Summary
Vascular endothelium is a dynamic cellular interface that displays a unique phenotypic plasticity. This plasticity is critical for vascular function and when dysregulated is pathogenic in several diseases. Human genotype-phenotype studies of endothelium are limited by the unavailability of patient-specific endothelial cells. To establish a cellular platform for studying endothelial biology, we have generated vascular endothelium from human induced pluripotent stem cells (iPSCs) exhibiting the rich functional phenotypic plasticity of mature primary vascular endothelium. These endothelial cells respond to diverse proinflammatory stimuli, adopting an activated phenotype including leukocyte adhesion molecule expression, cytokine production, and support for leukocyte transmigration. They maintain dynamic barrier properties responsive to multiple vascular permeability factors. Importantly, biomechanical or pharmacological stimuli can induce pathophysiologically relevant atheroprotective or atheroprone phenotypes. Our results demonstrate that iPSC-derived endothelium possesses a repertoire of functional phenotypic plasticity and is amenable to cell-based assays probing endothelial contributions to inflammatory and cardiovascular diseases.
Graphical Abstract

Highlights
-
•
Human iPSCs generate vascular ECs with a rich functional repertoire
-
•
iPSC-ECs can undergo endothelial activation and maintain dynamic permeability
-
•
Biomechanical forces direct iPSC-ECs to atheroprotective or atheroprone phenotypes
-
•
iPSC-ECs are directed to an atheroprotective phenotype via pharmacological stimulus
Introduction
The vascular endothelium, the single-cell layer lining blood vessels, is a multifunctional interface that displays a striking phenotypic plasticity necessary for maintaining vascular homeostasis. In this context, the vascular endothelium is critical to initiate an inflammatory response, trigger thrombosis, regulate vasomotor tone, and control vascular permeability. Dysfunction of the endothelium plays a significant pathogenic role in cardiovascular diseases, namely, atherosclerosis and its consequences: heart attacks and strokes (Gimbrone et al., 2000; Hansson, 2005). Notably, studies at the genetic and molecular level of human endothelium have been limited by the availability of relevant tissue derived from cadaveric, discarded surgical, or umbilical vasculature sources.
Recent developments in stem cell biology promise new resources for modeling genetic diseases. In particular, induced pluripotent stem cells (iPSCs) offer the ability to study the effects of genetic alterations and mechanisms of genetic diseases in currently inaccessible cell types (Takahashi and Yamanaka, 2006). Although iPSCs have been differentiated into many cell types including endothelium (Choi et al., 2009; Homma et al., 2010; Li et al., 2011; Park et al., 2010; Rufaihah et al., 2011, 2013; Taura et al., 2009; White et al., 2013), the fidelity and functional mimicry of stem cell-derived tissues and their relevance to human disease remain poorly characterized. This functionality must be carefully assessed before their scientific and therapeutic potential can be realized (Soldner and Jaenisch, 2012).
The goals of this study were to reproducibly generate human iPSC-derived vascular endothelial cells (iPSC-ECs) and to then assess whether they could acquire specific functions critical for vascular homeostasis displayed by primary cultures of human vascular endothelium. To this end, we differentiated human iPSCs as embryoid bodies (EBs) and isolated the endothelial population for detailed functional characterization. Significantly, in addition to displaying characteristic endothelial molecular and structural features, these ECs display phenotypic plasticity that allows them to mediate leukocyte transmigration and maintain a dynamic barrier. Furthermore, we have documented that the iPSC-ECs can be directed to acquire an atheroprotective or atheroprone phenotype in response to distinct biomechanical or pharmacological stimuli. Collectively, our results demonstrate that human iPSC-ECs support a spectrum of physiological endothelial functions and possess the relevant phenotypic plasticity to probe important features of human cardiovascular pathophysiology in a patient-specific manner.
Results
Human iPSC Differentiation into Vascular Endothelium
To generate ECs, we differentiated iPSCs of the BJ1 cell line as EBs in suspension by replacing iPSC medium with differentiation medium containing fetal calf serum. The specific serum chosen was selected from a screen optimizing proliferation and morphology of cultured human ECs (data not shown). To carefully characterize the timescale of EC differentiation, we performed quantitative real-time TaqMan PCR using RNA harvested daily from EBs from the time of their generation from iPSC colonies, day 0, through 18 days of differentiation. Under these conditions, we observed increasing expression of EC markers CDH5 (VE-cadherin), KDR (VEGFR2), and CD31 (PECAM1) (Figure 1A). To better define the EC population, we measured the expression of VE-cadherin, CD31, and KDR by flow cytometry within dissociated EBs after different durations of differentiation. As seen in Figure 1B, the VE-cadherin+|CD31+ EC fraction peaked at 18% ± 4% (mean ± SD) of the EBs after 10 days of differentiation. The EBs grew in large cystic tissues containing cord-like structures (Figure 1C). Two-photon confocal microscopy visualized fluorescently labeled VE-cadherin within the intact ∼1 mm diameter EBs indicating that most VE-cadherin+ cells reside in networked structures (Figure 1D).
