examined twice (the times of day for both measurements were scheduled to be as close as possible), at least 4 days apart, but within 30 days. Control subjects only underwent a pre-administration session.
Fig. 1.
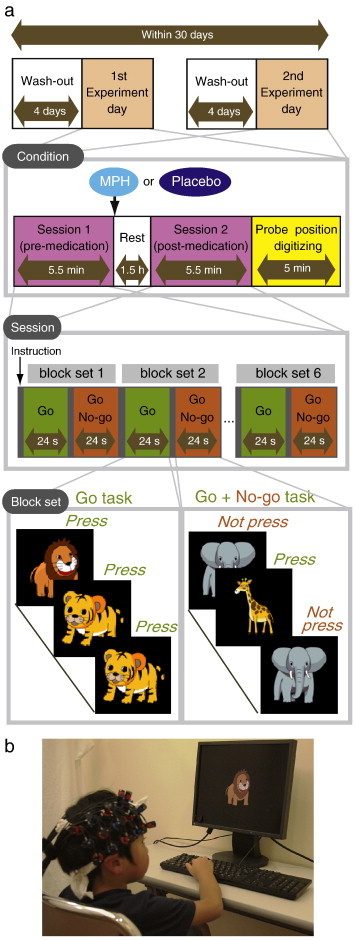
Experimental design. a. A schematic showing the flow of pre- and post-medication administration sessions for ADHD subjects. b. fNIRS measurements. Brain activity was measured while ADHD and control subjects performed the go/no-go task.
On each examination day, ADHD subjects underwent two sessions, one before drug (MPH or placebo) administration, and the other at 1.5 h after the drug administration. Each session consisted of 6 block sets, each containing alternating go (baseline) and go/no-go (target) blocks. Each block lasted 24 s and was preceded by instructions displayed for 3 s, giving an overall block-set time of 54 s and a total session time of 5.5 min. In the go block, subjects were presented a random sequence of two pictures and asked to press a button for both pictures. In the go/no-go block, subjects were presented with a no-go picture 50% of the time, thus being required to respond to half the trials (go trials) and inhibit their response to the other half (no-go trials). A go/no-go ratio of 50% was selected as it has been most often used in former neuroimaging studies (Dillo et al., 2010; Herrmann et al., 2005; Liddle et al., 2001; Menon et al., 2001; Vaidya et al., 1998). Pictures were presented with 1 Hz frequency during go and go/no-go blocks. At the beginning of each block, instructions (e.g., “press for tiger or elephant” for go conditions and “do not press for giraffe” for go/no-go conditions) were displayed for 3 s to inform the subject about the new block. Each subject performed a practice block before any measurements to ensure their understanding of the instructions.
After ADHD subjects performed the first session, either MPH (OROS-methylphenidate or Concerta) or a placebo was administered orally. Specific acute doses were the same as their daily dose as described in Table 1.
2.3. fNIRS measurements
We used the multichannel fNIRS system ETG-4000 (Hitachi Medical Corporation, Kashiwa, Japan), using two wavelengths of near-infrared light (695 and 830 nm). We analyzed the optical data based on the modified Beer–Lambert Law (Cope et al., 1988) as previously described (Maki et al., 1995).
We set the fNIRS probes to cover the lateral prefrontal cortices in reference to previous studies (Garavan et al., 1999; Herrmann et al., 2004, 2005; Liddle et al., 2001; Monden et al., 2012; Rubia et al., 2003), resulting in 22 channels (CH) per hemisphere. The specific setting was as previously described (Monden et al., 2012). After the fNIRS measurement, positional data of illuminators and detectors were obtained for both the ADHD and control subjects using a 3D-digitizer (Fastscan, Polhemus), and subjected to probabilistic registration of fNIRS channel positions to MNI space (Jurcak et al., 2007; Okamoto et al., 2004a; Okamoto and Dan, 2005; Singh et al., 2005; Tsuzuki et al., 2007, 2012) with reference to macroanatomical brain atlases (Rorden and Brett, 2000; Shattuck et al., 2008).
Oxy-Hb signals were used for further analysis due to its higher signal amplitude than that of deoxy-Hb (Strangman et al., 2002). Individual timeline data for the oxy-Hb signals of each channel were preprocessed with a first-degree polynomial fitting and high-pass filter using cut-off frequencies of 0.01 Hz to remove baseline drift, and a 0.8 Hz low-pass filter to remove heartbeat pulsations. After removal of blocks with marked motion-related artifacts, timeline data of the remaining blocks (where more than 4 blocks remained) were used. From the preprocessed time series data, we obtained channel-wise and subject-wise contrasts by calculating the inter-block means of difference between the target (4–24 s after go/no-go block onset) and baseline (14–24 s after go block onset) periods.
2.4. Statistical analyses
We performed statistical analyses in a channel-wise manner on oxy-Hb signals. Specifically, for control subjects who were examined only once, the target vs. baseline contrast of the session was generated. For ADHD subjects, the following contrasts were generated: (1) first-day, pre-medication contrast: target vs. baseline contrast for the pre-medication condition (either placebo or MPH administration) for the first day exclusively; (2) pre-medication contrasts: target vs. baseline contrast for the pre-placebo and pre-MPH conditions (for either first or second day measurements); (3) post-medication contrasts (specifically, post-placebo and post-MPH contrasts): target vs. baseline contrast for the post-placebo and post-MPH conditions; (4) intra-medication contrasts: difference between post- and pre-medication contrast for each medication (i.e., intra-placebo and intra-MPH contrasts); and (5) inter-medication contrast: difference between intra-MPH and intra-placebo contrasts. Note that (2) and (4) were generated temporally for calculating (5).
To screen the channels involved in go/no-go tasks in normal control subjects, target vs. baseline contrasts were subjected to one-sample t-tests against zero (two-tails). Statistical threshold was set at 0.05 with Bonferroni method for family-wise error correction. For thus-screened channels, comparisons between control and ADHD were performed for the following three ADHD contrasts: (1) first-day, pre-medication, (2) post-placebo, and (3) post-MPH. They were subjected to independent two-sample t-tests (two-tails) with a statistical threshold of p < 0.05. For examining medication effects on ADHD subjects, comparison between intra-MPH and intra-placebo (i.e., inter-medication contrast) was subjected to one-sample t-tests against zero (two-tails) with a statistical threshold of p < 0.05.
2.5. Behavioral data analysis
The reaction time (RT) of go trials, and accuracy (ACC) for go and no-go trials were computed for each go/no-go block. ACCs and RTs were averaged across go/no-go blocks, and subjected to statistical analyses as described in the previous section. Statistical threshold was set at 0.05 with Bonferroni method for multiple-comparison error correction (i.e., significant p was < 0.05/3).
3. Results
3.1. Behavioral performance
The average ACC for go and no-go trials and RT for correct go trials in the go/no-go block for control and ADHD subjects are summarized in Table 2. In ADHD subjects, the first-day data before administration of either a placebo or MPH was used as a representation of pre-medication contrast. Second-day data was excluded for this contrast.
Table 2.
