Abstract
Cellular reprogramming was recently “crowned” with the award of the Nobel Prize to two of its groundbreaking researchers, Sir John Gurdon and Shinya Yamanaka. The recent link between reprogramming and stem cells makes this appear almost a new field of research, but its historical roots have actually spanned more than a century. Here, the Nobel Prize in Physiology or Medicine 2012 is placed in its historical context.
Main Text
Introduction
Research is a gradual process offering flashes of brilliance and occasionally much more, as reward for tenacity. The physicist/historian/philosopher Thomas Kuhn described scientific advance as a series of interrelated bodies of work wherein discovery builds upon discovery (Kuhn, 1970). Kuhn articulated the vexation that arises when attempting to assign priority among scientists for a given breakthrough; one example considered whether it was Priestley or Lavoisier who legitimately “discovered” oxygen (you be the judge). The incremental nature of investigation also proves difficult when seeking to pin down the exact timing that an individual discovery was made: the so-called “Eureka moment.” Here, Kuhn discussed Roentgen’s work leading to the description of X-rays and the inability to define the moment of discovery along the trajectory of that research. Stem cell research is no less a product of cumulative, integrated effort between and within laboratories. Truly, experiencing the collaborative nature of research is among the greatest pleasures in a scientific career.
That said, there are bright lines in the history of any field, moments in which a particular observation drew away the curtain and set researchers on an exciting new course. In the 112 years since its inception, the Nobel Prize in Physiology or Medicine has recognized the contributions of luminaries within their respective disciplines. Pavlov, Cajal, Fleming, Luria, McClintock, Krebs, and many others, some of whom will be discussed below, were joined in 2012 by Sir John B. Gurdon and Shinya Yamanaka in recognition of their groundbreaking work showing that “…mature cells can be reprogrammed to become pluripotent” (2012 Nobel Prize winners in medicine, http://www.nobelprize.org/nobel_prizes/medicine/laureates/2012/) (Figure 1). Gurdon and Yamanaka’s work mark a new beginning in the study of development, cellular lineage determination, and our understanding of epigenesis. This review will briefly summarize milestones in the field’s history leading to the 2012 Nobel and offer a reading of tea leaves regarding things to come (for an abbreviated timeline, see Table S1 available online).
Figure 1.

Winners of the 2012 Nobel Prize in Physiology or Medicine: Sir John B. Gurdon and Shinya Yamanaka
The photo was taken at the ISSCR-Roddenberry International Symposium on Cellular Reprogramming only 10 days after the announcement of the laureates for 2012. Photo credit: Chris Goodfellow/Gladstone Institutes.
Before the Beginning
As Kuhn might well have observed, the question “When did stem cell research begin”? is interesting to ponder but difficult to answer. A response depends in part upon defining what one considers as stem cell research. What is clear is that the notion of replacing, repairing, or even regrowing damaged body parts is rooted in antiquity.
Although Aeschylus often receives the credit in his fifth century work Prometheus Bound, it was actually in the eighth century B.C. work Theogony that the Greek poet Hesiod first described the legend of Prometheus who gave fire to humans and was punished by Zeus by being chained to a rock so that a large eagle could swoop in and devour his liver. The cruelty of Prometheus’ sentence was compounded by the fact that his liver would fully regenerate by the next day so that the punishment could be repeated. What makes this ancient story incredible is that the liver actually has a tremendous capacity for postresection repair in which over 70% may be surgically removed only to regenerate (for review, see Duncan et al., 2009).
In the third century A.D., the twin brothers Damian and Cosmas, later Patron Saints of Physicians, would achieve fame (and martyrdom) by working as healers free of charge. Among their purported deeds was the successful grafting of an entire leg from one person onto another. To the modern reader, this procedure went so far as to include a form of cellular lineage tracing given that the transplanted leg bore dark skin, whereas the recipient’s flesh tone was white. Regardless of the veracity of stories such as these, the point remains that for a very long time, humankind has understood the concept of replacing diseased or damaged tissue with healthy counterparts. It is remarkable to note that of late, face, hand, even limb transplantations have actually taken place.
The years prior to the dawn of the 19th century brought additional advances; no doubt considered unrelated at the time but when looking back with the perfect vision of hindsight, nevertheless define a continuum of discovery leading to the 2012 Nobels. Among these are the first publications during the Renaissance describing human teratomas, benign tumors bearing representative tissues from all three somatic germ layers: ectoderm, mesoderm, and endoderm (e.g., Birch and Tyson, 1683; Scultetus, 1658; Yonge, 1706).
Today, we understand teratomas to derive from germ cell precursors (Teilum, 1965), arising primarily within the gonad of both sexes but also occurring throughout the mediastinum given the migratory route of primordial germ cells prior to their arrival in the genital ridge during embryogenesis (Witschi, 1948). Given their three germ layer composition, the tumor-initiating cell of a teratoma is termed “pluripotent” or capable of forming all tissue types found in the adult soma (for review, see Lensch et al., 2007). Flashing forward to the mid-1950s, it was Leroy Stevens working at the Jackson Laboratory who noted that the low frequency of testicular teratoma present in the inbred 129 mouse strain had a genetic basis that might be capable of amplification to the point of study at the cellular level (Stevens and Little, 1954). Stevens’ work would link the descriptive studies of mid-17th century medical curiosities to the clonal isolation of the first pluripotent stem cells in mice: the embryonal carcinoma (EC) cell (Kleinsmith and Pierce, 1964) (Figure 2), which has served as an invaluable resource capable of culture in vitro (Martin and Evans, 1974) and permitting investigators to probe many mysteries of early development (for review, see Andrews, 2002). More on pluripotency momentarily.
Figure 2.
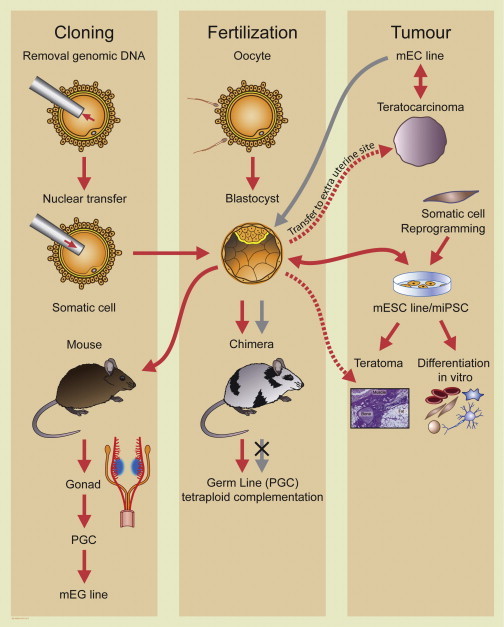
Relationships between Pluripotent Stem Cells and Embryos: 50 Years of History in Mice
Pluripotent stem cells can arise from NT-derived (cloned) blastocysts, fertilized embryos or teratocarcinomas, spontaneous tumors of the testis, or tumors induced by transferring early embryos to extrauterine sites. ESCs and EC cells will form chimeras if introduced into preimplantation embryos that are transferred to a pseudopregnant female mother. ESCs will be chimeric in the germline and give rise to sperm and eggs, but EC cells do not chimerize the germline. A less stringent test for pluripotency of ESCs than germline contribution is the ability to form benign teratomas after injection in immune-deficient mice. This test is also used to demonstrate pluripotency in human ESCs. A more stringent test is “tetraploid complementation,” where the entire postnatal animal is ESC derived. Teratocarcinomas are thought to derive spontaneously from deregulated primordial germ cells (PGCs) that give rise to the gametes. Pluripotent stem cell lines can also be derived as embryonic germ (EG) cells directly from PGCs. mEC, mouse embryonal carcinoma; mESC, mouse embryonic stem cell; miPSC, mouse induced pluripotent stem cell; mEG, mouse embryonic germ.