Figure 1.
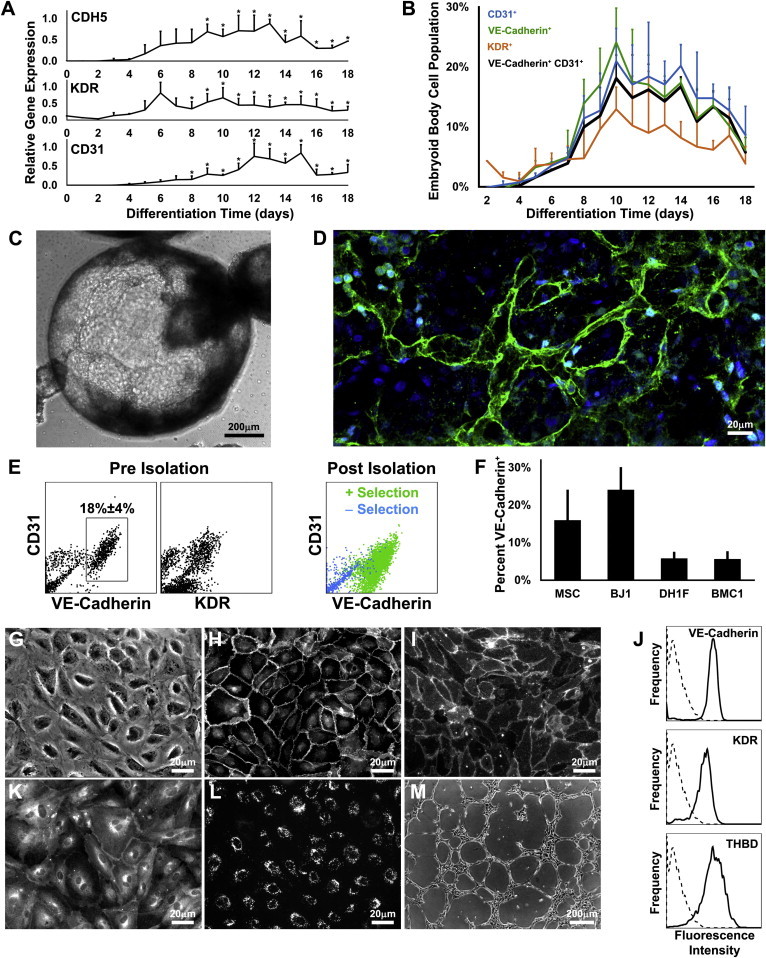
Derivation of iPSC-ECs
(A) Gene expression time profiles of CDH5 (VE-cadherin), KDR, and CD31 measured by quantitative real-time PCR (n = 3) with the asterisks (∗) denoting values different than day 0 (p < 0.05).
(B) CD31, VE-cadherin, and KDR expression in EB cells measured by flow cytometry (n = 3).
(C) Phase-contrast image of an EB after 10 days of differentiation.
(D) Reconstruction of two-photon confocal microscopy images of VE-cadherin (green) and nuclei (blue) within an EB after 10 days of differentiation.
(E) The isolation of VE-cadherin+ cells from the EB with magnetic bead sorting.
(F–M) Fraction of EB cells expressing VE-cadherin from different iPSC lines (F; n = 4). Isolated iPSC-ECs characterized by phase contrast (G), immunofluorescence for VE-cadherin (H) and CD31 (I), flow cytometry (J) for VE-cadherin, KDR, and thrombomodulin (sample in solid line; isotype control in dotted), immunofluorescence for eNOS (K) and endocytosed acetylated-LDL (L), and a phase-contrast image of the network formation on Matrigel (M).
All pooled data are represented as mean ± SD.
See also Figure S1.
Because the proportion of VE-cadherin+|CD31+ cells peaks at day 10, we isolated the EC population (iPSC-EC) from dissociated day 10 EBs for further analysis. Due to its endothelial specificity and to the presence of CD31+|VE-cadherin−, CD31+KDR−, and CD31−|KDR+ cells within the EBs (Figure 1E, left and center panels) and due to the fact that a subpopulation of CD31+ cells expresses CD45 (Figure S1A available online), we chose to isolate ECs based on VE-cadherin expression. Magnetic bead sorting produced a population with greater than 95% VE-cadherin+ cells (Figure 1E, right panel). To ensure that this endothelial differentiation protocol is generalizable, we tested additional human iPSC lines generated with different technologies. Importantly, each cell line was reproducibly able to generate ECs, although with different yields (Figure 1F).
