Abstract
We investigated the role of canonical WNT signaling in mesoderm and hematopoietic development from human embryonic stem cells (hESCs) using a recombinant human protein-based differentiation medium (APEL). In contrast to prior studies using less defined culture conditions, we found that WNT3A alone was a poor inducer of mesoderm. However, WNT3A synergized with BMP4 to accelerate mesoderm formation, increase embryoid body size, and increase the number of hematopoietic blast colonies. Interestingly, inclusion of WNT3A or a GSK3 inhibitor in methylcellulose colony-forming assays at 4 days of differentiation abrogated blast colony formation but supported the generation of mesospheres that expressed genes associated with mesenchymal lineages. Mesospheres differentiated into cells with characteristics of bone, fat, and smooth muscle. These studies identify distinct effects for WNT3A, supporting the formation of hematopoietic or mesenchymal lineages from human embryonic stem cells, depending upon differentiation stage at the time of exposure.
Graphical Abstract
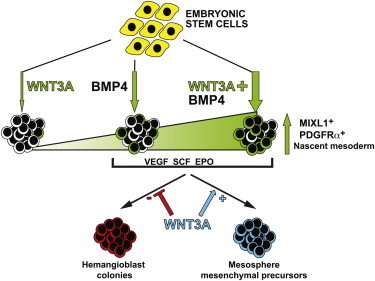
Highlights
-
•
WNT3A alone was a poor inducer of mesoderm from hESCs in defined medium
-
•
WNT3A and BMP4 synergized to generate hemangioblast colony-forming cells
-
•
Added later, WNT3A blocked hematopoiesis and generated mesenchymal colonies
-
•
The timing of growth factor addition significantly influences differentiation
Introduction
WNT signaling is involved in multiple processes during early development, including the maintenance and/or proliferation of stem and progenitor populations, cell fate specification, segmentation, and dorsal-ventral patterning (Logan and Nusse, 2004). During gastrulation in the mouse, WNT signaling plays a critical role in the generation of mesoderm, with Wnt3-null embryos failing to form a primitive streak (Liu et al., 1999), the structure from which hematopoietic progenitors and all other mesodermal and endodermal lineages emerge (Kinder et al., 1999).
The role of WNT signaling has also been examined at later stages of hematopoietic development. Mouse knockout studies indicate that WNT3A is required for the maintenance of long-term hematopoietic stem cell (HSC) and multipotent progenitors and that WNT3A is the critical ligand that activates canonical WNT signaling in fetal liver HSCs (Luis et al., 2010). These findings are in agreement with earlier work suggesting WNT3A can preserve the immature phenotype of HSCs in vitro or can induce stem cell characteristics in hematopoietic progenitors (Malhotra et al., 2008). Indeed, recent studies utilizing mice carrying hypomorphic alleles of the Apc gene, which binds the WNT signaling intermediate, β-catenin, showed that WNT levels regulate HSCs as well as myeloid and T lymphoid progenitors (Luis et al., 2011). These investigators determined that increasing levels of WNT signaling enhanced T cell differentiation and eventually depleted HSCs due to reduced self-renewal (Luis et al., 2011).
Although difficult to study in vivo, the critical early stages of hematopoietic lineage commitment and development can be modeled in vitro using embryonic stem cell (ESC) differentiation. Studies have confirmed that WNT signaling is required for mesoderm formation from differentiating ESCs and for the subsequent emergence of hematopoietic progenitors from mouse (Cheng et al., 2008; Gadue et al., 2006; Jackson et al., 2010; Lako et al., 2001; Lengerke et al., 2008; Lindsley et al., 2006; Nakanishi et al., 2009; Nostro et al., 2008) and human (Murry and Keller, 2008; Sumi et al., 2008; Vijayaragavan et al., 2009; Wang and Nakayama, 2009; Woll et al., 2008) ESCs.
The literature cited above underscores the requirement for WNT signaling at different points during the genesis of the hematopoietic system. However, many prior differentiation studies included either stromal layers or undefined media components (Cheng et al., 2008; Gadue et al., 2006; Lako et al., 2001; Lindsley et al., 2006; Vijayaragavan et al., 2009; Woll et al., 2008), raising the possibility that some of the observed effects of WNTs resulted from complex interactions with unknown factors. To address this issue, we developed a defined medium (APEL) that allows the activity of exogenously added factors to be assessed free from the influence of uncharacterized media components, including bovine serum albumin (BSA), knockout serum replacer (KOSR), or serum (Ng et al., 2008).
In this study, we employed APEL-based differentiation to assess the contribution of WNT signaling to hematopoietic development from human embryonic stem cells. Although mesoderm formation was dependent upon WNT signals, WNT3A alone was an inefficient inducer of mesoderm. However, WNT3A synergized strongly with BMP4 to generate MIXL1+ mesoderm from which hematopoietic blast colonies were generated in semisolid media. Surprisingly, the inclusion of WNT3A in the methylcellulose abrogated blast colony development and led instead to the formation of mesenchymal colonies, denoted mesospheres. These studies highlight the importance of the timing of growth factor exposure during development and show how WNT signaling during different temporal windows promotes either hematopoietic or mesenchymal differentiation.
Results
We examined the role of WNT signaling during the differentiation of human ESCs (hESCs) in APEL medium toward hematopoietic cells. To evaluate the role of WNT during mesoderm formation, hESCs were differentiated as spin embryoid bodies (EBs) (Ng et al., 2008), supplemented with WNT3A and/or BMP4. To facilitate the analysis, we employed two hESC lines (HES3 MIXL1GFP/w and MEL1 MIXL1GFP/w) in which GFP reports expression of MIXL1, a homeobox gene whose expression is restricted to mesoderm and endoderm precursors in the primitive streak (Davis et al., 2008). In contrast to the results of previous studies that employed BSA-, KOSR-, or N2B27-containing media (Gadue et al., 2006; Nakanishi et al., 2009; Sumi et al., 2008; Wang and Nakayama, 2009), we found that WNT3A alone was a poor inducer of mesendoderm, with few cells expressing MIXL1-GFP at day 4 (d4) (Figures 1A and 1B; Figures S1A and S1B available online). Instead, WNT3A-only-treated EBs resembled those formed in absence of growth factors, with approximately 80% of cells retaining high levels of the undifferentiated hESC/epiblast marker, E-CADHERIN (Figures 1B, 1C, and S1C). Indeed, transcriptional profiling indicated that EBs formed in WNT3A alone displayed a very similar pattern of gene expression to EBs formed in the absence of growth factors (Figure S1D). Nevertheless, in line with previous data from mouse and human ESC studies (Gadue et al., 2006; Jackson et al., 2010; Nostro et al., 2008; Sumi et al., 2008; Wang and Nakayama, 2009; Woll et al., 2008), we found that mesoderm induction by BMP4 was not only antagonized by NOGGIN and dependent on NODAL signaling, but was also WNT dependent, since inclusion of either FZD8 or DKK1 significantly reduced MIXL1-GFP expression (Figures 1D and S1E).
Figure 1.