Go/no-go task performance and functional data for control and ADHD subjects.
| Control |
ADHD |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st day pre-medication |
Post-placebo |
Post-MPH |
|||||||||||||
| Mean | SD | Mean | SD | t | p | Mean | SD | t | p | Mean | SD | t | p | ||
| ACC for go trials (%) | 96.5 | 5.48 | 86.2 | 21.9 | 1.829 | 0.085ns | 85.3 | 20.2 | 2.14 | 0.047ns | 92.3 | 12.0 | 1.266 | 0.219ns | |
| ACC for no-go trials (%) | 95.2 | 4.52 | 86.6 | 11.9 | 2.688 | 0.014⁎ | 86.5 | 8.46 | 3.634 | 0.001⁎⁎ | 89.5 | 8.51 | 2.347 | 0.028ns | |
| RT for correct go trials (ms) | 421.4 | 57.5 | 385.5 | 96.8 | 1.275 | 0.214ns | 377.0 | 83.6 | 1.753 | 0.091ns | 404.9 | 47.9 | 0.881 | 0.386ns | |
| Oxy-Hb right CH10 (mM·mm) | 0.075 | 0.074 | 0.008 | 0.084 | 2.374 | 0.024⁎ | 0.001 | 0.087 | 2.586 | 0.015⁎ | 0.077 | 0.060 | − 0.110 | 0.916ns | |
Performance data (ACC and RT) is presented for go and no-go trial data from go/no-go blocks. Oxy-Hb values for right CH10 are presented as functional data. For ADHD subjects, data for 1st day pre-medication, post-medication of placebo and MPH are shown. t-value, p-value and statistical significance were the results of t-tests between control and each ADHD condition. Abbreviations: SD, standard deviation; t, t-value; p, p-value. Statistical significances are presented as follows: ⁎, p < 0.05; ⁎⁎, p < 0.01; and ns, not significant.
First-day pre-medication, post-placebo, and post-MPH values of ADHD subjects were compared with values for control subjects (Table 2). Significant differences in ACC for no-go trials were found between control subjects and first-day pre-medication ADHD subjects and between control subjects and post-placebo ADHD subjects.
For analysis within each ADHD subject, the inter medication contrast comparing the effect of MPH against the placebo revealed no significant differences in behavioral parameters (Table 3).
Table 3.
ADHD inter-medication (placebo vs. MPH) comparison.
| Mean | SD | t | p | |
|---|---|---|---|---|
| ACC for go trials (%) | 1.98 | 8.12 | 0.975 | 0.345ns |
| ACC for no-go trials (%) | 0.03 | 11.2 | 0.011 | 0.991ns |
| RT for correct go trials (ms) | 0.84 | 54.3 | 0.062 | 0.951ns |
| Oxy-Hb right CH10 (mM·mm) | 0.084 | 0.088 | 3.809 | 0.002⁎⁎ |
Abbreviations: SD, standard deviation; t, t-value; p, p-value. Statistical significances are presented as follows: ⁎, p < 0.05; ⁎⁎, p < 0.01; and ns, not significant.
3.2. fNIRS analyses
We screened for any fNIRS channels involved in the go/no-go task for the control subjects. Significant oxyHb increase was found in the right CH 10 (mean 0.075, SD 0.074, p < 0.05, Bonferroni-corrected, Cohen's d = 1.009). This channel was located in the border region between the right MFG and IFG (MNI coordinates x,y,z (SD): 46,43,30 (14), MFG 78%, IFG 22% with reference to macroanatomical brain atlases (Rorden and Brett, 2000; Shattuck et al., 2008)). Thus, we set the right CH 10 as a region-of-interest (ROI) for the rest of the study. For reference, cortical activation patterns with all the channels are presented for control and ADHD subjects as supplementary material.
Comparison between oxy-Hb signals of the control and first-day pre-medicated ADHD subjects revealed significantly higher oxy-Hb signal in the right CH 10 in the control subjects (independent two-sample t-test, p < 0.05, Cohen's d = 0.839, Table 2). This indicates that the control subjects exhibited higher right prefrontal activation during go/no-go tasks than did the pre-medicated ADHD children.
Effects of medications were examined between control and post-placebo ADHD subjects, and between control and post-MPH ADHD subjects (independent two-sample t-test, thresholded at p < 0.05). Oxy-Hb signal in control was significantly higher than in post-placebo ADHD subjects, whereas no significant difference was found for those in control and post-MPH ADHD subjects (Table 2). This suggests that the impaired right prefrontal activation in pre-medicated ADHD subjects was normalized by the MPH administration.
Finally, we tested whether there was an MPH-induced, but not placebo-induced, right prefrontal activation in ADHD children. In the inter-medication contrast, the right CH 10 was found to be significant with a large effect size (one-sample t-test, p < 0.05, Cohen's d = 0.952, Table 3). This result indicates that the oxy-Hb signal increase during go/no-go tasks was induced by MPH but not by the placebo.
3.3. Oxy-Hb timeline data
Fig. 2 illustrates the grand average waveforms of all 16 control subjects and 16 ADHD subjects. For ADHD, oxy-Hb and deoxy-Hb signals are presented for pre-/post-placebo and pre-/post-MPH conditions on CH 10 of the right hemisphere. We observed more stable task-related oxy-Hb signals than deoxy-Hb signals, suggesting the robustness of oxy-Hb signals for our experimental conditions. An oxy-Hb increase in the right CH 10 was clearly observed for control and post-MPH administration of ADHD in the grand average waveform. Waveforms for individual subjects (subject 5: 6-year-old ADHD girl, and subject 1: 7-year-old ADHD boy) are also illustrated. Although the individual data resulted in somewhat noisy waveforms, the oxy-Hb activation in the post-MPH session is clearly presented even in the data of the 6-year-old ADHD subject.
Fig. 2.
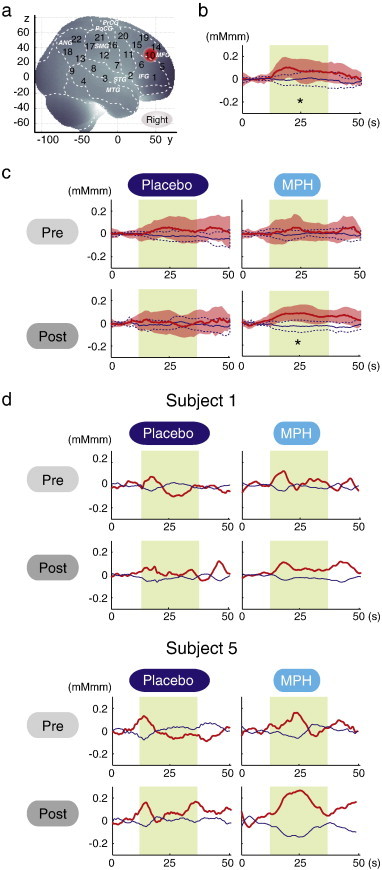
The channel location and waveforms of oxy-Hb (red line) and deoxy-Hb (blue line) signals for right CH 10. The green area indicates the go/no-go task period. Significant (one-sample t-test, p < .05) conditions are indicated by asterisks. a. On-brain channel locations (right hemisphere) are statistically estimated for the group of subjects (including both ADHD and control) and exhibited in MNI space. CH 10 is indicated in red. b. Grand averages for control subjects. Standard deviations among the 16 subjects are exhibited as pale red (oxy-Hb) and blue dotted (deoxy-Hb) areas. Each time line is adjusted to the average value for a baseline period of zero. Oxy-Hb and deoxy-Hb signals are shown in units of mM·mm. c. Grand averages for ADHD subjects for pre-/post- and placebo/MPH conditions are illustrated. d. Graphs for ADHD individuals for pre-/post- and placebo/MPH conditions. Subject 1 is a 7-year-old boy and subject 5 is a 6-year-old girl (corresponding to Table 1).
