Abstract
Nerve-derived neural crest cells are essential for regeneration in certain animals, such as newts. Here, we asked whether they play a similar role during mammalian tissue repair, focusing on Sox2-positive neural crest precursors in skin. In adult skin, Sox2 was expressed in nerve-terminal-associated neural crest precursor cells (NCPCs) around the hair follicle bulge, and following injury was induced in nerve-derived cells, likely dedifferentiated Schwann cell precursors. At later times postinjury, Sox2-positive cells were scattered throughout the regenerating dermis, and lineage tracing showed that these were all neural-crest-derived NCPCs. These Sox2-positive NCPCs were functionally important, since acute deletion of Sox2 prior to injury caused a decrease of NCPCs in the wound and aberrant skin repair. These data demonstrate that Sox2 regulates skin repair, likely by controlling NCPCs, and raise the possibility that nerve-derived NCPCs may play a general role in mammalian tissue repair.
Highlights
-
•
Sox2 regulates murine skin repair
-
•
Sox2 regulates the neural crest precursor response to tissue injury
-
•
Sox2 identifies a nerve terminal-associated neural crest precursor in hair follicles
Introduction
Insights into potential mechanisms for the regulation of mammalian tissue repair have come from studies of animals that can regenerate limbs, tails, and even the spinal cord, such as amphibians and reptiles. One major conclusion from these studies is that tissue regeneration requires nerve innervation (Kumar and Brockes, 2012). These findings have led to the idea that peripheral nerves may regulate tissue repair in mammals. This possibility has important implications because every tissue in the body is innervated. Indirect support for this intriguing idea comes from recent studies showing that innervation regulates the biology of some adult tissue precursors (Yamazaki et al., 2011; Brownell et al., 2011).
How might nerves regulate tissue repair and regeneration? In newts, neural-crest-derived Schwann cells migrate into the regenerating limb and secrete factors that regulate mesenchymal cell proliferation and regeneration itself (Kumar and Brockes, 2012). In mammals, nerve injury leads to a dramatic dedifferentiation of Schwann cells into a precursor cell state, which is important for appropriate nerve regeneration (Jessen and Mirsky, 2008). Here, we asked whether nerve-derived neural crest precursor cells (NCPCs) play a role in mammalian tissue repair, focusing on adult murine skin. We describe nerve-associated NCPCs in adult skin and show that NCPCs contribute to the regenerating dermis in a Sox2-dependent fashion, and that when Sox2 is ablated, this NCPC response is perturbed concomitantly with aberrant skin repair. Thus, Sox2 regulates skin repair, likely via its actions in Sox2-positive NCPCs, suggesting that nerve-derived NCPCs may play a general role in promoting mammalian tissue repair.
Results
Sox2 Defines Nerve Terminal-Associated NCPCs in Adult Skin
We first defined Sox2-expressing skin populations using mice with EGFP knocked in to the Sox2 allele (Sox2+/EGFP mice; Ellis et al., 2004). Immunostaining of back skin at 8 weeks, when hair follicles are in telogen, showed that Sox2-EGFP was limited to some highly distinctive cells around the bulge region of virtually all hair follicles (Figure 1A) as well as scattered K8-positive Merkel cells (Figure S1A available online), which were previously reported to express Sox2 (Driskell et al., 2009). Since previous studies identified NCPC activity in the bulge (Sieber-Blum and Hu, 2008; Amoh et al., 2005), we asked whether these Sox2-EGFP-positive cells expressed p75NTR, nestin, and S100β, all markers for NCPCs. Immunostaining showed that they coexpressed these proteins (Figures 1A–1C). Moreover, immunostaining for the synaptic protein synapsin1 showed that they were localized with axon terminals innervating the bulge (Figure 1D). These Sox2-EGFP-positive cells (which we call nerve terminal [NT] cells) were also present in anagen hair follicles at 4 weeks of age, when Sox2-EGFP was also expressed in mesenchymal hair follicle precursors (Figure S1B–S1E), as we previously reported (Biernaskie et al., 2009).
Figure 1.