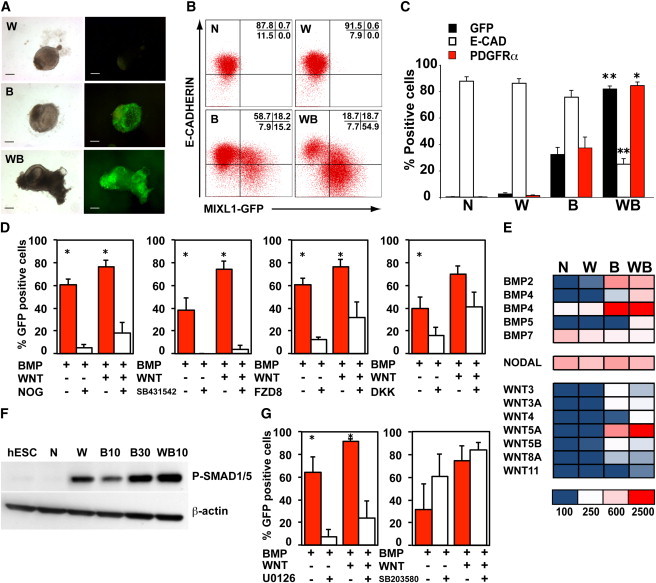
WNT3A Synergizes with BMP4 to Induce Mesoderm
(A) Bright-field and epifluorescence images showing MIXL1-GFP expression in d4 EBs formed in WNT3A/BMP4 (WB) and BMP4 (B) but not in WNT3A alone (W). Scale bar, 200 μm.
(B) Representative flow cytometric profiles of E-CADHERIN and MIXL1-GFP expression in d4 EBs differentiated in APEL medium supplemented with no growth factors (N), WNT3A (W), BMP4 (B), or WNT3A/BMP4 (WB) as indicated. The percentage of cells in each quadrant is shown in upper right of each panel.
(C) Histogram representing the mean frequency of MIXL1-GFP, E-CAD, and PDGFRα-expressing cells assayed by flow cytometry at d4 in EBs formed in the indicated growth factors. Data represent the mean ± SEM from 7 to 12 independent experiments. B and WB groups were compared using Student’s t test. *p < 0.01; **p < 0.001.
(D) Histogram representing the mean frequency of MIXL1-GFP-expressing cells assayed at d4 in EBs formed in BMP4 with or without WNT3A and NOGGIN (300 ng/ml), SB431542 (4 μM), FZD8 (2 μg/ml), or DKK1 (1 μg/ml). Data represent the mean ± SEM from three to five independent experiments. Groups with and without inhibitor were compared using Student’s t test. *p < 0.05; **p < 0.01.
(E) Heatmap showing mean signal intensity of BMPs, NODAL, and WNTs in d4 EBs differentiated in the indicated growth factors. The scale in arbitrary units is shown.
(F) Western blot examining the phosphorylation of SMAD1/5 in hESCs treated for 30 min with the growth factor combinations shown. Blots were probed with anti-β-actin antibodies to indicate that equal amounts of protein were loaded in each track. N, no growth factors; W, WNT3A; B, BMP4; WB, WNT3A/BMP4; P-SMAD 1/5, SMAD 1/5 phosphorylated on Ser463/465.
(G) Histogram representing the mean frequency of MIXL1-GFP-expressing cells assayed at d4 in EBs formed in BMP4 with or with out WNT3A and the ERK inhibitor, U0126 (10 μM) or the p38 inhibitor, SB203580 (10 μM). Data represent the mean ± SEM from three independent experiments. Groups with and without inhibitor were compared using Student’s t test. *p < 0.05.
See also Figures S1, S2, and Tables S1 and S2.
Analysis of d4 EBs revealed a strong synergy between WNT3A and BMP4, with 82% ± 2% of cells treated with both growth factors expressing MIXL1-GFP, compared to 32% ± 5% and 2.5% ± 0.8% GFP+ cells in cultures treated with 10 ng/ml of BMP4 alone or 100 ng/ml of WNT3A alone, respectively (Figure 1C). Induction of MIXL1-GFP was associated with a parallel increase in expression of the primitive streak and early mesoderm marker, platelet-derived growth factor receptor (PDGFR)α (Davis et al., 2008), and a reciprocal reduction in the proportion of E-CADHERIN+ cells. Similar responses were observed in both independent MIXL-GFP hESC lines (Figure S1C). Inhibitor studies confirmed that mesoderm induction in hESCs by the combination of WNT3A and BMP4 was also inhibited by antagonists of BMP, NODAL, and WNT signaling (Figures 1D and S1E). In agreement with this finding, microarray analysis demonstrated that BMP4- or WNT3A/BMP4-induced EBs expressed BMPs, NODAL, and WNT genes (Figure 1E), consistent with our observations that endogenously produced growth factors in differentiating mouse ESCs provided paracrine or autocrine mesoderm inducing signals (Jackson et al., 2010). The increased expression of these genes in WNT3A/BMP4-treated hESCs was explicable by the higher proportion of MIXL1-GFP+ cells generated under this condition. Microarray analysis indicated that this subpopulation expressed growth factor genes at the highest levels (Figure S1F).
Previous reports have suggested that WNTs modulate BMP signaling by promoting C-terminal phosphorylation of SMAD1/5, key components of the BMP signal transduction pathway (Fuentealba et al., 2007). Therefore, we compared the phosphorylation of SMAD1/5 in EBs formed in the combination of WNT3A/BMP4 to those differentiated with either factor alone (Figures 1F and S1G). This analysis confirmed that WNT3A led to SMAD1/5 phosphorylation and the combination of 100 ng/ml WNT3A and 10 ng/ml BMP4 resulted in levels of SMAD1/5 phosphorylation that exceeded those seen in EBs treated with either factor alone and was comparable to the SMAD1/5 phosphorylation observed in EBs formed in 30 ng/ml BMP4. Collectively, these results suggested that enhanced BMP4 signaling might be in part responsible for the synergistic activity of WNT3A during BMP4-induced hESC differentiation. However, the fact that WNT3A alone was unable to efficiently induce MIXL1 expression also indicated that signaling involving SMAD1/5 intermediates was not sufficient for robust mesoderm induction. Given that transforming growth factor (TGF)-β family proteins also signal via MAPK pathways in a SMAD-independent fashion (Derynck and Zhang, 2003), we examined the effects of ERK and p38 inhibition on the induction of MIXL1-GFP by BMP4 and WNT3A/BMP4. Interestingly, while inhibition of ERK by U0126 reduced the frequency of MIXL1-GFP+ cells, differentiation was augmented by inhibiting p38 MAPK with SB203580 (Figure 1G).
A recent report suggested that treatment with WNT3A primed cells to primitive endoderm in mouse ESCs and enhanced visceral endoderm differentiation (Price et al., 2012). In order to determine whether a similar situation existed in hESCs, we compared the transcriptional profiles of the small percentage of GFP+ cells that could be isolated from MIXL1-GFP hESCs differentiated in WNT3A for 4 days with the gene expression patterns of GFP+ cells sorted from parallel cultures stimulated by a combination of WNT3A/BMP4 (Figures S2A–S2C). Consistent with the reported mouse ESC data, we observed enhanced expression of endodermal genes such as FOXA1, FOXA2, SOX17, CCKBR, APOA2, and CXCR4 in the WNT3A-induced GFP+ cells, while the GFP+ cells purified from the WNT3A/BMP4 cultures expressed higher levels of primitive streak and mesoderm associated genes (Figure S2C; Tables S1 and S2).