3.4. Examination on the effects of IQ
Since we did not match the IQ of the ADHD and normal healthy control subjects, we performed additional analyses to find the possible effects of IQ. We examined the correlation between IQ and activation in the right CH 10 for ADHD children (ADHD 1st day pre-medication contrast) and normal healthy control children, respectively. In ADHD children, Pearson's correlation coefficient was 0.184 (p = 0.494), while that in control children was 0.010 (p = 0.969): Neither analysis yielded any significant correlation with a meaningful effect size. In addition, we examined whether the two correlation coefficients were different, but did not find any significant difference (Fischer's z = 0.45, p > 0.05). Thus we concluded there was no correlation between IQ and the activation in the right CH 10 in either group.
4. Discussion
The current study exploring fNIRS-based diagnosis of the effects of MPH administered to ADHD children revealed that the right IFG/MFG activation could serve as an objective neuro-functional biomarker for fNIRS measurement. First, relative to control subjects, unmedicated ADHD children exhibited reduced brain activation in the right IFG/MFG during go/no-go task blocks. Second, the reduced right IFG/MFG activation was acutely normalized after MPH administration, but not after placebo administration. Third, the MPH-induced right IFG/MFG activation was significantly larger than the placebo-induced activation.
4.1. Behavioral performance for go/no-go task
The current fNIRS analyses adopted the contrast of go/no-go against go tasks. In addition to response inhibition, this contrast is thought to commeasure additional cognitive functions, including decision making, response competition/response selection, conflict monitoring, and increased attentional demand (Liddle et al., 2001; Menon et al., 2001; Rubia et al., 2001, 2003). Thus, the fNIRS results are expected to reflect a rather wide spectrum of cognitive functions associated with ADHD. On the other hand, neuropsychological tests are expected to examine specific cognitive aspects of ADHD symptoms. Go errors (omission errors) are typically considered indicators of inattention to the task, while no-go errors (commission errors) and RT to go responses are considered indicators of impulsivity (Barkley, 1991; Newcorn et al., 2001). Numerous studies have demonstrated that MPH improves no-go indices in child and adult ADHD patients (Aron et al., 2003; Aron and Poldrack, 2005; Bedard et al., 2003; Tannock et al., 1995). However, MPH also affects the speed of go responses, go response variability, and discrimination errors in go trials (Aron and Poldrack, 2005; Bedard et al., 2003; Tannock et al., 1995). Moreover, Rubia et al. suggested that the beneficial MPH effects were more pronounced for inattention problems (reflected by omission errors) than impulsivity (reflected by commission errors) (Rubia et al., 2009).
In the current study, the comparison between controls and unmedicated ADHD patients showed a significantly higher commission error rate for ADHD subjects. This result was mostly in line with former studies. We detected no significant behavioral performance differences in the MPH-medicated ADHD children contrasted against control subjects, suggesting an MPH effect for no-go performance. However, the inter-condition contrast representing MPH effects against placebo failed to yield any significant behavioral performance change. Although behavioral parameters may often well reflect specific cognitive aspects of ADHD symptoms or MPH effects on them, the current study could not confirm a normalization effect of MPH (but not placebo) on behavioral parameters.
4.2. Right IFG/MFG activation as a robust neuro-functional biomarker
On the other hand, fNIRS may provide more robust measures of MPH effects. Previous neuroimaging studies have elucidated the neural correlates of go/no-go tasks (Simmonds et al., 2008), including the bilateral IFG, MFG and SFG (superior frontal gyrus), supplementary motor area, anterior cingulate gyrus, inferior parietal and temporal lobes, caudate nucleus, and cerebellum (Rubia et al., 2003). The IFG may be specifically related to motor response inhibition, while the MFG/SFG, medial prefrontal, and parietal cortices possibly mediate more general meta-motor executive control functions such as motor attention, conflict monitoring, and response selection, necessary for inhibition task performance (Rubia et al., 2001).
Among these regions, our fNIRS measurements covered the right and left IFG (BA 44/45), MFG (BA 46/9), and SMG (supramarginal gyrus, BA40), and we found activation in the right MFG and IFG (BA9, 46, 45) during the go/no-go task period in the control subjects, but not in the first-day, pre-medicated ADHD subjects. These results suggest that the right prefrontal function associated with the go/no-go task performance was impaired in ADHD children. The administration of a placebo did not result in right prefrontal activation, while that of MPH led the ADHD children to exhibit a degree of right prefrontal activation comparable to that of the normal control subjects. Moreover, the right prefrontal activation due to MPH administration was significantly higher than that due to placebo administration. These results led us to conclude that MPH had significant effects in normalizing right frontal dysfunctions in ADHD children.
The right prefrontal dysfunction and MPH-elicited recovery observed by fNIRS are consistent with former studies using other neuroimaging modalities. A recent ALE meta-analysis of go/no-go tasks (Buchsbaum et al., 2005) reported a mainly right-lateralized network associated with response inhibition, including the right MFG and IFG (BA46/44), the right SMG (BA40), and the superior medial frontal gyrus (BA6) (Simmonds et al., 2008). These regions have been implicated in the processes of stimulus recognition, maintenance and manipulation of stimulus–response associations and response selection, including selecting not to respond (Grafton et al., 1992; Law et al., 1997; Liddle et al., 2001; Mostofsky et al., 2003; Rubia et al., 2001), all of which are critical to the performance of go/no-go tasks (Simmonds et al., 2008). fMRI studies of the go/no-go task have consistently recruited frontal cortices; however, localization and the extent of frontal activation vary across these studies, with activation most often localized to the right IFG (BA 45/47) (Durston et al., 2002; Garavan et al., 1999; Konishi et al., 1999; Rubia et al., 2001), followed by the right MFG/SFG (BA9/46) (Garavan et al., 1999, 2002, 2003, 2006; Hester et al., 2004; Mostofsky and Simmonds, 2008). An fNIRS study also added further evidence to the involvement of the right lateral prefrontal cortex (more exactly, F4) during go/no-go tasks (Boecker et al., 2007). Another recent fNIRS study reported reduced prefrontal activation in ADHD children compared to normal controls during a go/no-go condition (albeit no laterality was reported) (Inoue et al., 2012). Moreover, recent fMRI studies on MPH-medicated children have provided more direct evidence for cortical activation and MPH treatment. Using a continuous performance task, Rubia et al. (2009) found that MPH treatment improved under-activation in ADHD children compared to normal children by adding activation in the right IFG and MFG along with several other regions (Monden et al., 2012). Therefore, it would be relevant to suggest that normalized right IFG/MFG activation induced by MPH administration during go/no-go task serves as a robust neuro-functional biomarker for fNIRS assessment of MPH effect on ADHD children.
4.3. Effects of IQ
We did not match the IQs of ADHD and normal healthy control children. However, this did not seem to cause any serious effects on the findings of the current study, as we did not find any correlation between IQ and the activation in the right CH 10 in either group. There have been arguments over whether to match IQ or not in ADHD studies. The IQs of ADHD children are lower than those of normal healthy children (Frazier et al., 2004; Kuntsi et al., 2004), and an extensive epidemiological study reported that the co-occurrence of ADHD and low IQ has a genetic overlap (de Zeeuw et al., 2012). Therefore, it is not considered appropriate to treat IQ as a covariant of ANCOVA type of studies (de Zeeuw et al., 2012; Dennis et al., 2009). If IQ had been used as a covariant, the differences in cortical activation in ADHD and normal healthy control children would have been over-corrected since IQ is relevant to the brain phenotype of the disorder (de Zeeuw et al., 2012).
Moreover, IQ measurement of ADHD children poses a problem intrinsic to ADHD symptoms: it is sometimes difficult for young ADHD children to execute IQ tests as they are not always sufficiently patient. Thus, the IQs of ADHD children might be underestimated, and not adequately reliable.