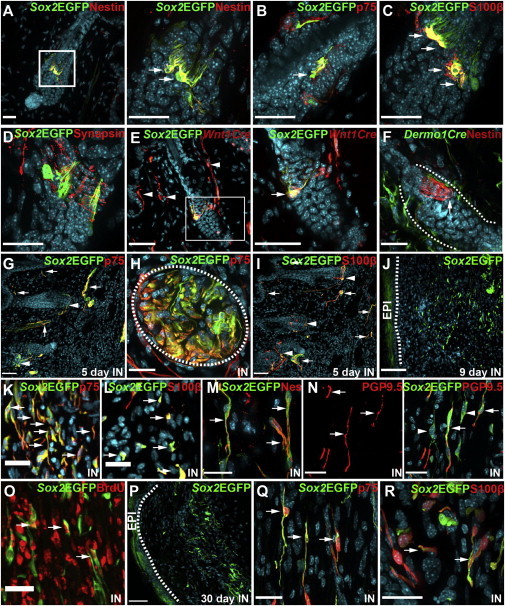
Sox2-Positive NCPCs in Intact and Injured Adult Murine Back Skin
(A–D) Telogen hair follicles in uninjured back skin from 8-week-old Sox2+/EGFP mice immunostained for Sox2-EGFP (Sox2EGFP; green in all panels), and for nestin (A), p75NTR (B), S100β (C), or synapsin (D; all red). Double-positive cells (yellow) are indicated by arrows. In (A), the boxed area is shown at higher magnification to the right.
(E) Telogen hair follicle of an 8-week-old Sox2+/EGFP;Wnt1-Cre;R26TdTfl/+ mouse immunostained for Sox2-EGFP (Sox2EGFP; green) and TdTomato (Wnt1Cre; red) showing a double-positive NT cell (yellow, arrow). Arrowheads denote skin nerves positive for only the Wnt1-Cre reporter.
(F) Telogen hair follicle of an 8-week-old Dermo1Cre+;R26YFPfl/+ mouse immunostained for nestin (red) to identify NT cells and YFP (Dermo1Cre; green). Dotted lines delineate the bulge.
(G–I) Adult Sox2+/EGFP mouse skin adjacent to a punch wound 5 days postinjury, immunostained for Sox2-EGFP (Sox2EGFP; green) and p75NTR (G and H) or S100β (I; both red). Double-labeled (yellow) NT cells and skin nerves are denoted with arrowheads and arrows, respectively.
(H) A skin nerve cut in cross-section, defined by the dotted oval.
(J–Q) Regenerating skin of Sox2+/EGFP mice 9 days (J–O) or 30 days (P–R) postinjury, immunostained for Sox2-EGFP (Sox2EGFP; green) and p75NTR (K and Q), S100β (L and R), nestin (M), PGP9.5 (N), or BrdU (O; all red). In (O), mice were treated with BrdU daily commencing at the time of injury.
(J and P) Overviews of the wound site, with the dotted line defining the epidermal/dermal boundary.
IN indicates sections from injured animals, and the arrows denote double-labeled (yellow) cells, except for (N), where arrows and arrowheads denote EGFP-positive NCPCs that are associated or not associated with PGP9.5-positive axons, respectively. All sections are counterstained with Hoechst 33258 (blue) to show nuclei. Scale bars in all panels, 25 μm, except (E), 50 μm; (G), (I), (J), and (P), 100 μm. See also Figure S1.
We performed lineage tracing to confirm that NT cells were neural-crest derived, crossing Sox2+/EGFP animals and mice carrying a Wnt1-Cre transgene (which marks neural crest progeny) and a floxed TdTomato reporter gene in the Rosa26 locus. Immunostaining of back skin from 8-week-old Sox2+/EGFP;Wnt1-cre;R26TdTfl/+ mice showed that TdTomato was expressed in Sox2-EGFP-positive NT cells and in EGFP-negative neural crest cells, including skin nerve cells (Figure 1E) and melanocytes (data not shown). To confirm that NT cells are of neural crest and not dermal origin, we also analyzed Dermo1Cre/+;R26YFPfl/+ mice in which Cre recombinase is knocked in to one allele of the Dermo1 transcription factor gene, which is expressed in dermal but not epidermal or neural-crest-derived cells (Yu et al., 2003). In these mice, nestin-positive NT cells did not express YFP (Figure 1F), but almost all dermal cells were yellow fluorescent protein (YFP) positive, including the telogen hair follicle dermal papilla and sheath cells (Figure S1F). Thus, Sox2 defines a unique population of neural-crest-derived NT cells in hair follicles that persist during all stages of the hair cycle.