Time-course analysis indicated that MIXL1-GFP hESCs differentiated in APEL supplemented with the combination of WNT3A and BMP4 more rapidly gained GFP and PDGFRα expression and more rapidly lost E-CADHERIN expression compared with cells treated with BMP4 alone (Figures 2A, 2B, and S3A). In the examples shown, differentiation proceeded rapidly and the accelerated onset of PDGFRα and MIXL1 expression and the loss of surface E-CADHERIN were evident by days 2 and 3 of differentiation. These conditions did not induce significant expression of surface CXCR4, a marker often used as an early indicator of endodermal differentiation. In addition to the synergy observed between WNT3A and BMP4 in enhancing the rate of mesoderm formation, d4 EBs formed in WNT3A/BMP4 containing medium were 2-to 3-fold larger, suggesting that WNT3A contributed to increased proliferation or decreased cell death (Figures 2C and S3B).
Figure 2.
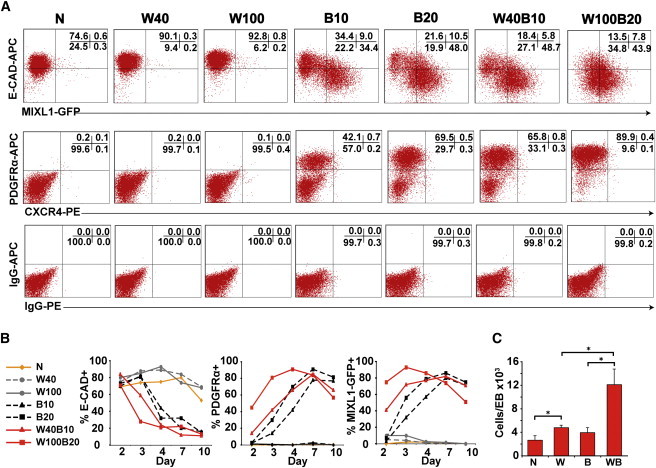
WNT3A Accelerates BMP4-Dependent Mesoderm Differentiation and Enhances EB Size
(A) Representative flow cytometry profiles showing the percentage of MIXL1-GFP+, E-CAD+, CXCR4+, and PDGFRα+ cells in d4 EBs differentiated in APEL medium supplemented with the indicated growth factors. These profiles form part of the time-course experiment shown in (B). The fraction of cells present in each quadrant is indicated.
(B) Graphical representation of flow cytometry data showing the percentage of MIXL1-GFP+, E-CAD+, and PDGFRα+ cells in EBs differentiated in APEL medium supplemented with the growth factors indicated at different time points. The concentration of growth factors in ng/ml is indicated. Data are shown from one representative experiment, with an independent example shown in Figure S3A.
(C) Histogram representing the mean cell number per EB at d4 in cultures induced with the growth factor combinations indicated. Data represent the mean ± SEM from five independent experiments. Groups were compared using Student’s t test. *p < 0.05.
N, no growth factors; W, WNT3A; B, BMP4; WB, WNT3A/BMP4. See also Figure S3.
We disaggregated d4 EBs and flow-sorted populations on the basis of E-CADHERIN and MIXL1-GFP expression (Figures 3A, 3B and S4). Day 4 EBs formed in WNT3A/BMP4-containing medium included a larger percentage of cells with a more mature phenotype (E−G+) than was observed in EBs differentiated in BMP4. Comparison of transcriptional profiles of the sorted cell populations revealed that genes expressed by corresponding fractions were very similar in both the BMP4- and WNT3A/BMP4-treated EBs, consistent with the notion that WNT3A primarily accelerated the differentiation observed with BMP4 alone (Figures 3C–3E and S5A–S5H). Examination of the transcription factors whose expression was most highly upregulated in the WNT3A/BMP4 E−G+ cells revealed that they were primitive streak or posterior mesodermal genes that were frequently expressed at a similar level in the corresponding BMP4 sorted fraction (Figure 3F).
Figure 3.
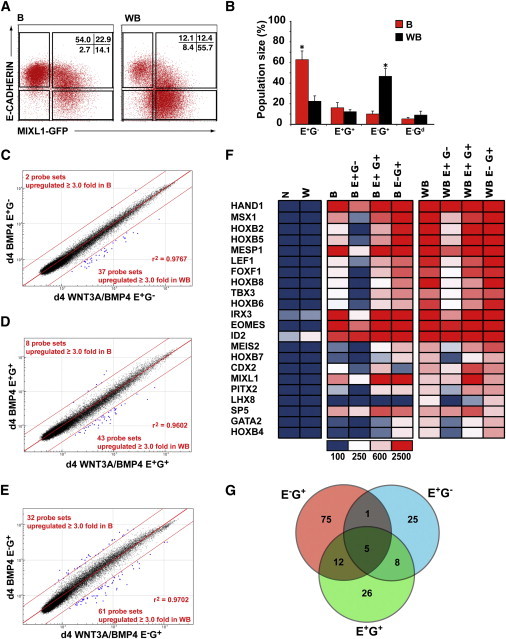
Transcriptional Profiles Confirm Accelerated Differentiation in WNT3A/BMP4 EBs
(A) Flow cytometry plots showing the position of gates used for sorting experiments based on E-CADHERIN and MIXL1-GFP expression in d4 EBs differentiated in APEL medium supplemented with BMP4 (B) and WNT3A/BMP4 (WB). The percentage of cells falling into each quadrant is shown.
(B) Histogram showing the mean percentage of cells in E-CADHERIN- (E) and MIXL1-GFP- (G) expressing populations. Data represent the mean ± SEM from four independent experiments. B and WB groups were compared using Student’s t test. *p < 0.05.
(C–E) Comparison of transcriptional profiles of sorted cell populations shown in (A) and (B) derived from BMP4- and WNT3A/BMP4-stimulated cultures. Enhanced dots outside the parallel red lines indicate probe sets differing by ≥3-fold from the mean.
(F) Heatmap showing the expression of transcription factors upregulated in d4 WNT3A/BMP4 E−G+ cells. These primitive streak or posterior mesodermal genes were frequently expressed at a similar level in the corresponding BMP4 sorted fraction. The scale for mean signal intensity in arbitrary units is shown.
(G) Venn diagram illustrating the overlap between the differentially expressed probe sets in the sorted populations identified in (C)–(E). The genes recognized by the E−G+ and E+G+ probe sets and their level of expression are listed in Tables S3 and S4.
See also Figures S4 and S5.
Of the small number of genes whose transcription differed by ≥3-fold between the two treatment groups (39, 51, and 93 out of 48,701 probe sets for E+G−, E+G+, and E−G+ fractions) (Figure 3G), most were upregulated in the WNT3A/BMP4-treated EBs (37, 43, and 61 probe sets, respectively). In the GFP+ fractions, these included transcripts for the WNT8 inhibitor RGS4 and for genes associated with anterior lateral and paraxial mesoderm, such as ACTC1, CDX2, LHX8, C6ORF32, and LUM, consistent with the role of WNT signaling in cardiac and somitic differentiation (Cohen et al., 2008; Geetha-Loganathan et al., 2008) (Tables S3 and S4).