Previous reports adopting an IQ match between ADHD and normal healthy children enrolled ADHD children with relatively high IQs (Aron et al., 2003; Booth et al., 2005; Chabernaud et al., 2012; Durston et al., 2007; Ma et al., 2012; Negoro et al., 2010; Schecklmann et al., 2010; Vaidya et al., 1998; Yerys et al., 2009). Most of these studies excluded low-IQ ADHD children with criteria such as IQ > 85 or IQ > 90 (Aron et al., 2003; Ma et al., 2012; Vaidya et al., 1998; Yerys et al., 2009). We calculated the average IQ of ADHD children in these studies and found that it was 108 ± 8 (mean ± SD). There is a possibility that low-IQ ADHD children with severe behavioral rating scores had been selectively excluded.
On the other hand, our IQ criterion was greater than 70, which is among the most lenient criterions together with two other recent studies (Inoue et al., 2012; Negoro et al., 2010). Such samples are expected to represent the ADHD population in a balanced manner as they include severe patients. While there might be other possibilities for matching ADHD IQs with those of normal healthy children with low IQs, it is difficult to match the IQs of normal healthy children to those of ADHD groups including children with low IQs, and no study has performed matching for such low scores. Moreover, even if IQ matching had been realized, such samples would not have represented general healthy children.
Although IQ matching for ADHD and control children poses several problems as mentioned above, we do not mean that it should be avoided. Rather, IQ-matching studies should be pursued with different perspectives, such as to assess the effects of IQ, and should be undertaken in the future. As de Zeeuw reported (de Zeeuw et al., 2012), the brains of low-IQ ADHD children might undergo different functional and anatomical development. If this is the case, subdividing the whole group to yield low-IQ groups would be of great clinical importance. For this purpose, the current system, which can measure severely ADHD children with low IQs, would serve as a valuable tool.
4.4. Limitations
A few limitations should be noted for adequate understanding of the current findings. First, a learning effect associated with go/no-go tasks cannot be excluded from the current experimental design: while control subjects underwent only one task session, ADHD children underwent two sessions in the same day. Thus, the effects of habituation (Fischer et al., 2003; Kiehl and Liddle, 2003; Loubinoux et al., 2001) and procedural learning (Eliassen et al., 2001) could be present. First-day pre-medicated data (i.e., either pre-placebo or pre-MPH) was used for the ADHD group to compare the two groups (ADHD and control) under conditions not affected by these factors. However, post-medication data are by necessity from the second sessions. For separate sessions of the same task, an activation of greater magnitude has been observed for the first session for go/no-go tasks in fMRI studies (Langenecker and Nielson, 2003). Thus, it was expected that the oxy-Hb amplitude of the second, post-medicated, sessions would be reduced. However, in the current study, MPH administration to ADHD children still led to increased oxy-Hb amplitude comparable to that of control children. This indicates that MPH exerted pharmacological effects beyond the level needed to compensate for the expected habituation and learning effects.
Second, the current study limited the analyses to the oxy-Hb parameter because we did not find any channels with significant activation with the deoxy-Hb parameter during the screening process performed on normal healthy control subjects. Thus, we concluded that deoxy-Hb was not suitable for further analysis in the current study.
Many fNIRS studies have solely reported the results of the oxy-Hb parameter, including an ADHD study by Negoro et al. (2010). There is a tendency that oxy-Hb is more sensitive than deoxy-Hb (e.g. Ehlis et al., 2008; Inoue et al., 2012; Weber et al., 2005), but the precise reason for the decreased sensitivity of deoxy-Hb has yet to be elucidated. Our previous study adopting a similar experimental paradigm also failed to detect activation with the deoxy-Hb parameter (Monden et al., 2012). Ehlis et al. (2008) reported that deoxy-Hb behavior was different between ADHD and normal subjects: deoxy-Hb decreases were larger in ADHD subjects than in normal subjects. In addition, even when oxy-Hb increased, the deoxy-Hb parameter either increased, remained unchanged or decreased depending on tasks, regions, age and so on (Ehlis et al., 2008; Sakatani et al., 1999), suggesting difficulty in dealing with the deoxy-Hb parameter. Further exploration is necessary to elucidate the role and applicability of the deoxy-Hb parameter.
4.5. Clinical utility of fNIRS-based examination
In the current study, we adopted a go/no-go task paradigm with alternating go and go/no-go blocks. Tsujii et al. (2011) and Cui et al. (2011) employed alternating go and go/no-go tasks, and used the go task as a baseline contrast for the go/no-go task fNIRS signal that they were interested in. Similarly, we used the go block as a baseline period, and did not adopt rest periods. This was primarily because it is extremely difficult for ADHD patients to stay still without performing any task: it may lead to unexpected movements or behaviors. Secondarily, we could save time by omitting rest blocks: a prolonged experiment time may bore ADHD subjects. Finally, experimental paradigms employing alternate go and go/no-go blocks have been commonly used in fMRI studies (Altshuler et al., 2005; Dillo et al., 2010; Ma et al., 2012; Vaidya et al., 1998) and also in fNIRS studies (Tsujii et al., 2011). Thus, considering comparisons across modalities, the choice of experimental paradigm in the current study is appropriate.
An additional merit of the alternative task paradigm is that go blocks can serve as a motor control for go/no-go blocks. Schecklmann et al. (2008) performed weekday reciting task and word fluency tasks and used the weekday reciting task as a baseline to which fNIRS signal during the word fluency tasks was compared. By using a control condition with a similar motor output, movement and muscle artifacts in a task condition are expected to be cancelled. Similarly, we adopted the go task as a baseline period. During the go task period, subjects are expected to press a button twice as often as in the go/no-go task period, assuming that they perform the tasks appropriately. Due to the nature of go/no-go tasks, that go periods require increased response, we cannot fully equalize the motor loads of go and go/no-go periods. However, since motor effects are considered larger during the go period, we can expect that a go/no-go vs. go contrast would rule out motion artifacts. Accordingly, activation in a go/no-go task block is considered to reflect response inhibition and target detection, and is therefore more appropriate than a rest block as a baseline. Although fNIRS studies often use a paradigm where rest and task blocks were alternately performed (Herrmann et al., 2005), we suggest that it would be more suitable for studies using younger ADHD children to adopt experimental paradigms employing alternate go and go/no-go blocks, which have been commonly used in fMRI studies (Altshuler et al., 2005; Dillo et al., 2010; Ma et al., 2012; Vaidya et al., 1998).
5. Conclusion
In the current study exploring fNIRS-based diagnosis on the effects of MPH administration to ADHD children using a double-blind, placebo-controlled, cross-over design, we presented the following findings: 1) Relative to control subjects, unmedicated ADHD children exhibited reduced brain activation in the right IFG and MFG during go/no-go task blocks. 2) The reduced activation in the right inferior and middle frontal gyri was acutely normalized after MPH administration, but not after placebo administration. 3) Compared to the placebo-induced activation, the MPH-induced right IFG/MFG activation was significantly larger. These findings led us to conclude that the activation in the right inferior and middle frontal gyri could serve as an objective neuro-functional biomarker to indicate the effects of MPH on ADHD children. fNIRS-based examination on the effect of MPH was applicable to ADHD children as young as 6 years old. This promising technique will enable the early clinical diagnosis and treatment of ADHD children.