Sox2-Positive NCPCs Contribute to the Regenerating Dermis following Skin Injury
To ask whether Sox2 was induced in dedifferentiated NCPCs in skin nerves following tissue injury, we performed full-thickness punch wounds on 8-week-old Sox2+/EGFP mice. Immunostaining 5 days postinjury identified many Sox2-EGFP-positive nerve cells that coexpressed p75NTR and S100β (Figures 1G–1I). At 7–9 days postinjury, many Sox2-EGFP-positive cells were also scattered throughout the regenerating dermis (Figure 1J). Virtually all of these coexpressed p75NTR and S100β (Figures 1K and 1L), and many expressed nestin (Figure 1M), consistent with an NCPC phenotype. Some, but not all, of these NCPCs were associated with axons that had sprouted into the healing tissue and expressed the axonal marker PGP9.5 (Figure 1N). Moreover, many were proliferating: when bromodeoxyuridine (BrdU) was administered once a day commencing at the time of injury; ∼11% of the Sox2-EGFP-positive cells were BrdU positive by 9 days (Figure 1O). In contrast, the vast majority of Sox2-EGFP-positive NT cells in hair follicles did not incorporate BrdU over this time course. These Sox2-EGFP-positive, NCPC-like cells persisted within the healed dermis for at least 4 weeks (Figure 1P), during which time they maintained their expression of p75NTR and S100β (Figures 1Q and 1R).
We definitively established that the Sox2-EGFP-positive cells within the wound were neural-crest derived by performing punch wounds on Sox2+/EGFP;Wnt1-Cre;R26TdTomatofl/+ mice. At 9 days postinjury, virtually all Sox2-EGFP-positive cells within the wound were positive for TdTomato and coexpressed p75NTR and S100β (Figures 2A–2C). These neural-crest-derived cells were still present within the regenerated dermis at 30 days postinjury (Figures 2D–2F). Similar experiments with Dermo1Cre/+;R26TdTomatofl/+ mice crossed to Sox2+/EGFP mice showed that none of the Sox2-EGFP-positive cells coexpressed the Dermo1 reporter (Figures 2G and 2H).
Figure 2.
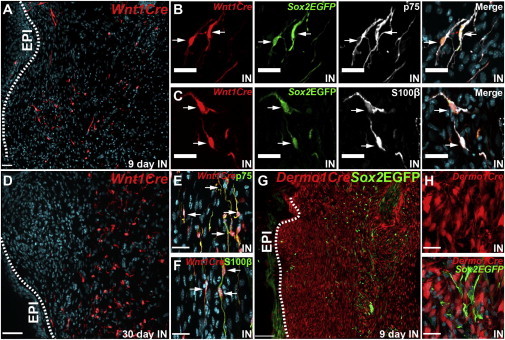
Lineage Tracing to Define the Origins of Sox2-Positive NCPCs within Regenerating Skin
(A–C) Regenerating skin from adult Sox2+/EGFP;Wnt1-Cre;R26TdTomatofl/+ mice 9 days postinjury, immunostained for Sox2-EGFP (Sox2EGFP; green), TdTomato (Wnt1Cre; red), and p75NTR (B) or S100β (C; both pseudocolored white), with arrows denoting triple-labeled cells.
(D–F) Regenerating skin from Wnt1-Cre;R26TdTomatofl/+ mice 30 days postinjury, immunostained for TdTomato (Wnt1Cre; red) and p75NTR (E) or S100β (F; both green). Arrows denote double-labeled cells.