We compared the frequency of hematopoietic blast colony forming cells (Bl-CFCs) in d4 EBs cultured in BMP4 alone and in WNT3A/BMP4. We have previously demonstrated that these mesodermally derived precursors displayed the capacity to differentiate into erythroid cells, endothelium, and smooth muscle (Davis et al., 2008; Yu et al., 2012), similar to results in mouse and human ESCs reported by other laboratories (Choi et al., 1998; Kennedy et al., 2007) and to hemangioblasts isolated from the mouse embryo (Huber et al., 2004). Examination of their hematopoietic potential revealed that d4 EBs formed in WNT3A/BMP4 medium generated Bl-CFCs at a slightly higher frequency than those differentiated in BMP4 alone (49 ± 12 and 27 ± 11 Bl-CFC/104 d4 EB cells, respectively), although the differences were not statistically significant (Figures 4A and 4B). Since they were cultured in MC supplemented with erythropoietin (EPO), most blast colonies contained a predominance of primitive erythroid cells (Figure 4A). Given their larger size, these data suggested that d4 EBs formed in WNT3A/BMP4 generated ∼5-fold greater number of Bl-CFCs per input hESC than those differentiated in BMP4 alone. Most Bl-CFCs were found in the MIXL1-GFP+ fractions as expected (Davis et al., 2008), with the highest frequency and greatest proportion in the E−G+ population (Figures 4B and 4C). In d4 WNT3A/BMP4 cultures, nearly 20% of the Bl-CFCs were localized to the most differentiated MIXL1-GFP dim (E−Gd) cells, a population not present in BMP4-only-treated cultures at that time. To test the hypothesis that the combination of WNT3A/BMP4 accelerated the generation of hematopoietic mesoderm, we performed experiments to examine Bl-CFC frequency at d2 and d3 of differentiation. While the maximal frequency of Bl-CFC in BMP4-induced cultures was seen at d3 of differentiation, inclusion of WNT3A accelerated the generation of these precursors such that maximal frequency was observed earlier, after just 2 days (Figure 4D).
Figure 4.
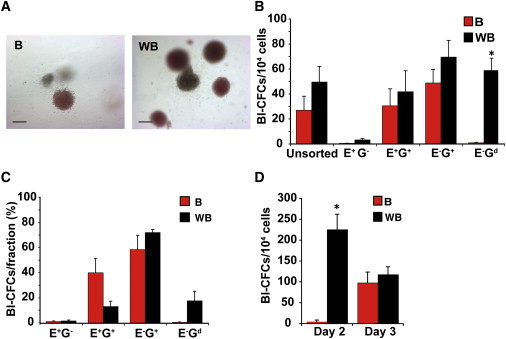
WNT3A Positively Influences Hematopoietic Development
(A) Representative images showing hemangioblast colonies in methylcellulose cultures derived from d4 EBs formed in APEL medium supplemented with BMP4 (B) or WNT3A/BMP4 (WB). Scale bar, 100 μm.
(B) Histogram showing the mean frequency of hemangioblast colony-forming cells (Bl-CFCs) in methylcellulose cultures seeded with cells from B and WB unsorted populations and the indicated sorted fractions.
(C) Percentage of the Bl-CFCs that fall within each sorted fraction.
(D) Histogram showing the mean frequency of Bl-CFCs in methylcellulose cultures seeded with cells from B and WB unsorted populations after 2 or 3 days of differentiation. Data in (B)–(D) represent the mean ± SEM from three to four independent experiments. Paired B and WB groups (B–D) were compared using Student’s t test. Asterisks indicate samples in which statistically significant differences in Bl-CFC frequency between B and WB differentiated cultures were observed (p < 0.05 in B and p < 0.01 in D).
To determine whether the WNT3A/BMP4 combination ultimately generated a greater frequency of more mature hematopoietic cells and progenitors, we analyzed d4 EBs that had been cultured for a further 7–9 days in medium supplemented with BMP4, vascular endothelial growth factor (VEGF), stem cell factor (SCF), interleukin (IL)-3, and EPO (Figures 5A–5C). In cultures initiated in either no growth factor or WNT3A alone, only small numbers of hematopoietic or endothelial cells were generated, as evidenced by a low percentage of cells expressing CD31, CD34, and CD45 and the detection of infrequent hematopoietic CFCs in methylcellulose (Figures 5A–5C). Conversely, a much higher percentage of hematopoietic cells were generated in cultures initiated in BMP4, and the inclusion of WNT3A further enhanced hematopoietic differentiation. Although the proportion of CD31+ and CD34+ was similar in cultures initiated in the presence of WNT3A/BMP4 or BMP4, the frequency of CD45+ cells and CFCs were consistently higher in WNT3A/BMP4-treated EBs (Figures 5B and 5C). Thus, the synergy between WNT3A and BMP4 during the first 4 days of EB differentiation and mesoderm formation resulted in an augmented generation of hematopoietic cells a week later, underscoring the importance of WNT signaling at the earliest commitment steps during hESC-derived hematopoiesis.
Figure 5.
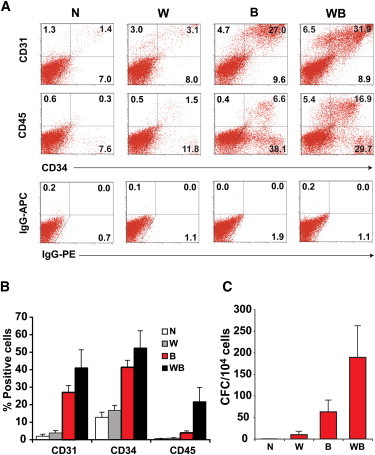
WNT3A Synergizes with BMP4 to Enhance Generation of Hematopoietic Progenitor Cells
(A) Representative flow cytometry profiles comparing the expression of CD31, CD34, and CD45 in d11 cultures derived from d4 EBs formed in the presence of the indicated growth factors. Cultures were supplemented with VEGF and SCF and received additional BMP4, VEGF, SCF, IL-3, and EPO from d4, as outlined in Table S1. Immunoglobulin (Ig)G-APC and IgG-PE represent the isotype control staining. The percentage of cells falling within each region is shown.
(B) Histograms summarizing data examining the percentage of cells positive for each antibody at d11–d13 of differentiation. Data represent the mean ± SEM from three independent experiments. Analysis using one-way ANOVA with Bonferroni post test indicated that cultures treated with different combinations of WNT3A and BMP4 growth factors differed significantly as measured by the percentage of cells expressing CD31 (p < 0.01), CD34 (p < 0.005), and CD45 (p < 0.05).
(C) Frequency of hematopoietic CFCs in methylcellulose cultures seeded with cells at d11–13 differentiated under the indicated growth factor conditions (n = 3 independent experiments). The mean CFC frequencies were significantly different between culture groups (p < 0.05), using one-way ANOVA with Bonferroni post test.
Because many growth factors function in a context-dependent manner, we explored the effects of adding WNT3A to the MC on the growth of Bl-CFCs. In cultures containing a combination of hematopoietic growth factors (VEGF, SCF, IL-3, IL-6, thrombopoietin [TPO], EPO, and FLT3L), 0.2%–0.6% of cells derived from EBs differentiated with BMP4 or WNT3A/BMP4 generated Bl-CFC colonies at d4 (Figures 6A–6D). Strikingly, addition of WNT3A to the methylcellulose dramatically reduced the frequency of hematopoietic blast colonies and instead supported the growth of compact, mesodermal colonies, termed “mesospheres,” at a similar frequency (0.4%–0.6%) to that observed for Bl-CFCs (Figures 6A, 6B, 6E–6G, S6A, and S6B). However, because mesospheres and blast colonies required mutually exclusive culture conditions, it was not possible to determine if they arose from common progenitor. We also demonstrated that the effects of adding WNT3A to the methylcellulose could be replicated with (2′Z,3′E)-6-bromoindirubin-3′-oxime (BIO), a canonical WNT signaling agonist that inhibits GSK3 (Figures S7A and S7B).