Acknowledgements
We appreciate ELCS for the English proofreading. We thank Illpop (http://illpop.com/animal_top01.htm) for kindly providing source pictures for the experimental materials. This work was supported in part by the Grant-in-Aid for Scientific Research from the Japan Society for Promotion of Science (22242012 to ID, 23390354 to EW, 23700885 to HD, 23650217 to ID, 80382951 to YM, and 70438662 to MN), and Health and Labor Sciences Research Grants, Research on Psychiatric and Neurological Diseases and Mental Health (to ID).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Cortical activation pattern of control subject (a) and post-MPH session of ADHD subjects (b). They are shown as t-maps of oxy-Hb signal, with significant t-values (one-sample t-test, p < .05 uncorrected) being shown according to the color bar. We screened for any channels involved in the go/no-go task for the control subjects. Significant increase was found in two channels on the left hemisphere (CHs 10 and 14) and five channels on the right hemisphere (CHs 1, 5, 6, 10, and 15). Among these channels, the right CH 10 exhibited the most significant increase with a large ES (1.009), and was the only channel remaining after family-wise error correction (Bonferroni, p < 0.05). Therefore, right CH 10 was determined as a channel of interest in the main analyses. In ADHD subjects, significant MPH-effects on oxy-Hb increase were found in four channels on the right hemisphere (CHs 5, 10, 18, and 22), whereas 1st-day pre-medication and post-placebo contrast revealed no significant channel (data not shown).
References
- Altshuler L.L., Bookheimer S.Y., Townsend J., Proenza M.A., Eisenberger N., Sabb F., Mintz J., Cohen M.S. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 4th ed. American Psychiatric Association; Washington, DC: 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Anderson C.M., Polcari A., Lowen S.B., Renshaw P.F., Teicher M.H. Effects of methylphenidate on functional magnetic resonance relaxometry of the cerebellar vermis in boys with ADHD. The American Journal of Psychiatry. 2002;159:1322–1328. doi: 10.1176/appi.ajp.159.8.1322. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31:2376–2383. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Poldrack R.A. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Dowson J.H., Sahakian B.J., Robbins T.W. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2003;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- Barkley R.A. The ecological validity of laboratory and analogue assessment methods of ADHD symptoms. Journal of Abnormal Child Psychology. 1991;19:149–178. doi: 10.1007/BF00909976. [DOI] [PubMed] [Google Scholar]
- Beauregard M., Levesque J. Functional magnetic resonance imaging investigation of the effects of neurofeedback training on the neural bases of selective attention and response inhibition in children with attention-deficit/hyperactivity disorder. Applied Psychophysiology and Biofeedback. 2006;31:3–20. doi: 10.1007/s10484-006-9001-y. [DOI] [PubMed] [Google Scholar]
- Bedard A.C., Ickowicz A., Logan G.D., Hogg-Johnson S., Schachar R., Tannock R. Selective inhibition in children with attention-deficit hyperactivity disorder off and on stimulant medication. Journal of Abnormal Child Psychology. 2003;31:315–327. doi: 10.1023/a:1023285614844. [DOI] [PubMed] [Google Scholar]
- Bedard A.C., Schulz K.P., Cook E.H., Jr., Fan J., Clerkin S.M., Ivanov I., Halperin J.M., Newcorn J.H. Dopamine transporter gene variation modulates activation of striatum in youth with ADHD. NeuroImage. 2010;53:935–942. doi: 10.1016/j.neuroimage.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J., Monuteaux M.C., Spencer T., Wilens T.E., Faraone S.V. Do stimulants protect against psychiatric disorders in youth with ADHD? A 10-year follow-up study. Pediatrics. 2009;124:71–78. doi: 10.1542/peds.2008-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker M., Buecheler M.M., Schroeter M.L., Gauggel S. Prefrontal brain activation during stop-signal response inhibition: an event-related functional near-infrared spectroscopy study. Behavioural Brain Research. 2007;176:259–266. doi: 10.1016/j.bbr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Booth J.R., Burman D.D., Meyer J.R., Lei Z., Trommer B.L., Davenport N.D., Li W., Parrish T.B., Gitelman D.R., Mesulam M.M. Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD) Journal of Child Psychology and Psychiatry. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- Buchsbaum B.R., Greer S., Chang W.L., Berman K.F. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Human Brain Mapping. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabernaud C., Mennes M., Kelly C., Nooner K., Di Martino A., Castellanos F.X., Milham M.P. Dimensional brain–behavior relationships in children with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2012;71:434–442. doi: 10.1016/j.biopsych.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope M., Delpy D.T., Reynolds E.O., Wray S., Wyatt J., van der Zee P. Methods of quantitating cerebral near infrared spectroscopy data. Advances in Experimental Medicine and Biology. 1988;222:183–189. doi: 10.1007/978-1-4615-9510-6_21. [DOI] [PubMed] [Google Scholar]
- Cui X., Bray S., Bryant D.M., Glover G.H., Reiss A.L. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. NeuroImage. 2011;54:2808–2821. doi: 10.1016/j.neuroimage.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zeeuw P., Schnack H.G., van Belle J., Weusten J., van Dijk S., Langen M., Brouwer R.M., van Engeland H., Durston S. Differential brain development with low and high IQ in attention-deficit/hyperactivity disorder. PLoS One. 2012;7:e35770. doi: 10.1371/journal.pone.0035770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M., Francis D.J., Cirino P.T., Schachar R., Barnes M.A., Fletcher J.M. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derefinko K.J., Adams Z.W., Milich R., Fillmore M.T., Lorch E.P., Lynam D.R. Response style differences in the inattentive and combined subtypes of attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2008;36:745–758. doi: 10.1007/s10802-007-9207-3. [DOI] [PubMed] [Google Scholar]
- Dibbets P., Evers L., Hurks P., Marchetta N., Jolles J. Differences in feedback- and inhibition-related neural activity in adult ADHD. Brain and Cognition. 2009;70:73–83. doi: 10.1016/j.bandc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Dillo W., Goke A., Prox-Vagedes V., Szycik G.R., Roy M., Donnerstag F., Emrich H.M., Ohlmeier M.D. Neuronal correlates of ADHD in adults with evidence for compensation strategies—a functional MRI study with a Go/No-Go paradigm. German Medical Science. 2010;8:Doc09. doi: 10.3205/000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmann R.W., Wehmeier P.M., Schacht A., Minarzyk A., Lehmann M., Sevecke K., Lehmkuhl G. Atomoxetine treatment and ADHD-related difficulties as assessed by adolescent patients, their parents and physicians. Child and Adolescent Psychiatry and Mental Health. 2009;3:21. doi: 10.1186/1753-2000-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsler R., Brandeis D., Foldenyi M., Imhof K., Steinhausen H.C. The course of neuropsychological functions in children with attention deficit hyperactivity disorder from late childhood to early adolescence. Journal of Child Psychology and Psychiatry. 2005;46:824–836. doi: 10.1111/j.1469-7610.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- Durston S., Thomas K.M., Worden M.S., Yang Y., Casey B.J. The effect of preceding context on inhibition: an event-related fMRI study. NeuroImage. 2002;16:449–453. doi: 10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- Durston S., Tottenham N.T., Thomas K.M., Davidson M.C., Eigsti I.M., Yang Y., Ulug A.M., Casey B.J. Differential patterns of striatal activation in young children with and without ADHD. Biological Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Durston S., Hulshoff Pol H.E., Schnack H.G., Buitelaar J.K., Steenhuis M.P., Minderaa R.B., Kahn R.S., van Engeland H. Magnetic resonance imaging of boys with attention-deficit/hyperactivity disorder and their unaffected siblings. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:332–340. doi: 10.1097/00004583-200403000-00016. [DOI] [PubMed] [Google Scholar]