The Renaissance also marked the first medically related transfer of cells into a human patient, the unfortunate Mr. Arthur Coga, in the form of blood transfusions using a rather surprising donor: a young sheep (Lower and King, 1667). The invention of this procedure also launched a furious priority of discovery battle between French and English physicians that played out within the pages of the Philosophical Transactions for several issues, despite the fact that animal-into-human blood transfusion proved to be a disappointing clinical practice.
Moving ahead less than 100 years, experiments began to be much better defined. Some regard Abraham Trembley as the legitimate forbearer of regeneration research (see Parson, 2004). A winner of the Copley Medal of the Royal Society of London in the year 1743 in recognition of his investigations of freshwater hydrozoans, Trembley would publish his master work in 1744 that detailed the hydra’s regenerative capacity following experimental dissections of tremendous variety (Trembley, 1744). The work set the stage for the fledgling field of experimental zoology in general and the empirical study of regeneration in particular.
Down the Rabbit Hole
1797 was a banner year in developmental biology. Cruikshank published his description of developing staged embryos in vivo within the rabbit fallopian tubes and uterus extending to the early somite stages (Cruikshank, 1797). The work within the Cruikshank paper was performed nearly 20 years prior to publication and stands as a milestone in the field of embryology. The study was facilitated in part by mentoring and funding from his senior colleague, the renowned scientist and surgeon John Hunter. The study would not have been possible but for improvements in optics, and earlier works detailing the features of the mammalian reproductive system. Cruikshank’s paper relies upon and cites prior studies, some in Latin, by Leuwenhoek, Harvey, and De Graaf, among others. It also highlights the importance of using appropriate model organisms in research when seeking to better understand the complexities of mammalian embryonic development.
It was the research of yet another rabbit fancier, Walter Heape, that profoundly altered scientific views on gestation and development and in a manner that runs counter to his present scientific obscurity. Working at Cambridge in the 1890s, Heape performed the first live-embryo transfer experiment when he mated purebred Angora rabbits (with white, fluffy fur), isolated the developing embryos 32 hr later at the four-cell stage, and placed them into the distal end of the fallopian tube of a purebred, Belgian rabbit doe mated for the first time only 3 hr earlier to a purebred Belgian buck (a breed with short, brown fur) (Heape, 1890).
The thinking of the day suggested that the uterine environment of the Belgian might have an inductive effect on the transferred embryos, perhaps contributing characteristics in a horizontal manner, conforming to the views of Lamarck among others, to the gestating Angoras provided they grew in the foster uterus at all. Heape’s paper of barely two pages likely caused a stir at the Royal Society when it reported the live birth of four Belgian offspring and two undeniable Angoras, the exact number he had transferred. Heape painstakingly built upon these studies, becoming an aficionado of artificial insemination techniques as he focused his later efforts on estrus. His work in some ways sounded the starter’s pistol for later research into entities such as embryonic chimeras and the derivation and culture of mammalian embryonic cell lines in vitro.
Heape was also a contemporary of August Weismann who would not only deliver the coup de grâce to the Lamarckian concept of the transmissibility of acquired characteristics but who would also throw down the proverbial gauntlet within the field to experimentally define the genetic basis of developmental specification within a growing organism.
Nuclear Equivalence—The Sine Qua Non of Cellular Reprogramming
The University of Freiburg’s August Weismann was an intellectual giant and champion of the germ-plasm theory, which states that characteristics are inherited only from cells in the germline, not the soma (Weismann, 1893). His 1889 landmark publication falsified Lamarck’s view that acquired characteristics, such as somatic mutilations, would be inherited by the offspring of the afflicted animal (Weismann, 1889). To prove this, Weismann performed a simple experiment: he cut off the tails of seven female and five male white mice and then mated them to one another. When their offspring were born, he measured their tail length, recorded it, and then snipped their tails as well. These F1s were raised to adulthood, bred, and their offspring were treated in the same manner. The process was repeated for five generations and a total of 901 mice. Despite his efforts, Weismann found that tail length did not decline, and whereas he would not state that it never could if there were an infinite number of iterations, he confidently concluded that over the span of a few generations, acquired mutilations to the soma had no measurable heritability. Whatever shaped subsequent generations, it came from the gametes alone.
Turning his attention to development, he then asked a related question: How does the cellular diversity present within a complicated multicellular organism arise from a single starting cell? Others had long wondered the same thing, and among the more prevalent theories was that of preformation that described an “unfolding” of structures present a priori: many small but incomplete individuals in the gametes that grew larger during development. Such a notion was in tension with tenets of the germ-plasm theory given that determinants must be present within the dividing zygote that would be allocated only to the germline and not the somatic cells. Weismann proposed that as the early embryo cleaved, the genes were divided among daughter cells, with the possible exception of the germline that would by necessity contain an entire complement (termed the idioplasm), and that this series of “qualitative divisions” was the basis of cellular lineage specification. The mechanisms by which this segregation would take place were difficult to envision, and noted biologists, including Theodor Boveri, were quick to point this out along with additional criticisms. However, such a theory had also been proposed by the experimentalist Wilhelm Roux, who set out to test the hypothesis.
Roux reasoned that if qualitative division accounted for different developmental trajectories within an embryo, then early removal of individual cells should prohibit formation of an entire organism. He tested this by pricking one cell of a two-celled frog embryo using a heated needle. Roux found that this procedure compromised the developmental capacity of entire embryos in support of the qualitative division theory (Roux, 1888). Work by others, including Thomas Hunt Morgan (Nobel Prize, 1933) (Morgan, 1895), arrived at similar conclusions though, importantly, would also suggest that experimental artifacts, such as whether or not one left the damaged cell in contact with the remaining intact cell, urged additional experiments. Among those taking up the question but employing alternative approaches were Oscar Hertwig, Hermann Endres, Amedeo Herlitzka, and Hans Driesch (see Spemann, 1938).
Driesch used a different model organism, the sea urchin, and a new technique to disaggregate the blastomeres at the two-cell stage. Employing the method of calcium-depleted sea water devised by the embryologist Curt Herbst, the sea urchin blastomeres were easily separated from one another following gentle agitation and developed into two complete organisms (Driesch, 1891). Not only was this perhaps the first cloning experiment, it also disagreed with Weismann and Roux.