Figure 6.
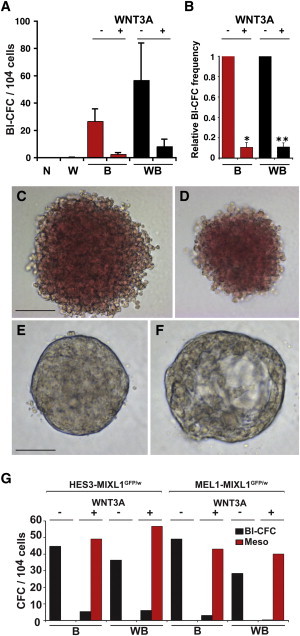
WNT3A Addition to Methylcellulose Cultures Suppresses Hematopoiesis and Promotes Mesodermal Colony Development from d4 EBs
(A) Frequency of hematopoietic Bl-CFCs in methylcellulose cultures containing hematopoietic growth factors with (+) or without (−) WNT3A addition to the methylcellulose. Cultures were seeded with cells derived from d4 EBs from the HES3-MIXL1GFP/w hESC line grown without added growth factors (N), or in the presence of WNT3A (W), BMP4 (B), or WNT3A/BMP4 (WB).
(B) Colony frequency was normalized to methylcellulose cultures supplemented with hematopoietic growth factors alone, demonstrating the suppressive effect of WNT3A. Data in (A) and (B) represent the mean ± SEM of four independent experiments. WNT3A (+) and (−) groups in (B) were compared using Student’s t test. *p < 0.01; **p < 0.001.
(C and D) Bright-field images showing the morphology of hemangioblast colonies formed in (C) BMP4-and (D) WNT3A/BMP4-stimulated d4EBs that were disaggregated and cultured with hematopoietic growth factors (see Table S1). Scale bar, 100 μM.
(E and F) Bright-field images of nonhematopoietic mesodermal colonies (mesospheres) formed in methylcellulose cultures supplemented with WNT3A. Cysts (F) were frequently observed. Scale bar, 200 μm.
(G) Frequency of hematopoietic blast colonies (Bl-CFC) and mesospheres (Meso) from EBs grown in the presence of either BMP4 (B) or WNT3A/BMP4 (WB) for 4 days that were subsequently disaggregated and cultured in methylcellulose containing hematopoietic growth factors with (+) or without (−) WNT3A addition to the methylcellulose. Results from representative experiments using HES3- and MEL1-MIXL1GFP/w cell lines are shown. See also Figures S6 and S7.
Gene profiling revealed that the WNT3A- and BIO-induced mesospheres contained cells that expressed early mesodermal genes such as FOXF1, MEOX1, PDGFR, HAND1, and SNAI2 and were highly enriched for transcripts associated with chondrocyte and bone differentiation, such as DLK1, LUM, MGP, COL15A1, COL3A1, FRZB, ITGA5, and CDH11 as well as many other extracellular matrix (ECM) proteins (Figure 7A; Table S5). Notably, several ECM proteins upregulated in WNT and BIO mesospheres (TNC, DCN, and FMOD) were recently shown to be highly expressed in OP9 cells engineered to constitutively express WNT3A (Ichii et al., 2012). The gene expression profiles of WNT and BIO mesospheres were very similar, with 689/979 (∼70%) of the probe sets upregulated in BIO mesospheres also upregulated in WNT mesospheres (Figures S7C and S7D; Table S5).
Figure 7.
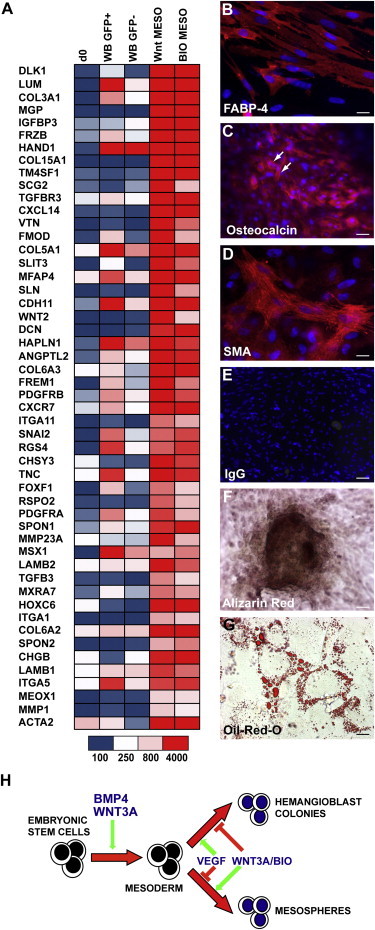
WNT3A-Induced Mesospheres Differentiate to Osteogenic, Adipogenic, and Smooth Muscle Lineages
(A) Heatmap showing that WNT3A- and BIO-induced mesospheres were enriched for transcripts associated with chondrocyte and bone differentiation. The expression of most of these genes differed in sorted d4 WNT3A/BMP GFP+ and GFP− fractions. The scale in arbitrary units is shown.
(B–G) Immunocytochemical and histochemical analysis of WNT3A-induced mesodermal colonies recultured and subjected to mesenchymal stem cell differentiation toward osteogenic and adipogenic fates. FABP-4 (B) and oil red O (G) reactivity confirmed adipogenic potential and osteogenic potential was confirmed by expression of OSTEOCALCIN (C, arrows) and alizarin red staining (F). The presence of smooth muscle was shown by SMA immunoreactivity (D). (E) IgG control for (B)–(D). Scale bars, 50 μM (B and D), 100 μM (C and G), and 200 μM (E and F).
(H) Model illustrating that the effects of WNT3A during mesoderm differentiation depend on the time of exposure. WNT3A synergizes with BMP4 to increase mesodermal differentiation. Methylcellulose cultures from BMP4- and WNT3A/BMP4-induced EBs stimulated with VEGF without addition of WNT3A or BIO form hemangioblast colonies, while cultures with WNT3A or BIO do not form hematopoietic colonies, but form mesospheres capable of differentiation toward bone, fat, and smooth muscle.
See also Table S5.
These gene expression profiles suggested nonhematopoietic mesodermal lineage potentials for the mesospheres. Indeed, we subsequently demonstrated that the mesospheres could differentiate toward adipocyte (marked by oil red O droplets and expression of FABP-4), osteoblast (marked by the formation of alizarin red aggregates and OSTEOCALCIN expression), and smooth muscle (marked by smooth muscle actin [SMA] expression) lineages (Figures 7B–7G). Whether the mesospheres contained multipotent progenitors or lineage restricted precursors for additional mesenchymal lineages remains to be determined.
Discussion
We explored the role of WNT signaling during hematopoietic differentiation from hESCs in APEL medium, identifying distinct activities at different stages of differentiation. First, WNT was required for the induction of mesoderm, an outcome anticipated from prior published literature, including our own studies in differentiating mouse ESCs (Gadue et al., 2006; Jackson et al., 2010; Lengerke et al., 2008; Murry and Keller, 2008; Woll et al., 2008). We confirmed the requirement for BMP and NODAL signals in mesoderm differentiation and demonstrated that inhibition of ERK and p38 MAP kinases mediated inhibitory or stimulatory signals, respectively, downstream of BMP4 that impacted on MIXL1-GFP expression. Our observation that ERK inhibition reduced mesoderm induction by BMP is consistent with the finding of Zhang and colleagues, who demonstrated BMP4-mediated ERK activation suppressed neural differentiation of mouse ESCs (Zhang et al., 2010).