(G and H) Regenerating skin from adult Sox2+/EGFP;Dermo1Cre/+;R26TdTomatofl/+ mice 9 days postinjury, immunostained for Sox2-EGFP (Sox2EGFP; green) and TdTomato (Dermo1Cre; red).
IN indicates sections from injured animals. Scale bars, 100 μm (A, D, and G) and 25 μm (all other panels).
Most Sox2-Positive NCPCs within the Regenerating Dermis Are Induced to Express Sox2 Following Injury
The above data indicate that there are several potential sources of NCPCs within injured skin, including NT cells, which always express Sox2, and cutaneous nerve cells, which are induced to express Sox2 following injury (Merkel cells are not neural-crest derived; Van Keymeulen et al., 2009). To ask which of these potential sources contributed Sox2-expressing NCPCs to the regenerating dermis, we performed lineage tracing with a mouse in which CreERT2 is knocked in to the Sox2 locus (Sox2CreERT2/+ mice; Arnold et al., 2011). We crossed these to R26TdTomatofl/+ mice and exposed them to tamoxifen to induce Cre-mediated recombination of the reporter gene in Sox2-positive cells. We did this either 3 weeks before a punch wound, thereby inducing expression of TdTomato in Sox2-positive NT cells and Merkel cells (Figures 3A and 3B), or at the time of injury, thereby inducing TdTomato in Sox2-expressing cells within the injured nerve (Figures 3C and 3D), in addition to NT cells and Merkel cells (Figures 3D–3F). No other cells in the skin were labeled under either condition.
Figure 3.
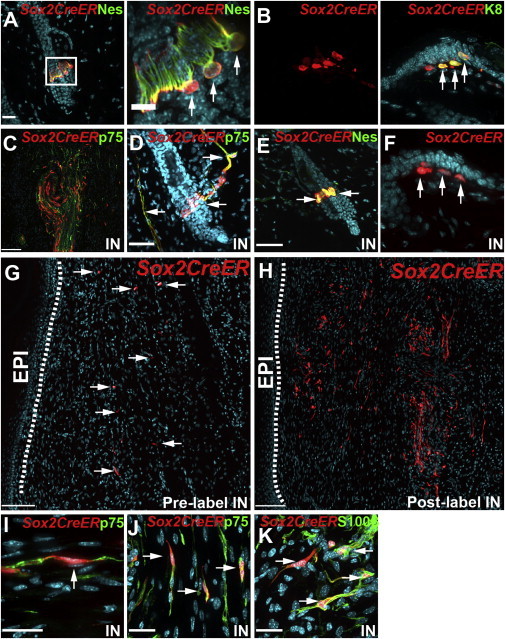
Lineage Tracing Demonstrates that Most NCPCs in the Regenerating Dermis Are Induced to Express Sox2 Following Injury
(A–K) Regenerating skin from adult Sox2CreERT2/+;R26TdTomatofl/+ mice that were induced with tamoxifen either 3 weeks prior to injury (A, B, G, and I) or at the time of injury (C–F, H, J, and K). Skin was immunostained for TdTomato (Sox2CreER; red) and cell-type-specific markers (green). Arrows denote double-labeled (all panels except F–H) or single-labeled (F–H) cells.
(A and B) Skin of pretreated mice on the day of injury, immunostained for the NT marker nestin (Nes; A) or the Merkel cell marker K8 (B).
(C–F) Skin of mice treated with tamoxifen at the time of injury 9 days postinjury.
(C and D) A nerve (C) and telogen hair follicle (D) adjacent to the wound site.
(E and F) A telogen hair follicle (E) and Merkel cells (F, arrows) far from the wound site.
(G and H) Low-magnification views of the wound bed 9 days postinjury in mice treated before (G) or at the time of (H) injury.
(I–K) Sections similar to those shown in (G) and (H) from mice treated before (I) or at the time of (J and K) injury were double labeled for p75NTR (I and J) or S100β (K).
Scale bars, 100 μm (G and H) and 25 μm (all other panels).