Yet, another approach and model organism would provide the most convincing evidence that Weismann’s theory was likely incorrect. Hans Spemann (Nobel Prize, 1935), also of the University of Freiburg, and his colleague Hilde Mangold were dedicated experimentalists interested in a wide variety of developmental phenomenon ranging from eye formation to early embryonic organizers and patterning. The work of Roux et al. was of great interest to Spemann, and he entered the fray using fertilized eggs of the common newt, Triton taeniatus. He also turned to an experimental approach developed by Oscar Hertwig, namely the use of thin, flexible fibers (ranging from silk threads to the hair from a baby’s head in practice) to constrict developing embryos into halves. Using this method and building upon earlier attempts by Endres and Herlitzka, Spemann was the first to clone a developing vertebrate (via “forced-twinning,” if you will) when he published results demonstrating the complete development of newts originating from the same egg (Spemann, 1928). Spemann’s experiment drove the nails into the coffin of Weismann and Roux’s position. The work suggested that the complement of genes in the various cells of developing organisms was the same, a concept termed “nuclear equivalence.” Although Spemann’s experiment fails to explain exactly how cellular lineage specification does occur, it rather importantly shows how it does not. The qualitative division theory was out. Thanks to Spemann’s work, we now know that developmental changes arise by epigenesis: the selective restriction of gene expression from among the entire genomic complement present within the many cell and tissue types in the body. Later investigators including the University of Edinburgh’s Conrad Hal Waddington would eloquently theorize about the effect of epigenetic restriction on cellular identity (Waddington, 1957). Defining the molecular details of lineage specification remains at the cutting edge of current science.
Ten years later, in his classical work Embryonic Development and Induction (Spemann, 1938), Spemann would issue marching orders to the next wave of researchers seeking to further test the validity of nuclear equivalence when he wrote (on page 211):
Decisive information about this question may perhaps be afforded by an experiment which appears, at first sight, to be somewhat fantastical … Probably the same effect could be attained if one could isolate the nuclei of the morula and introduce one of them into an egg or an egg fragment without an egg nucleus … This experiment might possibly show that even nuclei of differentiated cells can initiate normal development in the egg protoplasm.
Why didn’t Spemann attempt the experiment himself? The answer is that whereas he had ideas for how to isolate nuclei by grinding cells between glass slides, he did not know how to transfer a free nucleus into another cell. The idea would have to wait 14 years to be taken up in earnest by two investigators from Philadelphia.
When Fantasy Becomes Reality
The story goes that Robert Briggs had not heard of Spemann’s “fantastical” idea. However, a senior colleague of his, the Drosophila geneticist Jack Schultz (who himself had been a student of Morgan’s), brought the experiment to his attention. Briggs invited a young fellow, the embryologist Thomas J. King, to join him, and together with technical assistance from Marie DiBerardino, they put Spemann’s proposal to the test. Like Weismann’s determination that the conclusions made from his tail clipping experiments could not be extrapolated beyond the number of generations he had actually tested, Spemann likewise knew that his own data regarding nuclear equivalence extended only as far as the developmental stage of the embryos he had used. Was it possible that at some later developmental stage nuclear equivalence might be invalid? This was the hypothesis tested in Briggs and King’s nuclear transfer (NT) studies.
To develop the NT method, the model organism of choice for the majority of the work was the frog Rana pipiens, though among the many clever components in the paper was the intentional construction of R. pipiens/Rana catesbeiana hybrid nuclei as a validation of the transfer procedure (Briggs and King, 1952). The recipient egg is activated via needle prick, which causes the cytoplasm to rotate and enables the aspiration of the pronucleus with a glass needle. The jelly coating of the egg is then removed, and attention turns to obtaining donor nuclei. Animal pole cells within the donor blastula are individually dissected away from the mass so that single cells may be drawn into a glass pipette with an inner diameter less than that of the donor cell. Drawing the donor cell into the narrow pipette causes it to rupture, at which point it is injected into the recipient-enucleated egg.
The investigators obtained nuclei from the blastula stage of development when the cleaving structure contains thousands of cells. Their data indicate that of 194 eggs injected, 104 cleaved (52.8%), and 63 of these (60.6%) went on to reform complete blastulae. What is more, of 50 complete, “reconstituted” blastulae that were allowed to develop beyond the stage from which the nuclei had been obtained, roughly three-quarters completed normal gastrulation, and half of these went on successfully beyond the neurula stage, the point at which the neural tube forms. Thus, not only was nuclear equivalence maintained at a stage of organismal development containing thousands of cells, but the nuclei also remained fully capable of guiding integrated development onward in the majority of cases.
Caveats of the work, also discussed by the authors, include the transfer of a small amount of blastula cell cytoplasm along with the donor nucleus, which may have influenced the experimental outcome, perhaps by diluting the much greater volume of egg cytoplasm. Also, and as similarly observed by other experimentalists mentioned above, the interpretations of the work extended only so far as the age of the donor nuclei employed. The use of blastula nuclei, and not those from later stages of development, was intentional in the Briggs and King study because they wished to determine the efficiency of the technique using nuclei from an undifferentiated cell type bearing a high probability for supporting full development. Their goal in 1952 was not to see how far they could push the system but whether it would work at all. Still, the study was a tour de force, and the establishment of the NT technique would permit others to ask even bolder questions regarding nuclear equivalence. Among the earliest investigators attempting NT was a group at the University of Oxford that used it in another species of frog, Xenopus laevis (Fischberg et al., 1958). That team included a young graduate student named John Gurdon (Nobel Prize, 2012).
NT Comes of Age
Gurdon produced a cavalcade of high-impact work using NT to investigate the developmental potency of differentiating nuclei. Among these studies was the demonstration that despite a low frequency of success, highly specialized and differentiated cells from tissues such as the intestinal tract maintained the ability to complement the lost potential of the enucleated egg (Gurdon, 1962). Such NT-derived frogs, proven to be entirely donor nucleus derived via cellular lineage tracing, could even developmentally progress to the point of fertility provided that a “serial transplantation” scheme was employed in which NT embryos derived from intestinal cell donors were permitted to develop to the blastulae stage and then used in a second round of NT. In this subsequent stage, NT blastula-derived nuclei were obtained for another round of NT from which embryos were allowed to develop to adulthood and tested for reproductive capacity (Gurdon and Uehlinger, 1966). The correlation between declining nuclear potency and increasing developmental maturity of donor cells was another key insight (Gurdon, 1960). Despite the inefficiency of NT, the technique could be used to generate functioning organisms from additional types of differentiated cells as nuclear donors. Tissues as developmentally mature as keratinocytes (Gurdon et al., 1975) and lymphocytes (Wabl et al., 1975) would nevertheless prove capable of complementing enucleated frog eggs in rare cases.
Gurdon’s pioneering work paved the way for a wealth of studies demonstrating that even mammals like sheep (Campbell et al., 1996; Wilmut et al., 1997) and mice (Wakayama et al., 1998, 2000) could be cloned. In fact, it is important to point out that in examples such as Dolly the sheep (the first mature mammal to be directly cloned in a single round of NT; Wilmut et al., 1997), and the first cloned mice (Wakayama et al., 1998), the transferred nuclei were restored or reprogrammed to totipotency, i.e., the ability to form not only all of the cells of the adult organism (as is the case for pluripotency) but also the entire cadre of extraembryonic tissues including the trophectoderm of the placenta.