Studies have shown that BMP4-induced ERK phosphorylation was required in other cellular contexts, including BMP4-dependent capillary sprouting in HUVECs and stabilization of Runx2 expression during osteoblast differentiation of C2C12 cells (Jun et al., 2010; Zhou et al., 2007). Similar to our findings, opposing actions of ERK and p38 MAPK were reported for other mesodermal lineages. For example, ERK inhibition enhanced chondrogenesis in chick limb bud mesenchyme, while p38 inhibition had the opposite effect (Oh et al., 2000). Similarly, BMP4-stimulated generation of VEGF in mouse osteoblast-like MC3T3-E1 cells was reduced in the presence of p38 inhibition but was unaffected by blocking ERK (Tokuda et al., 2003).
Although WNT3A in isolation was a poor mesoderm inducer in hESCs differentiated in APEL medium, it synergized with BMP4 to efficiently induce MIXL1-GFP+ mesoderm, increasing the yield of Bl-CFCs and hematopoietic CFCs. Our data complement studies in which specific roles for BMP and WNT signaling during the formation of hematopoietic mesoderm from mouse ESCs were identified (Gadue et al., 2006; Jackson et al., 2010; Lengerke et al., 2008; Nostro et al., 2008). In contrast to our findings, a number of studies have demonstrated that primitive streak induction in differentiating mouse ESCs could be mediated by WNT3A alone (Gadue et al., 2006; Jackson et al., 2010; Nostro et al., 2008). While there may be differences between mouse and human ESC differentiation, we speculate that this discrepancy may reflect the influence of components of mouse ESC differentiation medium that are absent from APEL (such as BMP-like activity associated with serum or BSA). This hypothesis is consistent with the observed reduction in the hematopoietic-inducing activity of WNT3A by the BMP antagonist NOGGIN when human ESCs were differentiated in BSA-based medium (Wang and Nakayama, 2009).
The synergy between WNT3A and BMP4 in the generation of human hematopoietic cells was consistent with the observations that BMP4 induced ventral-posterior mesoderm and subsequently directed mesodermal cells toward a blood fate by activating WNT3A signaling and Cdx gene expression in mouse ESCs (Lengerke et al., 2008). Similarly, Nostro et al. showed that BMP4 had a strong posteriorizing effect on mesendodermal progenitors (Nostro et al., 2008), an effect that was likely to promote the generation of hematopoietic progenitors. Our study complements this work, showing that synergy between WNT3A and BMP during mesoderm induction enhanced the subsequent formation of hematopoietic progenitors through an increased rate of differentiation toward MIXL1+ mesendoderm and by an increase in cell numbers within EBs.
Other studies have examined the role of noncanonical and canonical WNT signaling during different stages of hematopoietic development from human pluripotent stem cells. Woll and colleagues examined the influence of WNT1, WNT5, and the WNT inhibitor, DKK1, on hematopoietic development from hESCs (Woll et al., 2008). Using stromal cell coculture, they demonstrated that hematoendothelial precursors were suppressed by DKK1, while their development was accelerated following exposure to WNT1, but not WNT5. The results of their study suggested that canonical but not noncanonical signaling supported the development of a hemogenic precursor, but did not specifically address the stage of differentiation at which the WNT signaling was required (Woll et al., 2008). Our results, indicating synergy between canonical WNT and BMP4 signaling in the generation of hemangioblasts, are consistent with these data.
Conversely, results from a second study examining hematopoietic development from hESCs argued that noncanonical signaling by WNT11 was important in guiding hESC differentiation toward mesoderm, while canonical signaling mediated by WNT3A acted only later in differentiation to promote proliferation of hematopoietic progenitors (Vijayaragavan et al., 2009). This latter observation is consistent with studies demonstrating a regulatory role of canonical WNT signaling on hematopoietic stem and progenitor cells in mouse bone marrow (Luis et al., 2011; Trowbridge et al., 2010). Indeed, recent studies examining factors regulating hematopoietic regeneration in the zebrafish and mouse demonstrated the requirement for both BMP and WNT signals (Trompouki et al., 2011).
Although it is difficult to readily reconcile all the findings from these studies, the data could be interpreted to suggest that there may be several windows during which WNT signaling differentially affects hematopoietic development. First, we have shown that the early addition of WNT3A synergistically expanded and accelerated the development of BMP4-induced hematopoietic mesoderm, but that the clonal growth of hemangioblast colonies required the removal of WNT3A. This is analogous to the sequential roles of WNT during cardiogenesis elucidated in vertebrate embryos and differentiating mouse and human ESCs (Mummery et al., 2012; Tzahor, 2007). Second, canonical WNT signaling promotes the growth of hematopoietic stem cells and committed hematopoietic progenitors, both during development and in the adult. Finally, the complexity of WNT interactions influencing hematopoiesis is increased by recent data that indicate that WNT signaling also impacts on the bone marrow stromal niche, inducing secretion of a range of mediators that influence hematopoietic stem and progenitor activity (Ichii et al., 2012).
Concomitant with its suppressive effect on blast colony formation, the inclusion of WNT3A, or the WNT agonist BIO, in methylcellulose cultures promoted the emergence of colonies we termed “mesospheres” (Figure 7H). These colonies were capable of osteogenic, adipogenic, and smooth muscle differentiation and were enriched for mesoderm markers including APLNR, PDGFRA, PDGFRB, FOXF1, HAND1, SNAI2, and CDH11, reminiscent of the mesenchymal stromal cells (MSCs) differentiated from hESCs reported by a number of laboratories (Karlsson et al., 2009). In contrast to mesospheres, that emerged in serum-free MC in response to WNT3A, most hESC-MSCs were derived in medium supplemented with serum or serum replacer plus FGF2.
It is interesting to speculate on the relationship between mesosphere-forming cells and a mesoderm-derived precursor cell, the mesenchymoangioblast, that gives rise to endothelium and mesenchymal stem cells (Slukvin and Vodyanik, 2011; Vodyanik et al., 2010). Interestingly, formation of mesenchymal cells was inhibited by VEGF, which has been postulated to block the necessary endothelial-mesenchymal transition (EndMT) (Medici and Olsen, 2012; Slukvin and Vodyanik, 2011). In contrast, mesosphere development in methylcellulose cultures occurred in the presence of VEGF. A plausible hypothesis linking the WNT3A dependence of mesospheres with the prior findings of Slukvin and colleagues, is that WNT3A stimulated the endothelial-mesenchymal transition from a mesenchymoangioblast-derived VEGF-dependent angiogenic precursor, thus overcoming the inhibitory effect of VEGF on EndMT. There is evidence for involvement of WNT signaling in both epithelial-mesenchymal transitions (Wu et al., 2012) and EndMT (von Gise and Pu, 2012), with canonical WNT signaling required for the endocardial-mesenchymal transition during endocardial cushion formation in mice (Liebner et al., 2004). However, an alternative hypothesis would postulate that mesospheres represent the expanded progeny of a distinct mesenchymal progenitor cell that did not pass through an endothelial precursor stage.