- Durston S., Davidson M.C., Mulder M.J., Spicer J.A., Galvan A., Tottenham N., Scheres A., Xavier Castellanos F., van Engeland H., Casey B.J. Neural and behavioral correlates of expectancy violations in attention-deficit hyperactivity disorder. Journal of Child Psychology and Psychiatry. 2007;48:881–889. doi: 10.1111/j.1469-7610.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- Ehlis A.C., Bahne C.G., Jacob C.P., Herrmann M.J., Fallgatter A.J. Reduced lateral prefrontal activation in adult patients with attention-deficit/hyperactivity disorder (ADHD) during a working memory task: a functional near-infrared spectroscopy (fNIRS) study. Journal of Psychiatric Research. 2008;42:1060–1067. doi: 10.1016/j.jpsychires.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Eliassen J.C., Souza T., Sanes J.N. Human brain activation accompanying explicitly directed movement sequence learning. Experimental Brain Research. 2001;141:269–280. doi: 10.1007/s002210100822. [DOI] [PubMed] [Google Scholar]
- Epstein J.N., Casey B.J., Tonev S.T., Davidson M., Reiss A.L., Garrett A., Hinshaw S.P., Greenhill L.L., Vitolo A., Kotler L.A., Jarrett M.A., Spicer J. Assessment and prevention of head motion during imaging of patients with attention deficit hyperactivity disorder. Psychiatry Research. 2007;155:75–82. doi: 10.1016/j.pscychresns.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Posner J., Nagel B.J., Bathula D., Dias T.G., Mills K.L., Blythe M.S., Giwa A., Schmitt C.F., Nigg J.T. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2010;68:1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H., Wright C.I., Whalen P.J., McInerney S.C., Shin L.M., Rauch S.L. Brain habituation during repeated exposure to fearful and neutral faces: a functional MRI study. Brain Research Bulletin. 2003;59:387–392. doi: 10.1016/s0361-9230(02)00940-1. [DOI] [PubMed] [Google Scholar]
- Frazier T.W., Demaree H.A., Youngstrom E.A. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18:543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- Garavan H., Ross T.J., Stein E.A. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H., Ross T.J., Murphy K., Roche R.A., Stein E.A. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. NeuroImage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Garavan H., Ross T.J., Kaufman J., Stein E.A. A midline dissociation between error-processing and response-conflict monitoring. NeuroImage. 2003;20:1132–1139. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- Garavan H., Hester R., Murphy K., Fassbender C., Kelly C. Individual differences in the functional neuroanatomy of inhibitory control. Brain Research. 2006;1105:130–142. doi: 10.1016/j.brainres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Grafton S.T., Mazziotta J.C., Woods R.P., Phelps M.E. Human functional anatomy of visually guided finger movements. Brain. 1992;115(Pt 2):565–587. doi: 10.1093/brain/115.2.565. [DOI] [PubMed] [Google Scholar]
- Herrmann M.J., Ehlis A.C., Fallgatter A.J. Bilaterally reduced frontal activation during a verbal fluency task in depressed patients as measured by near-infrared spectroscopy. The Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:170–175. doi: 10.1176/jnp.16.2.170. [DOI] [PubMed] [Google Scholar]
- Herrmann M.J., Plichta M.M., Ehlis A.C., Fallgatter A.J. Optical topography during a Go–NoGo task assessed with multi-channel near-infrared spectroscopy. Behavioural Brain Research. 2005;160:135–140. doi: 10.1016/j.bbr.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Hester R.L., Murphy K., Foxe J.J., Foxe D.M., Javitt D.C., Garavan H. Predicting success: patterns of cortical activation and deactivation prior to response inhibition. Journal of Cognitive Neuroscience. 2004;16:776–785. doi: 10.1162/089892904970726. [DOI] [PubMed] [Google Scholar]
- Hock C., Villringer K., Müller-Spahn F., Wenzel R., Heekeren H., Schuh-Hofer S., Hofmann M., Minoshima S., Schwaiger M., Dirnagl U., Villringer A. Decrease in parietal cerebral hemoglobin oxygenation during performance of a verbal fluency task in patients with Alzheimer's disease monitored by means of near-infrared spectroscopy (NIRS) — correlation with simultaneous rCBF-PET measurements. Brain Research. 1997;755:293–303. doi: 10.1016/s0006-8993(97)00122-4. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Sakihara K., Gunji A., Ozawa H., Kimiya S., Shinoda H., Kaga M., Inagaki M. Reduced prefrontal hemodynamic response in children with ADHD during the go/nogo task: a NIRS study. Neuroreport. 2012;23:55–60. doi: 10.1097/WNR.0b013e32834e664c. [DOI] [PubMed] [Google Scholar]
- Jourdan Moser S., Cutini S., Weber P., Schroeter M.L. Right prefrontal brain activation due to Stroop interference is altered in attention-deficit hyperactivity disorder — a functional near-infrared spectroscopy study. Psychiatry Research. 2009;173:190–195. doi: 10.1016/j.pscychresns.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Jurcak V., Tsuzuki D., Dan I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. NeuroImage. 2007;34:1600–1611. doi: 10.1016/j.neuroimage.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Karch S., Thalmeier T., Lutz J., Cerovecki A., Opgen-Rhein M., Hock B., Leicht G., Hennig-Fast K., Meindl T., Riedel M., Mulert C., Pogarell O. Neural correlates (ERP/fMRI) of voluntary selection in adult ADHD patients. European Archives of Psychiatry and Clinical Neuroscience. 2010;260:427–440. doi: 10.1007/s00406-009-0089-y. [DOI] [PubMed] [Google Scholar]
- Kiehl K.A., Liddle P.F. Reproducibility of the hemodynamic response to auditory oddball stimuli: a six-week test–retest study. Human Brain Mapping. 2003;18:42–52. doi: 10.1002/hbm.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S., Nakajima K., Uchida I., Kikyo H., Kameyama M., Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122(Pt 5):981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Kuntsi J., Eley T.C., Taylor A., Hughes C., Asherson P., Caspi A., Moffitt T.E. Co-occurrence of ADHD and low IQ has genetic origins. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2004;124B:41–47. doi: 10.1002/ajmg.b.20076. [DOI] [PubMed] [Google Scholar]
- Langenecker S.A., Nielson K.A. Frontal recruitment during response inhibition in older adults replicated with fMRI. NeuroImage. 2003;20:1384–1392. doi: 10.1016/S1053-8119(03)00372-0. [DOI] [PubMed] [Google Scholar]
- Law I., Svarer C., Holm S., Paulson O.B. The activation pattern in normal humans during suppression, imagination and performance of saccadic eye movements. Acta Physiologica Scandinavica. 1997;161:419–434. doi: 10.1046/j.1365-201X.1997.00207.x. [DOI] [PubMed] [Google Scholar]
- Liddle P.F., Kiehl K.A., Smith A.M. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubinoux I., Carel C., Alary F., Boulanouar K., Viallard G., Manelfe C., Rascol O., Celsis P., Chollet F. Within-session and between-session reproducibility of cerebral sensorimotor activation: a test–retest effect evidenced with functional magnetic resonance imaging. Journal of Cerebral Blood Flow and Metabolism. 2001;21:592–607. doi: 10.1097/00004647-200105000-00014. [DOI] [PubMed] [Google Scholar]