Later studies would sharpen the nuclear equivalency point by demonstrating that cells as fully differentiated as murine B and T lymphocytes were capable of producing monoclonal mice following a multistep procedure wherein NT was performed to generate blastocysts from which embryonic stem cells (ESCs) were derived that in turn were used to chimerize diploid or tetraploid embryos (Hochedlinger and Jaenisch, 2002) (Figure 2). In the case of tetraploid complementation, murine embryos fused at the two-cell stage (yielding a 4n embryo, which contains four haploid genome equivalents) will support the growth of the trophectoderm, but not the inner cell mass of the embryo from which the embryonic mouse arises; transferring diploid (2n) pluripotent stem cells into 4n blastocysts complements their inability to complete development (Nagy et al., 1990). In the case of the tetraploid studies, the lymphocyte origin of the resulting animals was verified by immunoglobulin gene rearrangement signatures in all tissues. Even the nuclei of sensory neurons retain the rare ability to produce mice via a similar, multistep approach and tetraploid complementation, where once again, the origins of the cells in the resulting mice were verified via cellular lineage tracing (Eggan et al., 2004).
A good experiment generates questions as well as answers. Although decades of work provide overwhelming support to the validity of nuclear equivalence, they fail to explain why NT works at all. What is the mechanism by which the egg cytoplasm “instructs” the incoming nucleus to reset its epigenetic state to a much earlier form? What factors are involved? What are the central genetic regulators of pluripotency or even totipotency? Although many have pondered these same questions, it would be investigators working at Kyoto University in the mid-2000s who offered some rather provocative responses. Before we get to that, it is worth dipping back briefly into the history books once again.
The First Isolation of Native ESCs
Going back to the mouse experiments on teratomas mentioned earlier and the isolation of pluripotent EC cells, it was known that despite being obtained from abnormal tissue growths (see Lensch and Ince, 2007), EC could nevertheless contribute to the soma once transferred into normal embryos (Brinster, 1974). It was a natural step to then consider whether or not pluripotent cells were capable of isolation from normal tissues, i.e., the early, preimplantation embryo. The answer to this question was a resounding “yes,” and in 1981, Martin Evans (Nobel Prize, 2007) and Matthew Kaufman from the University of Cambridge (Evans and Kaufman, 1981) and their colleague Gail Martin from the University of California-San Francisco (Martin, 1981) independently published papers describing the generation, extended culture, and differentiation capacity of lines of ESCs.
For Evans’ ESC isolations from the 129 mouse strain, a state of diapause or arrest of embryonic development (for review, Lopes et al., 2004) was imposed via ovariectomy 2.5 days after mating. This caused embryos hatched from the zona pellucida to increase their cell numbers somewhat without implantation prior to recovery less than 1 week later. Explanted blastocysts were then cocultured on a feeder layer of immortalized murine fibroblast STO cells in serum-containing media, yielding lines of cells resembling EC cells but with a normal karyotype. The investigators also demonstrated their developmental capacity via in vitro differentiation as cystic embryoid bodies, teratoma formation in vivo, and, though not detailed in this first publication, mouse chimeras.
Investigators have long been able to also obtain and study the gametes and developing concepti of many other species including humans (e.g., Jordan, 1918). Extensive study of ovulation, fertilization, and embryo transfer (for review, see Biggers, 2012; Johnson, 2010) would prove capable of clinical application when Patrick Steptoe and Robert Edward (Nobel Prize, 2010) assisted the formerly childless Brown family to bring Louise into the world; the first human being in history to arise via in vitro fertilization (IVF) (Steptoe and Edwards, 1978).
In 1994, Arif Bongso, an IVF specialist at the University Hospital of Singapore, managed to obtain stem cell-like colonies from surplus IVF embryos (Bongso et al., 1994) but had little experience in culture of pluripotent cells or access to their markers so was unable to establish lines and prove their identity. However, in 1998, James A. Thomson and colleagues from the University of Wisconsin demonstrated that stem cell colonies could likewise be obtained by culturing human embryos, which were generated by IVF for implantation but then donated to research (Thomson et al., 1998). Thomson’s group managed to establish these colonies as cell lines. The culture conditions used were similar to those employed for their murine counterparts. A total of 14 inner cell masses were obtained, and five distinct lines of human ESCs arising from five different embryos were derived. Each had a normal karyotype and proved capable of teratoma formation in immunodeficient murine hosts. Both murine and human ESCs express a variety of markers similar to proteins found in EC cells as well as normal cells present in the early embryo, including TRA-1-60, various stage-specific embryonic antigens (SSEAs), and alkaline phosphatase. ESCs also express telomerase and maintain telomere length provided they are cultured in conditions supporting the maintenance of pluripotency, which for murine ESCs, includes culture medium containing leukemia-inhibitory factor or LIF (Smith et al., 1988; Williams et al., 1988).
Beyond the value of their contribution to the growing lexicon of species from which ESCs might be derived, the generation of human ESCs permitted study of the earliest stages of human development in an empirical, hypothesis-driven manner. Never before had it been possible to study human tissue genesis, from the very first stages of uncommitted precursor cells through the elaboration of differentiated cell types, as it happened in vitro. Furthermore, if combined with NT in a platform where the donor nuclei were obtained from patient biopsies bearing genetic disease, then one might additionally be able to probe the impact of disease-causing genetic lesions on development or even use the technology to define how to regenerate “matched” tissue for direct replacement as a cellular therapy. As such, it is impossible to overstate the excitement, potential impact, and value of human ESCs to the study of human development, disease, and decay.
Following years of study, human NT was finally successful provided the egg pronucleus was left in place; lines of human NT-derived ES cells were derived albeit containing triploid genomes (Noggle et al., 2011). However, while this review was in press, human cellular reprogramming studies took a leap forward when the laboratory of Shoukhrat Mitalipov at Oregon Health & Science University published the highly efficient derivation of multiple lines of diploid hESC via NT, a process that was successful (in part) due to the use of 1.25 mM caffeine to protect oocytes from premature activation during spindle removal (Tachibana et al., 2013). What if it were possible to generate disease- and patient-specific lines of human pluripotent stem cells in a manner that did not rely on NT?
Cellular Reprogramming Changes the Game
A wealth of fascinating research was presented by scientists from around the world at the 2006 meeting of the International Society for Stem Cell Research (ISSCR) in Toronto, Ontario. Among the hundreds of posters and oral presentations delivered that year, the work of two investigators from Kyoto University, Kazutoshi Takahashi and Shinya Yamanaka (Nobel Prize, 2012), would not only fundamentally alter the field for years to come but with a degree of rapidity unparalleled in modern science. Simply put, their methodological approach to generate lines of “induced pluripotent stem” or iPS cells was a saltatory breakthrough of massive proportions that took the world of cell and developmental biology by storm.
Publishing their full manuscript later that year, the researchers demonstrated that a combination of four retrovirally delivered factors, Oct4, Klf4, Sox2, and cMyc, was capable of reprogramming murine adult and embryonic fibroblasts to pluripotency (Takahashi and Yamanaka, 2006). Theirs was not the first time that scientists had demonstrated that nuclear equivalence permits “lineage reassignment” by forced gene expression.