We then compared the number and phenotype of TdTomato-positive cells within the regenerating dermis 9 days postinjury. In mice treated with tamoxifen before the injury, relatively few TdTomato-positive cells were present in the wound bed (Figure 3G). In contrast, in mice treated with tamoxifen at the time of injury, many TdTomato-positive cells were scattered throughout the regenerating dermis (Figure 3H), as seen with the Sox2-EGFP reporter (Figure 1J). In both cases, virtually all of the TdTomato-positive cells expressed p75NTR and S100β (Figures 3I–3K), consistent with an NCPC phenotype. Thus, some NCPCs within the wound bed originate from NT cells, but most derive from NCPCs that are induced to express Sox2 following injury, likely from cutaneous nerves since other neural crest cells within the skin (such as melanocytes) do not express Sox2 either before or after injury (data not shown). Consistent with this interpretation, when tamoxifen was given at the time of injury, many TdTomato-positive cells appeared to be migrating from local nerves into the wound bed (Figure 3C).
Acute Deletion of Sox2 in Adult Mice Dysregulates the NCPC Response and Causes Aberrant Skin Repair
To ask whether Sox2-positive NCPCs were functionally important for skin repair, we conditionally deleted Sox2 in adult mice and asked whether this impaired wound healing. Specifically, we crossed Sox2fl/fl mice to mice expressing a constitutively expressed CreERT2 in the Rosa26 locus (Sox2fl/fl;R26 CreERT2/+ mice; Seibler et al., 2003, Taranova et al., 2006). We injected mice with tamoxifen at 9 months of age, confirmed that this caused recombination of the floxed Sox2 alleles in the skin (Figure 4A), and performed punch wounds 5 weeks later. Measurement of these punch wounds showed that Sox2 ablation significantly decreased the rate of wound closure over 9 days relative to three different control groups (Figure 4B). Morphological analysis (Figure 4C) confirmed this deficit: Sox2fl/fl;R26 CreERT2/+ mice treated with tamoxifen were significantly impaired with regard to the epithelial gap, wound width, and dermal tissue regeneration relative to controls (Figures 4D–4F). Moreover, the proportion of proliferating, Ki67-positive cells in the regenerating dermis was robustly decreased (Figures 4G and 4H). Deficits in wound healing were also observed in Sox2 heterozygous mice (Figure S2), supporting the conclusion that Sox2 is necessary for skin repair.
Figure 4.
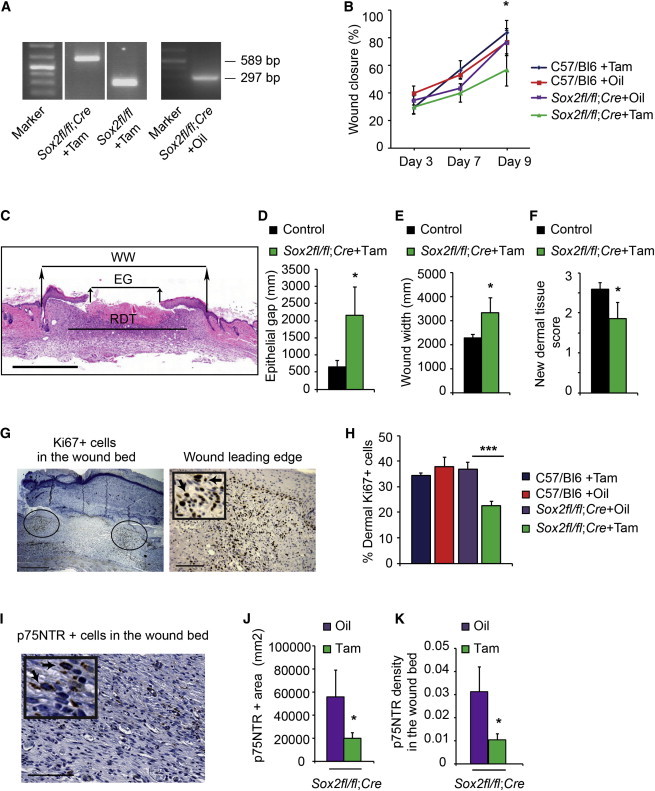
Genetic Ablation of Sox2 in Adult Mice Causes Aberrant Skin Repair Concomitantly with Deficits in the NCPC Injury Response
(A–K) Sox2fl/fl;R26CreERT2/+ mice were treated with tamoxifen (Sox2fl/fl;Cre+Tam, n = 7) or vehicle (Sox2fl/fl;Cre+Oil, n = 6) at 9 months, and punch wounds were performed 5 weeks later. As additional controls, wild-type mice of the same genetic background were treated with tamoxifen (C57/Bl6+Tam, n = 7) or oil (C57/Bl6+Oil, n = 5).