In summary, our data demonstrate that modulation of the timing of WNT signaling plays a critical role in the genesis of the hematopoietic system and the formation of other mesodermal derivatives. It will be of interest to determine whether this temporally dependent action of WNT involves altering the fate of a common precursor or the selection specific progenitors from a pool of cells with a spectrum of different potentials.
Experimental Procedures
The experiments using human embryonic stem cells performed in this study were approved by the Monash University Human Research Ethics Committee (2002/225MC).
Cell Culture, Differentiation, and Flow Cytometric Analysis
MIXL1-GFP reporter lines (HES3 MIXL1GFP/w and MEL1 MIXL1GFP/w) were passaged as previously described (Davis et al., 2008) and differentiated as spin EBs in APEL medium (Ng et al., 2008) supplemented with growth factors or inhibitors as indicated in the Supplemental Experimental Procedures. Methylcellulose (MC) hematopoietic colony-forming assays were performed in serum-free MC as described (Ng et al., 2008). For hematopoietic differentiation cultures, d4 EBs were cultured for 7–9 days on gelatinized 6-well dishes in AEL medium (Ng et al., 2008) containing growth factors as listed in the Supplemental Experimental Procedures. Flow cytometric analysis (FACSCalibur BD) and cell sorting (FACSDiva BD) employing the antibodies listed in the Supplemental Experimental Procedures was performed as described (Davis et al., 2008).
Western Blotting and Global Gene Expression
Nuclear extraction and western blotting were performed as described (Lim et al., 2009) using antibodies listed in the Supplemental Experimental Procedures. Total RNA (RNeasy kit, QIAGEN) was amplified, labeled, and hybridized to Human WG-6v2.0 Sentrix or HT-12v3 BeadChips (Illumina) by the Australian Genome Research Facility (http://www.agrf.org.au/). Data were analyzed with Beadstudio Gene Expression Module v3.4 (Illumina) using average normalization across all samples and differential expression analysis using GeneSpring GX10 (Agilent Technologies).
Mesenchymal Differentiation
Colonies formed in MC cultures supplemented with 100 ng/ml WNT3A or 5 μM (2′Z,3′E)-6-bromoindirubin-3′-oxime (BIO) (Calbiochem) were assayed for osteogenic and adipogenic potential using the Human Mesenchymal Stem Cell Functional Identification Kit (R&D Systems) according to the manufacturer’s instructions. Cells generated using this assay were analyzed by immunofluorescence using anti-SMA (BD Biosciences), anti-OSTEOCALCIN, and anti-FABP-4 antibodies (R&D Systems), the histochemical stain alizarin red, and lipophilic dye oil red O (Sigma-Aldrich).
Acknowledgments
The authors thank Robyn Mayberry and Amanda Bruce for provision of cells and Andrew Fryga and Darren Ellemor for flow cytometric sorting. This work was supported by the Australian Stem Cell Centre, Stem Cells Australia, the Juvenile Diabetes Research Foundation, the Qatar National Research Foundation, and the National Health and Medical Research Council (NHMRC) of Australia. E.G.S. and A.G.E. are Senior Research Fellows of the NHMRC. K.G. designed and performed experiments, collected, analyzed and interpreted data, made figures, and wrote the manuscript; L.A.P., E.S.N., C.E.H., and Q.C.Y. designed and performed experiments and made figures; R.P.D. contributed reagents and analytical tools; E.G.S. and A.G.E. designed experiments, collected, analyzed and interpreted data, made figures, provided financial support, and wrote the manuscript. A.G.E., E.G.S., and E.S.N. entered into consultancy agreements with STEMCELL Technologies after the completion of work in this manuscript. No support from the company was provided for these studies.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Accession Numbers
The Array Express (http://www.ebi.ac.uk/arrayexpress/) accession number for the microarray data reported here is E-MEXP-2495.
Supplemental Information
References
- Cheng X., Huber T.L., Chen V.C., Gadue P., Keller G.M. Numb mediates the interaction between Wnt and Notch to modulate primitive erythropoietic specification from the hemangioblast. Development. 2008;135:3447–3458. doi: 10.1242/dev.025916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K., Kennedy M., Kazarov A., Papadimitriou J.C., Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- Cohen E.D., Tian Y., Morrisey E.E. Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135:789–798. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- Davis R.P., Ng E.S., Costa M., Mossman A.K., Sourris K., Elefanty A.G., Stanley E.G. Targeting a GFP reporter gene to the MIXL1 locus of human embryonic stem cells identifies human primitive streak-like cells and enables isolation of primitive hematopoietic precursors. Blood. 2008;111:1876–1884. doi: 10.1182/blood-2007-06-093609. [DOI] [PubMed] [Google Scholar]
- Derynck R., Zhang Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Fuentealba L.C., Eivers E., Ikeda A., Hurtado C., Kuroda H., Pera E.M., De Robertis E.M. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadue P., Huber T.L., Paddison P.J., Keller G.M. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha-Loganathan P., Nimmagadda S., Scaal M., Huang R., Christ B. Wnt signaling in somite development. Ann. Anat. 2008;190:208–222. doi: 10.1016/j.aanat.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Huber T.L., Kouskoff V., Fehling H.J., Palis J., Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- Ichii M., Frank M.B., Iozzo R.V., Kincade P.W. The canonical Wnt pathway shapes niches supportive of hematopoietic stem/progenitor cells. Blood. 2012;119:1683–1692. doi: 10.1182/blood-2011-07-369199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S.A., Schiesser J., Stanley E.G., Elefanty A.G. Differentiating embryonic stem cells pass through ‘temporal windows’ that mark responsiveness to exogenous and paracrine mesendoderm inducing signals. PLoS ONE. 2010;5:e10706. doi: 10.1371/journal.pone.0010706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J.H., Yoon W.J., Seo S.B., Woo K.M., Kim G.S., Ryoo H.M., Baek J.H. BMP2-activated Erk/MAP kinase stabilizes Runx2 by increasing p300 levels and histone acetyltransferase activity. J. Biol. Chem. 2010;285:36410–36419. doi: 10.1074/jbc.M110.142307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson C., Emanuelsson K., Wessberg F., Kajic K., Axell M.Z., Eriksson P.S., Lindahl A., Hyllner J., Strehl R. Human embryonic stem cell-derived mesenchymal progenitors-Potential in regenerative medicine. Stem Cell Res. (Amst.) 2009;3:39–50. doi: 10.1016/j.scr.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Kennedy M., D’Souza S.L., Lynch-Kattman M., Schwantz S., Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinder S.J., Tsang T.E., Quinlan G.A., Hadjantonakis A.K., Nagy A., Tam P.P. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development. 1999;126:4691–4701. doi: 10.1242/dev.126.21.4691. [DOI] [PubMed] [Google Scholar]
- Lako M., Lindsay S., Lincoln J., Cairns P.M., Armstrong L., Hole N. Characterisation of Wnt gene expression during the differentiation of murine embryonic stem cells in vitro: role of Wnt3 in enhancing haematopoietic differentiation. Mech. Dev. 2001;103:49–59. doi: 10.1016/s0925-4773(01)00331-8. [DOI] [PubMed] [Google Scholar]