Working in the 1980s at the Fred Hutchinson Cancer Research Center, Harold “Hal” Weintraub and colleagues had successfully converted mouse fibroblasts to muscle-forming myoblasts via the enforced expression of a master muscle transcription factor they had identified: MyoD (Davis et al., 1987; Lassar et al., 1986; Tapscott et al., 1988). The fulcrum around which reprogramming capability appears to revolve is the correct identification of proximal transcriptional regulators within a given lineage, those capable of imposing a larger transcriptional profile specific to the intended tissue. The team from Kyoto theorized that similarly acting transactivators likely existed in pluripotent cells that given the proper context and culture conditions, might prove capable of reprogramming somatic cells to earlier stages of development. These insights were gleaned from the aforementioned NT studies as well as the use of cell fusion to study the “contingencies of phenotype” in hybrid cells (Miller and Ruddle, 1976).
For several decades prior to the turn of the 21st century, researchers investigated the capacity of various cell types to functionally influence or reprogram one another following cell fusion (for review, see Graf, 2011). Although early attempts to probe the developmental plasticity of fusions between mouse teratocarcinoma-derived EC cells and mature cell types such as fibroblasts were inconclusive, perhaps due to the specific EC lines used (e.g., Finch and Ephrussi, 1967; Jami et al., 1973), other studies would clearly demonstrate that the resulting hybrids were pluripotent (e.g., Miller and Ruddle, 1976; Andrews and Goodfellow, 1980). Despite abnormal ploidy, cell fusion hybrids were capable of forming multilineage teratomas, a measure of potency arising from the EC component, while simultaneously (and unambiguously) demonstrating continued expression of genes from the fusion partner such as glucose phosphate isomerase (Miller and Ruddle, 1976).
Later work showed that mouse ESCs were likewise capable of imposing pluripotency onto hybrids generated using a diverse array of somatic cell fusion partners including T cells (Tada et al., 2001), splenocytes (Matveeva et al., 1998), bone marrow (Terada et al., 2002), and neural progenitors (Ying et al., 2002). Human ESC-fibroblast fusion products are also pluripotent (Cowan et al., 2005). What is more, despite the fact that all components from each parent cell are present in the resulting hybrid, fusion experiments following density gradient centrifugation to obtain either ESC karyoplasts or cytoplasts revealed that it is not the cytoplasm but rather the nucleus that contains whatever factors are responsible for “reactivating” embryonic gene expression in the somatic partner (Do and Schöler, 2004). Identifying these factors would permit virtually any type of cell to be reprogrammed to pluripotency.
The approach used by Takahashi and Yamanaka was ingenious and involved compiling a set of 24 “candidate factors”: genes that were known to be highly associated with pluripotency via prior studies in knockout mice, ES, EC, and germ cells. All 24 factors were delivered to fibroblasts in a selection-based system in which the gene Fbx15 drove a cassette conferring resistance to the antibiotic neomycin. The choice of the Fbx15 gene was important as though it is expressed in ESCs and the early embryo it is not expressed in fibroblasts and thus, only reprogrammed cells would be drug resistant. Additionally, Fbx15 knockout mice are viable, and thus, gene targeting to introduce the neo-cassette was unlikely to impair pluripotency while at the same time ensuring that reprogramming-induced expression of Fbx15 would produce an efficient system with a low false-positive rate. The 24-factor approach produced a certain threshold of colony formation that permitted the investigators to initiate a subtraction assay. One by one, single members of the set of 24 were removed to evaluate the remaining 23 in order to identify which genes were indispensable for colony growth. This resulted in the final set of four “Yamanaka factors.”
The first iPS cells met many of the functional standards of mouse ESCs. They contained hypomethylated promoters relative to the parent fibroblasts for pluripotency-associated genes including Nanog and Fbx15, grew in colonies in vitro that were morphologically similar to mouse ESCs, expressed SSEA-1 and alkaline-phosphatase, had a normal karyotype, clustered with mouse ESCs and away from fibroblasts in gene expression microarray analysis, demonstrated expression of tissue-specific markers such as smooth muscle actin and β-III tubulin when differentiated in vitro, formed teratomas when injected into murine hosts, and chimerized recipient embryos as far as E13.5.
However, there were important measures of performance that the first iPS cells failed to meet including that there were no live-born chimeric mice, and no studies were capable of demonstrating definitive germline contribution, even among midgestation embryos. The reprogramming frequency was also very low, hovering somewhere around one colony per 6,000 starting fibroblasts. By the following year, investigators would refine the approach by driving drug-resistance/selection from other pluripotency-associated genes, a change that permitted live-born chimeras with germline contributions (Maherali et al., 2007; Okita et al., 2007; Wernig et al., 2007). Importantly, the basic four-factor approach remained otherwise unaltered, suggesting that the process likely produced a distribution of cell types reprogrammed to different degrees and capable of isolation or enrichment using alternative techniques such as Fbx15 or Nanog-driven drug selection.
The application of cellular reprogramming to human cells followed quite rapidly, also taking place at the conclusion of the year 2007 (Park et al., 2008b, which was published online December 23, 2007; Takahashi et al., 2007; Yu et al., 2007). Interestingly, the human iPS cells from the Thomson lab were generated using a somewhat different combination of factors, namely OCT4, SOX2, NANOG, and LIN28 (Yu et al., 2007). LIN28 is a protein demonstrated to be a central player in the maintenance of pluripotency via the modulation of the let7 family of microRNAs, which in turn regulate a variety of cellular oncogenes (Viswanathan et al., 2008). Apparently, there are many roads leading to pluripotency. Additionally, whereas the cocktail of four genes appears at first glance to be a fairly simple recipe for imposing such a profound developmental change onto cells, it is worth pointing out that OCT4 and SOX2 each impact hundreds of other genes in an extensive regulatory network (Boyer et al., 2005; Kim et al., 2008; Loh et al., 2006).
Considering the goal of being able to generate lines of human pluripotent stem cells that are matched to specific patients, either to study genetic disease or as a possible resource for regenerative therapy, iPS cells have a great deal to offer. The first lines of disease-specific human iPS cells included a sizeable compendium representing a wide variety of complex, inherited, multifactorial, and single-gene human conditions including Parkinson disease, type I diabetes, Gaucher disease, Down syndrome, and others (Park et al., 2008a) along with those derived from a patient with ALS (Dimos et al., 2008).
The refinement of iPS cell methods and applications has been nothing short of inspired. Given that these subjects have been extensively reviewed elsewhere, we will not focus upon them here but will provide an overview of the most immediate applications (Figure 3). Observing that between Yamanaka’s first announcement of his revolutionary reprogramming methodology in Toronto and his naming as a Nobel Laureate in Physiology or Medicine, along with Sir John Gurdon, a short 6 years later, stands as a testament to the robustness of his approach, its rapid and wide-ranging acceptance within the field, and the vast array of exciting opportunities it presents to basic science and biomedicine.
Figure 3.