(A) Genomic DNA PCR analysis showing the 297 nt product from the intact floxed allele, and the 589 nt product generated from the floxed allele after Cre-mediated recombination.
(B) Extent of wound closure 3–9 days postinjury. Two-way ANOVA; *p < 0.05 for group effect.
(C) Representative hematoxylin-and-eosin-stained section through the center of the wound bed 9 days postinjury showing the epithelial gap (EG), wound width (WW), and regenerating dermal tissue (RDT).
(D–F) Sections similar to that in (C) were analyzed for the epithelial gap (D), wound width (E), and new dermal tissue (F). Student’s t test; *p < 0.05 for the comparison between Sox2fl/fl;Cre+Tam and controls (the three control groups were pooled because they were statistically similar).
(G and H) Sections similar to that in (C) were immunostained for Ki67, and the percentage of positive cells at the leading edge of the regenerating dermis (ovals in G, magnified in the right panel) was quantified (H). Arrows indicate Ki67-positive cells. One-way ANOVA; ***p = 0.0001 relative to control groups; n = 5 C57/Bl6+Oil, 3 C57Bl6+Tam,4 Sox2fl/fl;Cre+Oil, 7 Sox2fl/fl;Cre+Tam.
(I–K) Sections adjacent to those used for proliferation analysis were immunostained for p75NTR (I) and analyzed for the total area (J) and density (K) of p75NTR-positive cells in the wound bed. Arrows in the inset indicate p75NTR-positive cells. Student’s t test; *p < 0.05; n = 4 Sox2fl/fl;Cre+Oil and 7 Sox2fl/fl;Cre+Tam.
Scale bars, 1 mm (C), 500 μm and 125 μm (G, right and left panels, respectively), 85 μm (I), and 45 μm (inset). See also Figure S2.
Since NCPCs are the only cells within the regenerating skin that express Sox2, these findings suggest that the decreased skin repair is due to a deficit in NCPCs. To test this idea, we quantified the relative proportion of p75NTR-positive NCPCs in the regenerating dermis (Figure 4I). This analysis showed that despite the larger wound area when Sox2 was conditionally ablated (1.62 ± 0.27 mm2 in Sox2fl/fl;R26 CreERT2/+ mice treated with oil versus 2.57 ± 0.59 mm2 in Sox2fl/fl;R26 CreERT2/+ mice treated with tamoxifen), the area covered by p75NTR-positive cells and their relative density were both decreased 2- to 3-fold (Figures 4J and 4K). Immunostaining for SOX2 on adjacent sections confirmed that in the Sox2fl/fl;R26 CreERT2/+ mice treated with oil, many cells within the wound bed and adjacent nerves were SOX2 positive, whereas positive cells were not observed in either the wound or nerves in the Sox2fl/fl;R26CreERT2/+ mice treated with tamoxifen. Thus, Sox2 regulates the NCPC response to tissue injury, and this response is necessary for appropriate skin repair.
Discussion
The data presented here support a number of conclusions. First, we identify a population of Sox2-positive neural-crest-derived NT cells around the hair follicle bulge. These cells express an NCPC phenotype and contribute cells to the regenerating dermis following skin injury. Second, we show that skin injury induces expression of Sox2 in skin nerve cells (likely dedifferentiated Schwann cells), and that these cells likely provide the major source of NCPCs in the regenerating dermis. Third, we show that Sox2 is important for this NCPC response, because when Sox2 is genetically ablated, the number of NCPCs within the regenerating dermis is reduced 2- to 3-fold. Finally, we show that an aberrant NCPC injury response, caused by genetic ablation of Sox2, is coincident with significant deficits in skin repair. Thus, Sox2-positive NCPCs contribute to the regenerating dermis. This contribution depends upon normal levels of Sox2, and when this injury response is perturbed, skin repair is aberrant.