- Lengerke C., Schmitt S., Bowman T.V., Jang I.H., Maouche-Chretien L., McKinney-Freeman S., Davidson A.J., Hammerschmidt M., Rentzsch F., Green J.B. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell. 2008;2:72–82. doi: 10.1016/j.stem.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Liebner S., Cattelino A., Gallini R., Rudini N., Iurlaro M., Piccolo S., Dejana E. Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J. Cell Biol. 2004;166:359–367. doi: 10.1083/jcb.200403050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S.M., Pereira L., Wong M.S., Hirst C.E., Van Vranken B.E., Pick M., Trounson A., Elefanty A.G., Stanley E.G. Enforced expression of Mixl1 during mouse ES cell differentiation suppresses hematopoietic mesoderm and promotes endoderm formation. Stem Cells. 2009;27:363–374. doi: 10.1634/stemcells.2008-1008. [DOI] [PubMed] [Google Scholar]
- Lindsley R.C., Gill J.G., Kyba M., Murphy T.L., Murphy K.M. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- Liu P., Wakamiya M., Shea M.J., Albrecht U., Behringer R.R., Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat. Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Logan C.Y., Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Luis T.C., Naber B.A., Fibbe W.E., van Dongen J.J., Staal F.J. Wnt3a nonredundantly controls hematopoietic stem cell function and its deficiency results in complete absence of canonical Wnt signaling. Blood. 2010;116:496–497. doi: 10.1182/blood-2010-04-282624. [DOI] [PubMed] [Google Scholar]
- Luis T.C., Naber B.A., Roozen P.P., Brugman M.H., de Haas E.F., Ghazvini M., Fibbe W.E., van Dongen J.J., Fodde R., Staal F.J. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell. 2011;9:345–356. doi: 10.1016/j.stem.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Malhotra S., Baba Y., Garrett K.P., Staal F.J., Gerstein R., Kincade P.W. Contrasting responses of lymphoid progenitors to canonical and noncanonical Wnt signals. J. Immunol. 2008;181:3955–3964. doi: 10.4049/jimmunol.181.6.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici D., Olsen B.R. The role of endothelial-mesenchymal transition in heterotopic ossification. J. Bone Miner. Res. 2012;27:1619–1622. doi: 10.1002/jbmr.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery C.L., Zhang J., Ng E.S., Elliott D.A., Elefanty A.G., Kamp T.J. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ. Res. 2012;111:344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry C.E., Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Nakanishi M., Kurisaki A., Hayashi Y., Warashina M., Ishiura S., Kusuda-Furue M., Asashima M. Directed induction of anterior and posterior primitive streak by Wnt from embryonic stem cells cultured in a chemically defined serum-free medium. FASEB J. 2009;23:114–122. doi: 10.1096/fj.08-111203. [DOI] [PubMed] [Google Scholar]
- Ng E.S., Davis R., Stanley E.G., Elefanty A.G. A protocol describing the use of a recombinant protein-based, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nat. Protoc. 2008;3:768–776. doi: 10.1038/nprot.2008.42. [DOI] [PubMed] [Google Scholar]
- Nostro M.C., Cheng X., Keller G.M., Gadue P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2008;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh C.D., Chang S.H., Yoon Y.M., Lee S.J., Lee Y.S., Kang S.S., Chun J.S. Opposing role of mitogen-activated protein kinase subtypes, erk-1/2 and p38, in the regulation of chondrogenesis of mesenchymes. J. Biol. Chem. 2000;275:5613–5619. doi: 10.1074/jbc.275.8.5613. [DOI] [PubMed] [Google Scholar]
- Price F.D., Yin H., Jones A., van Ijcken W., Grosveld F., Rudnicki M.A. Canonical wnt signaling induces a primitive endoderm metastable state in mouse embryonic stem cells. Stem Cells. 2012;31:752–764. doi: 10.1002/stem.1321. [DOI] [PubMed] [Google Scholar]
- Slukvin I.I., Vodyanik M. Endothelial origin of mesenchymal stem cells. Cell Cycle. 2011;10:1370–1373. doi: 10.4161/cc.10.9.15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi T., Tsuneyoshi N., Nakatsuji N., Suemori H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development. 2008;135:2969–2979. doi: 10.1242/dev.021121. [DOI] [PubMed] [Google Scholar]
- Tokuda H., Hatakeyama D., Shibata T., Akamatsu S., Oiso Y., Kozawa O. p38 MAP kinase regulates BMP-4-stimulated VEGF synthesis via p70 S6 kinase in osteoblasts. Am. J. Physiol. Endocrinol. Metab. 2003;284:E1202–E1209. doi: 10.1152/ajpendo.00300.2002. [DOI] [PubMed] [Google Scholar]
- Trompouki E., Bowman T.V., Lawton L.N., Fan Z.P., Wu D.C., DiBiase A., Martin C.S., Cech J.N., Sessa A.K., Leblanc J.L. Lineage regulators direct BMP and Wnt pathways to cell-specific programs during differentiation and regeneration. Cell. 2011;147:577–589. doi: 10.1016/j.cell.2011.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge J.J., Guezguez B., Moon R.T., Bhatia M. Wnt3a activates dormant c-Kit(-) bone marrow-derived cells with short-term multilineage hematopoietic reconstitution capacity. Stem Cells. 2010;28:1379–1389. doi: 10.1002/stem.457. [DOI] [PubMed] [Google Scholar]
- Tzahor E. Wnt/beta-catenin signaling and cardiogenesis: timing does matter. Dev. Cell. 2007;13:10–13. doi: 10.1016/j.devcel.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Vijayaragavan K., Szabo E., Bossé M., Ramos-Mejia V., Moon R.T., Bhatia M. Noncanonical Wnt signaling orchestrates early developmental events toward hematopoietic cell fate from human embryonic stem cells. Cell Stem Cell. 2009;4:248–262. doi: 10.1016/j.stem.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodyanik M.A., Yu J., Zhang X., Tian S., Stewart R., Thomson J.A., Slukvin I.I. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7:718–729. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gise A., Pu W.T. Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circ. Res. 2012;110:1628–1645. doi: 10.1161/CIRCRESAHA.111.259960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Nakayama N. WNT and BMP signaling are both required for hematopoietic cell development from human ES cells. Stem Cell Res. (Amst.) 2009;3:113–125. doi: 10.1016/j.scr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Woll P.S., Morris J.K., Painschab M.S., Marcus R.K., Kohn A.D., Biechele T.L., Moon R.T., Kaufman D.S. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood. 2008;111:122–131. doi: 10.1182/blood-2007-04-084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.Y., Tsai Y.P., Wu M.Z., Teng S.C., Wu K.J. Epigenetic reprogramming and post-transcriptional regulation during the epithelial-mesenchymal transition. Trends Genet. 2012;28:454–463. doi: 10.1016/j.tig.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Yu Q.C., Hirst C.E., Costa M., Ng E.S., Schiesser J.V., Gertow K., Stanley E.G., Elefanty A.G. APELIN promotes hematopoiesis from human embryonic stem cells. Blood. 2012;119:6243–6254. doi: 10.1182/blood-2011-12-396093. [DOI] [PubMed] [Google Scholar]
- Zhang K., Li L., Huang C., Shen C., Tan F., Xia C., Liu P., Rossant J., Jing N. Distinct functions of BMP4 during different stages of mouse ES cell neural commitment. Development. 2010;137:2095–2105. doi: 10.1242/dev.049494. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Heinke J., Vargas A., Winnik S., Krauss T., Bode C., Patterson C., Moser M. ERK signaling is a central regulator for BMP-4 dependent capillary sprouting. Cardiovasc. Res. 2007;76:390–399. doi: 10.1016/j.cardiores.2007.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