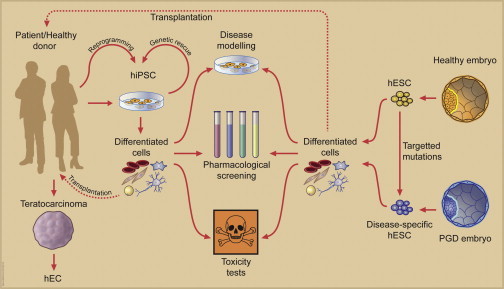
Derivation and Use of Human Pluripotent Stem Cells
Human ES cells (hESCs) and iPS cells (hiPSCs) have immediate applications in modeling disease, drug discovery, and safety pharmacology. Genetic or other correction provides the appropriate control cells for these studies. hESCs can be targeted genetically to create disease models and introduce different mutations on an isogenic background. Alternatively, disease-specific hESCs can be derived from embryos that are rejected after preimplantation genetic diagnosis (PGD). Longer-term applications are thought to be in cell transplantation therapy. The prototype human pluripotent stem cells are EC stem cells (hECs) derived from spontaneous teratocarcinomas. As in mice, pluripotent stem cells can also be derived from primordial germ cells in humans as human embryonic germ cells (hEGCs), but these have usually not become stable lines (data not shown).
Looking Ahead
Many authors have and will provide conjecture regarding the future of this field. Among the more provocative twists and turns of late are the papers indicating that cellular reprogramming need not necessarily transit through a pluripotent cell intermediate. Rather “direct reprogramming” from and to a variety of mature or progenitor cell types is possible via forced expression of sets of lineage-associated genes. Examples include converting fibroblasts to neurons in mouse cells via the genes Brn2, Myt1l, and Ascl1 (Vierbuchen et al., 2010) and in human cells using a slightly different mix of BRN2 and MYT1L plus the miR-124 microRNA (Ambasudhan et al., 2011). Again, the outward simplicity of a handful of genes capable of reprogramming cells hides the deeper truth of extensive chromatin rearrangements that take place when cells adopt a new identity.
Beyond experiments such as these, it is interesting to wonder what the “outer limits” of cellular reprogramming might be. Can any type of cell be converted to any other type of cell? Given the correct genetic inducements along with culture conditions capable of fostering cellular intermediates during the transition, perhaps the answer is “yes.” That said, single cells do exist that present a rather high bar for reprogramming including those with nondiploid genetic content like red blood cells (which have no nucleus at all) and megakaryocytes, which may contain up to 128 or more haploid equivalents because their genome endoreduplicates without cytokinesis during their maturation toward platelet production.
Taking this question one step further, and in a more provocative vein, we observe that the mammalian zygote is a single cell with a diploid genome. Might it be possible to one day reprogram adult somatic cells to totipotency? In other words, given the appropriate technology, might every cell in the body acquire the developmental potential of a fertilized egg? Given that cellular reprogramming is based upon changing the gene expression of one cell type to that of another, the answer would have to be “no.” Why? It is because of the curious state of gene expression in the zygote. It has none.
The earliest cellular cleavages and stages of postfertilization development are directed by the action of proteins and mRNAs stored in the egg during oogenesis—a process involving meiosis and occurring in a completely maternal environment (see Mayer et al., 2000; Stitzel and Seydoux, 2007; Tadros and Lipshitz, 2009). In humans, zygotic gene expression appears to be activated somewhere near to or after the eight-cell stage. In mice, it is even earlier at the two-cell stage, but in the single-cell zygote, the genome is silent. Fascinating recent work in mice shows that at least four preimplantation pluripotent cells are required for developmental progression in utero, though half embryos are capable of being stimulated to duplicate the requisite number of cells via modulation of fibroblast growth factor (FGF) and Wnt signaling such that forced monozygotic twins may even be produced (rather as Spemann) (Morris et al., 2012). However, though the authors managed to enhance the potency of half embryos, their work did not impose zygotic identity onto single cells. Thus, we end by suggesting that cellular reprogramming to totipotency is not possible. The gauntlet has been thrown down.
Final Word
The growing interest in stem cells among the scientific community and patient groups led to the formation of the ISSCR by Leonard I. Zon and a few enthusiastic supporters just over 10 years ago. This fully fledged society now welcomes almost 4,000 delegates to its annual meeting with thousands more following online from their home labs. Its current president is Shinya Yamanaka. The Society anticipates an exponential growth of the field in the coming decade and is now ready for its own journal, Stem Cell Reports, which launched at the ISSCR’s annual meeting in 2013. It is only fitting that the inaugural issue of the journal should include an article that reflects upon the history of the field, celebrates some of its heroes, and looks forward in eager anticipation of future work that will improve the quality of life for those with tissue damage, degeneration, or other forms of disease for which stem cell research promises relief.
Acknowledgments
The authors wish to thank the following individuals for helpful discussions relating to the preparation of this work: George Q. Daley, Heather Rooke, and Samantha Morris for comments on the manuscript; Lucretia McClure, Master Librarian at the Countway Library of Medicine, for assistance in locating classical works; and the generous suggestions of two anonymous reviewers. The authors regret that a more extensive discussion and bibliography are not possible due to space limitations. M.W.L. additionally thanks Grover C. Bagby for encouraging a lifelong interest in the blood. We also thank Bas Blankevoort for figure design. M.W.L. is supported by a Howard Hughes Medical Institute Investigator Award to George Q. Daley, and C.L.M. is supported by an ERC Advanced Award (STEMCARDIOVASC, ERC-2012-AdG-323182). M.W.L. created the concept and text, and C.L.M. designed the figures and edited the manuscript.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information
References
- Ambasudhan R., Talantova M., Coleman R., Yuan X., Zhu S., Lipton S.A., Ding S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9:113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P.W. From teratocarcinomas to embryonic stem cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002;357:405–417. doi: 10.1098/rstb.2002.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P.W., Goodfellow P.N. Antigen expression by somatic cell hybrids of a murine embryonal carcinoma cell with thymocytes and L cells. Somatic Cell Genet. 1980;6:271–284. doi: 10.1007/BF01538801. [DOI] [PubMed] [Google Scholar]