One question that arises from this work involves the nature of the NT cells. We show here that these bulge-associated cells are located at nerve terminals, that they resemble NCPCs phenotypically, and that they contribute cells to the regenerating skin. Intriguingly, previous publications have identified NCPC stem cell activity in the hair follicle bulge region (Sieber-Blum and Hu, 2008; Amoh et al., 2005). Moreover, during development, NCPCs migrate into the skin via nerves, where they contribute melanocytes to hair follicles (Adameyko et al., 2009). We therefore propose that NT cells are NCPCs that arrive in the embryonic skin via nerves, are maintained in a precursor state by their hair follicle niche, and function as a reservoir of adult NCPC activity.
A second question involves the origin of the Sox2-positive NCPCs within the regenerating dermis. The Sox2-CreERT2-mediated lineage tracing shows that a large majority of these NCPCs are induced to express Sox2 following skin injury. Because the only neural-crest-derived skin cells that express Sox2 following injury are NT cells and cutaneous nerve cells, it is likely that many of these NCPCs derive from the injured nerve, perhaps from dedifferentiated Schwann cell precursors that express Sox2 (Parrinello et al., 2010; Jessen and Mirsky, 2008). It is, however, formally possible that these Sox2-positive NCPCs originate outside of the skin and are trafficked into the regenerating dermis from a distance, perhaps via the circulation.
Importantly, our findings define a role for NCPCs in promoting skin repair. How then do NCPCs regulate skin repair? Based upon work in amphibians (Kumar and Brockes, 2012), we propose that following injury, nerve-derived, Sox2-positive NCPCs proliferate and migrate together with adjacent mesenchymal cells into injured tissues (e.g., the skin), where they secrete growth factors that regulate mesenchymal cell proliferation and potentially other aspects of wound healing. We also posit that (1) the initial NCPC proliferation and migration are Sox2 dependent (data shown here; Le et al., 2005), and (2) at later repair stages, NCPCs in the injured tissue associate with newly grown axons, differentiate into mature Schwann cells, and once again comprise an essential component of skin nerve innervation. In such a model, nerve-derived NCPCs play two essential roles: they act in a paracrine fashion to enhance tissue repair, and they provide a source of Schwann cells for the newly remodeled innervation. Intriguingly, nerves innervate every tissue in the body, and Schwann cell dedifferentiation is a general response to nerve injury, raising the possibility that nerve-derived NCPCs may play a role in promoting mammalian tissue repair throughout the body.
Experimental Procedures
Animals and Tamoxifen Treatments
This study was approved by the HSC Animal Care Committee in accordance with CCAC guidelines. Sox2+/EGFP (Ellis et al., 2004), Dermo1Cre/+ (Yu et al., 2003), Wnt1-Cre (Danielian et al., 1998), Sox2CreERT2/+ (Arnold et al., 2011), R26TdTomatofl/fl (Madisen et al., 2010), R26YFPfl/fl (Srinivas et al., 2001), Sox2fl/fl (Taranova et al., 2006), and R26CreERT2/+ (Seibler et al., 2003) mice are described in the Supplemental Experimental Procedures. Mice were injected intraperitoneally daily for 5 consecutive days with tamoxifen (3 mg/day) or with sunflower oil. Punch wounds were performed as previously described (Biernaskie et al., 2009; Supplemental Experimental Procedures), and in some experiments, 100 mg/kg BrdU was injected daily commencing on the day of injury.
Tissue Preparation and Immunostaining
We performed morphometric analysis on paraffin-embedded sections and immunofluorescence on cryosections (Biernaskie et al., 2009). Fluorescence images were captured by confocal microscopy. For further details and antibodies, see the Supplemental Experimental Procedures.