- Ma J., Lei D., Jin X., Du X., Jiang F., Li F., Zhang Y., Shen X. Compensatory brain activation in children with attention deficit/hyperactivity disorder during a simplified go/no-go task. Journal of Neural Transmission. 2012;119:613–619. doi: 10.1007/s00702-011-0744-0. [DOI] [PubMed] [Google Scholar]
- Maki A., Yamashita Y., Ito Y., Watanabe E., Mayanagi Y., Koizumi H. Spatial and temporal analysis of human motor activity using noninvasive NIR topography. Medical Physics. 1995;22:1997–2005. doi: 10.1118/1.597496. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Kato T., Fukuda M., Kato N. Alteration of hemoglobin oxygenation in the frontal region in elderly depressed patients as measured by near-infrared spectroscopy. The Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:465–471. doi: 10.1176/jnp.12.4.465. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Taneichi K., Matsumoto A., Ohtani T., Yamasue H., Sakano Y., Sasaki T., Sadamatsu M., Kasai K., Iwanami A., Asukai N., Kato N., Kato T. Hypoactivation of the prefrontal cortex during verbal fluency test in PTSD: a near-infrared spectroscopy study. Psychiatry Research. 2003;124:1–10. doi: 10.1016/s0925-4927(03)00093-3. [DOI] [PubMed] [Google Scholar]
- Menon V., Adleman N.E., White C.D., Glover G.H., Reiss A.L. Error-related brain activation during a go/nogo response inhibition task. Human Brain Mapping. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monden Y., Dan H., Nagashima M., Dan I., Kyutoku Y., Okamoto M., Yamagata T., Momoi M.Y., Watanabe E. Clinically-oriented monitoring of acute effects of methylphenidate on cerebral hemodynamics in ADHD children using fNIRS. Clinical Neurophysiology. 2012;123:1147–1157. doi: 10.1016/j.clinph.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Moriai-Izawa A., Dan H., Dan I., Sano T., Oguro K., Yokota H., Tsuzuki D., Watanabe E. Multichannel fNIRS assessment of overt and covert confrontation naming. Brain and Language. 2012;121:185–193. doi: 10.1016/j.bandl.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Mostofsky S.H., Simmonds D.J. Response inhibition and response selection: two sides of the same coin. Journal of Cognitive Neuroscience. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Mostofsky S.H., Schafer J.G., Abrams M.T., Goldberg M.C., Flower A.A., Boyce A., Courtney S.M., Calhoun V.D., Kraut M.A., Denckla M.B., Pekar J.J. fMRI evidence that the neural basis of response inhibition is task-dependent. Brain Research. Cognitive Brain Research. 2003;17:419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Mulligan R.C., Knopik V.S., Sweet L.H., Fischer M., Seidenberg M., Rao S.M. Neural correlates of inhibitory control in adult attention deficit/hyperactivity disorder: evidence from the Milwaukee longitudinal sample. Psychiatry Research. 2011;194:119–129. doi: 10.1016/j.pscychresns.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negoro H., Sawada M., Iida J., Ota T., Tanaka S., Kishimoto T. Prefrontal dysfunction in attention-deficit/hyperactivity disorder as measured by near-infrared spectroscopy. Child Psychiatry and Human Development. 2010;41:193–203. doi: 10.1007/s10578-009-0160-y. [DOI] [PubMed] [Google Scholar]
- Newcorn J.H., Halperin J.M., Jensen P.S., Abikoff H.B., Arnold L.E., Cantwell D.P., Conners C.K., Elliott G.R., Epstein J.N., Greenhill L.L., Hechtman L., Hinshaw S.P., Hoza B., Kraemer H.C., Pelham W.E., Severe J.B., Swanson J.M., Wells K.C., Wigal T., Vitiello B. Symptom profiles in children with ADHD: effects of comorbidity and gender. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:137–146. doi: 10.1097/00004583-200102000-00008. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Dan I. Automated cortical projection of head-surface locations for transcranial functional brain mapping. NeuroImage. 2005;26:18–28. doi: 10.1016/j.neuroimage.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Dan H., Sakamoto K., Takeo K., Shimizu K., Kohno S., Oda I., Isobe S., Suzuki T., Kohyama K., Dan I. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. NeuroImage. 2004;21:99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Dan H., Shimizu K., Takeo K., Amita T., Oda I., Konishi I., Sakamoto K., Isobe S., Suzuki T., Kohyama K., Dan I. Multimodal assessment of cortical activation during apple peeling by NIRS and fMRI. NeuroImage. 2004;21:1275–1288. doi: 10.1016/j.neuroimage.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Matsunami M., Dan H., Kohata T., Kohyama K., Dan I. Prefrontal activity during taste encoding: an fNIRS study. NeuroImage. 2006;31:796–806. doi: 10.1016/j.neuroimage.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Peterson B.S., Potenza M.N., Wang Z., Zhu H., Martin A., Marsh R., Plessen K.J., Yu S. An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. The American Journal of Psychiatry. 2009;166:1286–1294. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak R.H., Mollica C.M., Maruff P., Snyder P.J. Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. Neuroscience and Biobehavioral Reviews. 2006;30:1225–1245. doi: 10.1016/j.neubiorev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Rorden C., Brett M. Stereotaxic display of brain lesions. Behavioural Neurology. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rubia K., Overmeyer S., Taylor E., Brammer M., Williams S.C., Simmons A., Bullmore E.T. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. The American Journal of Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Rubia K., Russell T., Overmeyer S., Brammer M.J., Bullmore E.T., Sharma T., Simmons A., Williams S.C., Giampietro V., Andrew C.M., Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. NeuroImage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Brammer M.J., Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Brammer M.J., Toone B., Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. The American Journal of Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Rubia K., Halari R., Cubillo A., Mohammad A.M., Brammer M., Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Sakatani K., Lichty W., Xie Y., Li S., Zuo H. Effects of aging on language-activated cerebral blood oxygenation changes of the left prefrontal cortex: Near infrared spectroscopy study. Journal of Stroke and Cerebrovascular Diseases. 1999;8:398–403. doi: 10.1016/s1052-3057(99)80047-0. [DOI] [PubMed] [Google Scholar]
- Schecklmann M., Ehlis A.C., Plichta M.M., Romanos J., Heine M., Boreatti-Hummer A., Jacob C., Fallgatter A.J. Diminished prefrontal oxygenation with normal and above-average verbal fluency performance in adult ADHD. Journal of Psychiatric Research. 2008;43:98–106. doi: 10.1016/j.jpsychires.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Schecklmann M., Romanos M., Bretscher F., Plichta M.M., Warnke A., Fallgatter A.J. Prefrontal oxygenation during working memory in ADHD. Journal of Psychiatric Research. 2010;44:621–628. doi: 10.1016/j.jpsychires.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Schulz K.P., Fan J., Tang C.Y., Newcorn J.H., Buchsbaum M.S., Cheung A.M., Halperin J.M. Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: an event-related FMRI study. The American Journal of Psychiatry. 2004;161:1650–1657. doi: 10.1176/appi.ajp.161.9.1650. [DOI] [PubMed] [Google Scholar]