- Biggers J.D. IVF and embryo transfer: historical origin and development. Reprod. Biomed. Online. 2012;25:118–127. doi: 10.1016/j.rbmo.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Birch S., Tyson E. An extract of two letters from Mr. Sampson Birch, an Alderman and Apothecary at Stafford, concerning an extraordinary birth in Staffordshire, with reflections thereon by Edw. Tyson M.D. Fellow of the Coll. of Physitians, and of the R. Society. Philos. Trans. R. Soc. London. 1683;13:281–284. [Google Scholar]
- Bongso A., Fong C.Y., Ng S.C., Ratnam S. Isolation and culture of inner cell mass cells from human blastocysts. Hum. Reprod. 1994;9:2110–2117. doi: 10.1093/oxfordjournals.humrep.a138401. [DOI] [PubMed] [Google Scholar]
- Boyer L.A., Lee T.I., Cole M.F., Johnstone S.E., Levine S.S., Zucker J.P., Guenther M.G., Kumar R.M., Murray H.L., Jenner R.G. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R., King T.J. Transplantation of living nuclei from blastula cells into enucleated frogs’ eggs. Proc. Natl. Acad. Sci. USA. 1952;38:455–463. doi: 10.1073/pnas.38.5.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R.L. The effect of cells transferred into the mouse blastocyst on subsequent development. J. Exp. Med. 1974;140:1049–1056. doi: 10.1084/jem.140.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K.H., McWhir J., Ritchie W.A., Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–66. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- Cowan C.A., Atienza J., Melton D.A., Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369–1373. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- Cruikshank W. Experiments in which, on the third day after impregnation, the ova of rabbits were found in the fallopian tubes; and on the fourth day after impregnation in the uterus itself; with the first appearances of the foetus. Philos. Trans. R. Soc. London. 1797;87:197–214. [Google Scholar]
- Davis R.L., Weintraub H., Lassar A.B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Dimos J.T., Rodolfa K.T., Niakan K.K., Weisenthal L.M., Mitsumoto H., Chung W., Croft G.F., Saphier G., Leibel R., Goland R. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Do J.T., Schöler H.R. Nuclei of embryonic stem cells reprogram somatic cells. Stem Cells. 2004;22:941–949. doi: 10.1634/stemcells.22-6-941. [DOI] [PubMed] [Google Scholar]
- Driesch H. Entwicklungsmechanische Studien I. Der Wert der ersten beiden Furchungszellen in der Echinodermenentwickelung. Experimentelle Erzeugung von Teil und Doppelbildungen. Ztschr. f. Wiss. Zool. 1891;53:160–183. [Google Scholar]
- Duncan A.W., Dorrell C., Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009;137:466–481. doi: 10.1053/j.gastro.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan K., Baldwin K., Tackett M., Osborne J., Gogos J., Chess A., Axel R., Jaenisch R. Mice cloned from olfactory sensory neurons. Nature. 2004;428:44–49. doi: 10.1038/nature02375. [DOI] [PubMed] [Google Scholar]
- Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Finch B.W., Ephrussi B. Retention of multiple developmental potentialities by cells of a mouse testicular teratocarcinoma during prolonged culture in vitro and their extinction upon hybridization with cells of permanent lines. Proc. Natl. Acad. Sci. USA. 1967;57:615–621. doi: 10.1073/pnas.57.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischberg M., Gurdon J.B., Elsdale T.R. Nuclear transplantation in Xenopus laevis. Nature. 1958;181:424. [PubMed] [Google Scholar]
- Graf T. Historical origins of transdifferentiation and reprogramming. Cell Stem Cell. 2011;9:504–516. doi: 10.1016/j.stem.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Gurdon J.B. Factors responsible for the abnormal development of embryos obtained by nuclear transplantation in Xenopus laevis. J. Embryol. Exp. Morphol. 1960;8:327–340. [PubMed] [Google Scholar]
- Gurdon J.B. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J. Embryol. Exp. Morphol. 1962;10:622–640. [PubMed] [Google Scholar]
- Gurdon J.B., Uehlinger V. “Fertile” intestine nuclei. Nature. 1966;210:1240–1241. doi: 10.1038/2101240a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J.B., Laskey R.A., Reeves O.R. The developmental capacity of nuclei transplanted from keratinized skin cells of adult frogs. J. Embryol. Exp. Morphol. 1975;34:93–112. [PubMed] [Google Scholar]
- Heape W. Preliminary note on the transplantation and growth of mammalian ova within a uterine foster-mother. Proc. R. Soc. Lond. 1890;48:457–458. [Google Scholar]
- Hochedlinger K., Jaenisch R. Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature. 2002;415:1035–1038. doi: 10.1038/nature718. [DOI] [PubMed] [Google Scholar]
- Jami J., Failly C., Ritz E. Lack of expression of differentiation in mouse teratoma-fibroblast somatic cell hybrids. Exp. Cell Res. 1973;76:191–199. doi: 10.1016/0014-4827(73)90435-7. [DOI] [PubMed] [Google Scholar]
- Johnson, M.H. (2010). Robert Edwards: Nobel Laureate in Physiology or Medicine, Nobel Lecture/Nobel Prize Symposium in Honour of Robert G. Edwards. Nobel Foundation, http://www.nobelprize.org/nobel_prizes/medicine/laureates/2010/edwards_lecture.pdf.
- Jordan H.E. A study of a 7mm. human embryo; with special reference to its peculiar spirally twisted form, and its large aortic cell-clusters. Anat. Rec. 1918;14:479–492. [Google Scholar]
- Kim J., Chu J., Shen X., Wang J., Orkin S.H. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinsmith L.J., Pierce G.B., Jr. Multipotentiality of single embryonal carcinoma cells. Cancer Res. 1964;24:1544–1551. [PubMed] [Google Scholar]
- Kuhn T.S. Second Edition. University of Chicago Press; Chicago: 1970. The Structure of Scientific Revolutions. [Google Scholar]
- Lassar A.B., Paterson B.M., Weintraub H. Transfection of a DNA locus that mediates the conversion of 10T1/2 fibroblasts to myoblasts. Cell. 1986;47:649–656. doi: 10.1016/0092-8674(86)90507-6. [DOI] [PubMed] [Google Scholar]
- Lensch M.W., Ince T.A. The terminology of teratocarcinomas and teratomas. Nat. Biotechnol. 2007;25:1211. doi: 10.1038/nbt1107-1211a. author reply 1211–1212. [DOI] [PubMed] [Google Scholar]
- Lensch M.W., Schlaeger T.M., Zon L.I., Daley G.Q. Teratoma formation assays with human embryonic stem cells: a rationale for one type of human-animal chimera. Cell Stem Cell. 2007;1:253–258. doi: 10.1016/j.stem.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Loh Y.H., Wu Q., Chew J.L., Vega V.B., Zhang W., Chen X., Bourque G., George J., Leong B., Liu J. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- Lopes F.L., Desmarais J.A., Murphy B.D. Embryonic diapause and its regulation. Reproduction. 2004;128:669–678. doi: 10.1530/rep.1.00444. [DOI] [PubMed] [Google Scholar]
- Lower R., King E. An account of the experiment of transfusion, practised upon a man in London. Philos. Trans. R. Soc. 1667;2:557–559. [Google Scholar]
- Maherali N., Sridharan R., Xie W., Utikal J., Eminli S., Arnold K., Stadtfeld M., Yachechko R., Tchieu J., Jaenisch R. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G.R., Evans M.J. The morphology and growth of a pluripotent teratocarcinoma cell line and its derivatives in tissue culture. Cell. 1974;2:163–172. doi: 10.1016/0092-8674(74)90090-7. [DOI] [PubMed] [Google Scholar]