Quantitative Analyses and Statistics
Wound closure (calculated as the percentage of healed area relative to the initial wound size) and quantitative morphometric analyses (performed on central sections where wound diameter was largest) are described in the Supplemental Experimental Procedures. The proportion of Ki67-positive cells was measured at the leading edges of the newly formed dermis, and the p75NTR-positive cell area was measured throughout the entire dermal portion of the wound bed. Quantitative analyses were performed blind. Statistics were obtained using Student’s t test (one- or two-tailed as appropriate) or one- or two-way ANOVA as indicated in the text. Error bars indicate SEM.
Acknowledgments
We thank Derek van der Kooy, Brian DeVeale, and Michael Sefton for their intellectual input, and Smitha Paul, Benigno Aquino, Vania Ariosa, and Lily Morikawa for technical advice and assistance. This work was funded by CIHR grant MOP-64211 to F.D.M. A.P.W.J. is funded by an Ontario Stem Cell Initiative fellowship, F.D.M. and D.R.K. hold Canada Research Chairs, and F.D.M. is an HHMI International Research Scholar.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information
References
- Adameyko I., Lallemend F., Aquino J.B., Pereira J.A., Topilko P., Müller T., Fritz N., Beljajeva A., Mochii M., Liste I. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell. 2009;139:366–379. doi: 10.1016/j.cell.2009.07.049. [DOI] [PubMed] [Google Scholar]
- Amoh Y., Li L., Katsuoka K., Penman S., Hoffman R.M. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc. Natl. Acad. Sci. USA. 2005;102:5530–5534. doi: 10.1073/pnas.0501263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K., Sarkar A., Yram M.A., Polo J.M., Bronson R., Sengupta S., Seandel M., Geijsen N., Hochedlinger K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J., Paris M., Morozova O., Fagan B.M., Marra M., Pevny L., Miller F.D. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell. 2009;5:610–623. doi: 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell I., Guevara E., Bai C.B., Loomis C.A., Joyner A.L. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian P.S., Muccino D., Rowitch D.H., Michael S.K., McMahon A.P. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Driskell R.R., Giangreco A., Jensen K.B., Mulder K.W., Watt F.M. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–2823. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis P., Fagan B.M., Magness S.T., Hutton S., Taranova O., Hayashi S., McMahon A., Rao M., Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev. Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Jessen K.R., Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- Kumar A., Brockes J.P. Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci. 2012;35:691–699. doi: 10.1016/j.tins.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Le N., Nagarajan R., Wang J.Y.T., Araki T., Schmidt R.E., Milbrandt J. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc. Natl. Acad. Sci. USA. 2005;102:2596–2601. doi: 10.1073/pnas.0407836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello S., Napoli I., Ribeiro S., Wingfield Digby P., Fedorova M., Parkinson D.B., Doddrell R.D., Nakayama M., Adams R.H., Lloyd A.C. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143:145–155. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibler J., Zevnik B., Küter-Luks B., Andreas S., Kern H., Hennek T., Rode A., Heimann C., Faust N., Kauselmann G. Rapid generation of inducible mouse mutants. Nucleic Acids Res. 2003;31:e12. doi: 10.1093/nar/gng012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber-Blum M., Hu Y. Epidermal neural crest stem cells (EPI-NCSC) and pluripotency. Stem Cell Rev. 2008;4:256–260. doi: 10.1007/s12015-008-9042-0. [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.-S., William C.M., Tanabe Y., Jessell T.M., Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taranova O.V., Magness S.T., Fagan B.M., Wu Y., Surzenko N., Hutton S.R., Pevny L.H. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keymeulen A., Mascre G., Youseff K.K., Harel I., Michaux C., De Geest N., Szpalski C., Achouri Y., Bloch W., Hassan B.A., Blanpain C. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J. Cell Biol. 2009;187:91–100. doi: 10.1083/jcb.200907080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S., Ema H., Karlsson G., Yamaguchi T., Miyoshi H., Shioda S., Taketo M.M., Karlsson S., Iwama A., Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- Yu K., Xu J., Liu Z., Sosic D., Shao J., Olson E.N., Towler D.A., Ornitz D.M. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