- Sebastian A., Gerdes B., Feige B., Kloppel S., Lange T., Philipsen A., Tebartz van Elst L., Lieb K., Tuscher O. Neural correlates of interference inhibition, action withholding and action cancelation in adult ADHD. Psychiatry Research. 2012;202:132–141. doi: 10.1016/j.pscychresns.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Shattuck D.W., Mirza M., Adisetiyo V., Hojatkashani C., Salamon G., Narr K.L., Poldrack R.A., Bilder R.M., Toga A.W. Construction of a 3D probabilistic atlas of human cortical structures. NeuroImage. 2008;39:1064–1080. doi: 10.1016/j.neuroimage.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinba T., Nagano M., Kariya N., Ogawa K., Shinozaki T., Shimosato S., Hoshi Y. Near-infrared spectroscopy analysis of frontal lobe dysfunction in schizophrenia. Biological Psychiatry. 2004;55:154–164. doi: 10.1016/s0006-3223(03)00547-x. [DOI] [PubMed] [Google Scholar]
- Simmonds D.J., Pekar J.J., Mostofsky S.H. Meta-analysis of go/no-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.K., Okamoto M., Dan H., Jurcak V., Dan I. Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. NeuroImage. 2005;27:842–851. doi: 10.1016/j.neuroimage.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Siniatchkin M., Glatthaar N., von Muller G.G., Prehn-Kristensen A., Wolff S., Knochel S., Steinmann E., Sotnikova A., Stephani U., Petermann F., Gerber W.D. Behavioural treatment increases activity in the cognitive neuronal networks in children with attention deficit/hyperactivity disorder. Brain Topography. 2012;25:332–344. doi: 10.1007/s10548-012-0221-6. [DOI] [PubMed] [Google Scholar]
- Slifer K.J., Koontz K.L., Cataldo M.F. Operant-contingency-based preparation of children for functional magnetic resonance imaging. Journal of Applied Behavior Analysis. 2002;35:191–194. doi: 10.1901/jaba.2002.35-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.B., Taylor E., Brammer M., Toone B., Rubia K. Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. The American Journal of Psychiatry. 2006;163:1044–1051. doi: 10.1176/ajp.2006.163.6.1044. [DOI] [PubMed] [Google Scholar]
- Solanto M.V., Schulz K.P., Fan J., Tang C.Y., Newcorn J.H. Event-related FMRI of inhibitory control in the predominantly inattentive and combined subtypes of ADHD. Journal of Neuroimaging. 2009;19:205–212. doi: 10.1111/j.1552-6569.2008.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T.J. ADHD treatment across the life cycle. The Journal of Clinical Psychiatry. 2004;65(Suppl. 3):22–26. [PubMed] [Google Scholar]
- Strangman G., Boas D.A., Sutton J.P. Non-invasive neuroimaging using near-infrared light. Biological Psychiatry. 2002;52:679–693. doi: 10.1016/s0006-3223(02)01550-0. [DOI] [PubMed] [Google Scholar]
- Suto T., Fukuda M., Ito M., Uehara T., Mikuni M. Multichannel near-infrared spectroscopy in depression and schizophrenia: cognitive brain activation study. Biological Psychiatry. 2004;55:501–511. doi: 10.1016/j.biopsych.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Tamm L., Menon V., Ringel J., Reiss A.L. Event-related fMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- Tannock R., Schachar R., Logan G. Methylphenidate and cognitive flexibility: dissociated dose effects in hyperactive children. Journal of Abnormal Child Psychology. 1995;23:235–266. doi: 10.1007/BF01447091. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Anderson C.M., Polcari A., Glod C.A., Maas L.C., Renshaw P.F. Functional deficits in basal ganglia of children with attention-deficit/hyperactivity disorder shown with functional magnetic resonance imaging relaxometry. Nature Medicine. 2000;6:470–473. doi: 10.1038/74737. [DOI] [PubMed] [Google Scholar]
- Tsujii T., Sakatani K., Nakashima E., Igarashi T., Katayama Y. Characterization of the acute effects of alcohol on asymmetry of inferior frontal cortex activity during a go/no-go task using functional near-infrared spectroscopy. Psychopharmacology. 2011;217:595–603. doi: 10.1007/s00213-011-2318-0. [DOI] [PubMed] [Google Scholar]
- Tsuzuki D., Jurcak V., Singh A.K., Okamoto M., Watanabe E., Dan I. Virtual spatial registration of stand-alone fNIRS data to MNI space. NeuroImage. 2007;34:1506–1518. doi: 10.1016/j.neuroimage.2006.10.043. [DOI] [PubMed] [Google Scholar]
- Tsuzuki D., Cai D.S., Dan H., Kyutoku Y., Fujita A., Watanabe E., Dan I. Stable and convenient spatial registration of stand-alone NIRS data through anchor-based probabilistic registration. Neuroscience Research. 2012;72:163–171. doi: 10.1016/j.neures.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Vaidya C.J., Austin G., Kirkorian G., Ridlehuber H.W., Desmond J.E., Glover G.H., Gabrieli J.D. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya C.J., Bunge S.A., Dudukovic N.M., Zalecki C.A., Elliott G.R., Gabrieli J.D. Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. The American Journal of Psychiatry. 2005;162:1605–1613. doi: 10.1176/appi.ajp.162.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasic, N., Plichta, M.M., Wolf, R.C., Fallgatter, A.J., Sosic-Vasic, Z., Gron, G., in press. Reduced neural error signaling in left inferior prefrontal cortex in young adults with ADHD. Journal of Attention Disorders. [DOI] [PubMed]
- Weber P., Lutschg J., Fahnenstich H. Cerebral hemodynamic changes in response to an executive function task in children with attention-deficit hyperactivity disorder measured by near-infrared spectroscopy. Journal of Developmental and Behavioral Pediatrics. 2005;26:105–111. doi: 10.1097/00004703-200504000-00005. [DOI] [PubMed] [Google Scholar]
- Wehmeier P.M., Schacht A., Wolff C., Otto W.R., Dittmann R.W., Banaschewski T. Neuropsychological outcomes across the day in children with attention-deficit/hyperactivity disorder treated with atomoxetine: results from a placebo-controlled study using a computer-based continuous performance test combined with an infra-red motion-tracking device. Journal of Child and Adolescent Psychopharmacology. 2011;21:433–444. doi: 10.1089/cap.2010.0142. [DOI] [PubMed] [Google Scholar]
- Wilens T.E. Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. Journal of Clinical Psychopharmacology. 2008;28:S46–S53. doi: 10.1097/JCP.0b013e318173312f. [DOI] [PubMed] [Google Scholar]
- Yerys B.E., Jankowski K.F., Shook D., Rosenberger L.R., Barnes K.A., Berl M.M., Ritzl E.K., Vanmeter J., Vaidya C.J., Gaillard W.D. The fMRI success rate of children and adolescents: typical development, epilepsy, attention deficit/hyperactivity disorder, and autism spectrum disorders. Human Brain Mapping. 2009;30:3426–3435. doi: 10.1002/hbm.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.Z., Zang Y.F., Cao Q.J., Yan C.G., He Y., Jiang T.Z., Sui M.Q., Wang Y.F. Fisher discriminative analysis of resting-state brain function for attention-deficit/hyperactivity disorder. NeuroImage. 2008;40:110–120. doi: 10.1016/j.neuroimage.2007.11.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cortical activation pattern of control subject (a) and post-MPH session of ADHD subjects (b). They are shown as t-maps of oxy-Hb signal, with significant t-values (one-sample t-test, p < .05 uncorrected) being shown according to the color bar. We screened for any channels involved in the go/no-go task for the control subjects. Significant increase was found in two channels on the left hemisphere (CHs 10 and 14) and five channels on the right hemisphere (CHs 1, 5, 6, 10, and 15). Among these channels, the right CH 10 exhibited the most significant increase with a large ES (1.009), and was the only channel remaining after family-wise error correction (Bonferroni, p < 0.05). Therefore, right CH 10 was determined as a channel of interest in the main analyses. In ADHD subjects, significant MPH-effects on oxy-Hb increase were found in four channels on the right hemisphere (CHs 5, 10, 18, and 22), whereas 1st-day pre-medication and post-placebo contrast revealed no significant channel (data not shown).