- Matveeva N.M., Shilov A.G., Kaftanovskaya E.M., Maximovsky L.P., Zhelezova A.I., Golubitsa A.N., Bayborodin S.I., Fokina M.M., Serov O.L. In vitro and in vivo study of pluripotency in intraspecific hybrid cells obtained by fusion of murine embryonic stem cells with splenocytes. Mol. Reprod. Dev. 1998;50:128–138. doi: 10.1002/(SICI)1098-2795(199806)50:2<128::AID-MRD2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Mayer W., Niveleau A., Walter J., Fundele R., Haaf T. Demethylation of the zygotic paternal genome. Nature. 2000;403:501–502. doi: 10.1038/35000656. [DOI] [PubMed] [Google Scholar]
- Miller R.A., Ruddle F.H. Pluripotent teratocarcinoma-thymus somatic cell hybrids. Cell. 1976;9:45–55. doi: 10.1016/0092-8674(76)90051-9. [DOI] [PubMed] [Google Scholar]
- Morgan T.H. Half embryos and whole embryos from one of the first two blastomeres. Anat. Anz. 1895;10:623–638. [Google Scholar]
- Morris S.A., Guo Y., Zernicka-Goetz M. Developmental plasticity is bound by pluripotency and the Fgf and Wnt signaling pathways. Cell Rep. 2012;2:756–765. doi: 10.1016/j.celrep.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A., Gócza E., Diaz E.M., Prideaux V.R., Iványi E., Markkula M., Rossant J. Embryonic stem cells alone are able to support fetal development in the mouse. Development. 1990;110:815–821. doi: 10.1242/dev.110.3.815. [DOI] [PubMed] [Google Scholar]
- Noggle S., Fung H.L., Gore A., Martinez H., Satriani K.C., Prosser R., Oum K., Paull D., Druckenmiller S., Freeby M. Human oocytes reprogram somatic cells to a pluripotent state. Nature. 2011;478:70–75. doi: 10.1038/nature10397. [DOI] [PubMed] [Google Scholar]
- Okita K., Ichisaka T., Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Park I.H., Arora N., Huo H., Maherali N., Ahfeldt T., Shimamura A., Lensch M.W., Cowan C., Hochedlinger K., Daley G.Q. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I.H., Zhao R., West J.A., Yabuuchi A., Huo H., Ince T.A., Lerou P.H., Lensch M.W., Daley G.Q. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Parson A.B. First Edition. Joseph Henry Press; Washington, D.C.: 2004. The Proteus Effect: Stem Cells and Their Promise for Medicine. [Google Scholar]
- Roux W. Beiträge zur Entwickelungsmechanik des Embryo. Über die künstliche Hervorbringung “halber” Embryonen durch Zerstörung einer der beiden ersten Furchungskugeln, sowie über die Nachentwickelung (Postgeneration) der fehlenden Körperhälfte: Gesammelte Abhandlungen II. Virchows Arch. 1888;114:419–521. [Google Scholar]
- Scultetus J. literis Michaelis Enderi; Norimbergae, Germany: 1658. Trichiasis admiranda, sive Morbus pilaris mirabilis observatus a Johanne Sculteto. [Google Scholar]
- Smith A.G., Heath J.K., Donaldson D.D., Wong G.G., Moreau J., Stahl M., Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- Spemann H. Die Entwicklung seitlicher und dorso-ventraler Keimhälften bei verzögerter Kernversorgung. Ztschr. f. Wiss. Zool. 1928;132:105–134. [Google Scholar]
- Spemann H. Yale University Press; New Haven, CT: 1938. Embryonic Development and Induction. [Google Scholar]
- Steptoe P.C., Edwards R.G. Birth after the reimplantation of a human embryo. Lancet. 1978;2:366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- Stevens L.C., Little C.C. Spontaneous testicular teratomas in an inbred strain of mice. Proc. Natl. Acad. Sci. USA. 1954;40:1080–1087. doi: 10.1073/pnas.40.11.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzel M.L., Seydoux G. Regulation of the oocyte-to-zygote transition. Science. 2007;316:407–408. doi: 10.1126/science.1138236. [DOI] [PubMed] [Google Scholar]
- Tachibana M., Amato P., Sparman M., Gutierrez N.M., Tippner-Hedges R., Ma H., Kang E., Fulati A., Lee H.S., Sritanaudomchai H. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013 doi: 10.1016/j.cell.2013.05.006. Published online on May 15, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Takahama Y., Abe K., Nakatsuji N., Tada T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr. Biol. 2001;11:1553–1558. doi: 10.1016/s0960-9822(01)00459-6. [DOI] [PubMed] [Google Scholar]
- Tadros W., Lipshitz H.D. The maternal-to-zygotic transition: a play in two acts. Development. 2009;136:3033–3042. doi: 10.1242/dev.033183. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tapscott S.J., Davis R.L., Thayer M.J., Cheng P.F., Weintraub H., Lassar A.B. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988;242:405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- Teilum G. Classification of endodermal sinus tumour (mesoblatoma vitellinum) and so-called “embryonal carcinoma” of the ovary. Acta Pathol. Microbiol. Scand. 1965;64:407–429. doi: 10.1111/apm.1965.64.4.407. [DOI] [PubMed] [Google Scholar]
- Terada N., Hamazaki T., Oka M., Hoki M., Mastalerz D.M., Nakano Y., Meyer E.M., Morel L., Petersen B.E., Scott E.W. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Trembley A. Jean & Herman Verbeek; Leiden, the Netherlands: 1744. Mémoires pour Servir à L’histoire d’un Genre de Polypes d’eau Douce. [Google Scholar]
- Vierbuchen T., Ostermeier A., Pang Z.P., Kokubu Y., Südhof T.C., Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan S.R., Daley G.Q., Gregory R.I. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabl M.R., Brun R.B., Du Pasquier L. Lymphocytes of the toad Xenopus laevis have the gene set for promoting tadpole development. Science. 1975;190:1310–1312. doi: 10.1126/science.1198115. [DOI] [PubMed] [Google Scholar]
- Waddington C.H. Allen and Unwin; London: 1957. The Strategy of the Genes: A Discussion of Some Aspects of Theoretical Biology. [Google Scholar]
- Wakayama T., Perry A.C., Zuccotti M., Johnson K.R., Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- Wakayama T., Shinkai Y., Tamashiro K.L., Niida H., Blanchard D.C., Blanchard R.J., Ogura A., Tanemura K., Tachibana M., Perry A.C. Cloning of mice to six generations. Nature. 2000;407:318–319. doi: 10.1038/35030301. [DOI] [PubMed] [Google Scholar]
- Weismann A. First Edition. Volume 1. Clarendon Press; Oxford: 1889. (Essays upon Heredity and Kindred Biological Problems). [Google Scholar]
- Weismann A. Charles Scribner’s Sons; New York: 1893. The Germ-Plasm: A Theory of Heredity. [Google Scholar]
- Wernig M., Meissner A., Foreman R., Brambrink T., Ku M., Hochedlinger K., Bernstein B.E., Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- Williams R.L., Hilton D.J., Pease S., Willson T.A., Stewart C.L., Gearing D.P., Wagner E.F., Metcalf D., Nicola N.A., Gough N.M. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Wilmut I., Schnieke A.E., McWhir J., Kind A.J., Campbell K.H. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- Witschi E. Migration of the germ cells of human embryos from the yolk sac to the primitive gonadal folds. Contrib. Embryol. 1948;32:67–80. [Google Scholar]
- Ying Q.L., Nichols J., Evans E.P., Smith A.G. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- Yonge J. An account of balls of hair taken from the uterus and ovaria of several women; by Mr. James Yonge, F.R.S. communicated to Dr. Hans Sloane, R.S. Secr. Philos. Trans. R. Soc. 1706;25:2387–2392. [Google Scholar]
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