Next, we assessed whether isolated iPSC-ECs exhibit characteristic EC molecular markers and cellular behavior after in vitro culture. As seen in Figures 1G–1M, iPSC-ECs displayed typical cobblestone morphology, expressed VE-cadherin and CD31 at cell junctions, expressed VE-cadherin, KDR, and thrombomodulin on their cell surface, expressed endothelial nitric oxide synthase (eNOS) at the perinuclear region and plasma membrane, endocytosed fluorescent acetylated LDL, and could form networks on Matrigel in the presence of VEGF.
We also observed that iPSC-ECs displayed punctate von Willebrand factor (vWF) and angiopoietin-2 staining (Figure S1B). Because vWF and angiopoietin-2 are normally stored in endothelia-restricted organelles termed Weibel-Palade bodies (WPBs), we investigated via electron microscopy the presence of these organelles in iPSC-ECs. We identified electron-dense striated structures typical of WPBs that stained for vWF by immunogold labeling (Figure S1B). Functionally, WPBs store and rapidly exocytose vWF and P-selectin to initiate a hemostatic response (Wagner and Frenette, 2008). In order to verify that the WPBs were competent for rapid exocytosis, we treated the iPSC-ECs with histamine and observed rapid and transient presentation of P-selectin to the cell surface (Figure S1B).
Proinflammatory Endothelial Activation
After documenting essential molecular features of vascular endothelium, we investigated whether iPSC-ECs can display multiple functional phenotypes characteristic of mature endothelium. A pathophysiologically essential property of endothelium is the response to proinflammatory stimuli and participation in inflammatory responses. To assess this activated functional phenotype, we exposed iPSC-ECs to the proinflammatory stimuli, interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), and lipopolysaccharide (LPS), and assessed the cell surface expression of the adhesion molecules E-selectin, intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1), critical for endothelial-leukocyte interactions (Figure 2A; for fluorescence intensity values, see Figure S2A). After 6 hr, IL-1β, TNF-α, and LPS induced the expression of E-selectin and ICAM-1 in the majority of the cell population, and of VCAM-1 in a fraction. After 24 hr of IL-1β, TNF-α, or LPS stimulation, the iPSC-ECs expressed E-selectin to a lesser degree, and ICAM-1 and VCAM-1. Additionally, treatment with interferon-gamma (IFNγ) induced the ECs to upregulate ICAM-1 expression synergistically with TNF-α in a time-dependent manner as reported in studies of primary EC cultures (Doukas and Pober, 1990) (Figure S2B).
Figure 2.

Assessment of Endothelial Activation in iPSC-ECs
(A) Surface expression of E-selectin, ICAM-1, and VCAM-1 after 6 or 24 hr of 10 U/ml IL-1β, 10 ng/ml TNF-α, or 1 μg/ml LPS treatment measured by flow cytometry.
(B) Culture supernatant concentration of chemokines after 24 hr treatment of TNF-α, IL-1β, IFNγ, LPS, or TNF-α+IFNγ with the asterisks (∗) indicating difference to vehicle (veh) (p < 0.05; n = 3–4).
(C) Time profile of transmigrated neutrophils and T cells after 4 hr TNF-α treatment.
(D) Fraction of neutrophils or T cells transmigrated across TNF-α- stimulated iPSC-ECs and HUVECs (n = 3; n.s., not significant).
(E–H) Transmigration of neutrophils after pretreatment of neutrophils with anti-CD18 antibody (E) or pretreatment of iPSC-ECs with anti- ICAM-1 antibody (F). Transmigration of T cells after pretreatment of T cells with anti-CD18 antibody (G) or pretreatment of iPSC-ECs with anti- ICAM-1 antibody (H). A binding nonblocking anti-MHC class I antibody was used as a control (n = 2).
All pooled data are represented as mean ± SD.
In addition to presenting adhesion molecules, the activated endothelial phenotype includes the secretion of proinflammatory cytokines. Therefore, we examined whether iPSC-ECs have this capability by measuring the concentration of several proinflammatory cytokines in the culture supernatant. Treatment for 24 hr with IL-1β, TNF-α, or LPS induced the iPSC-ECs to secrete monocyte chemotactic protein-1 (MCP1), IL-8, RANTES, and IFNγ-induced protein 10 (IP10), potent chemoattractants that act on T cells, neutrophils, and monocytes (Figure 2B), whereas treatment with IFNγ induced the ECs to secrete IP10 and monokine induced by gamma interferon (MIG).
Importantly, the coordinated expression of adhesion molecules and secretion of proinflammatory cytokines by the vascular endothelium orchestrates the transendothelial migration of leukocytes. The iPSC-ECs, after treatment for 4 hr with 10 ng/ml TNF-α, were able to coordinate the integrated behavior of inducing human neutrophil and T lymphocyte rolling, arrest, and transmigration under laminar fluid flow (Figure 2C; Movies S1 and S2). The fractions of transmigrating leukocytes were similar to those seen with primary HUVECs, the human model currently most commonly used for leukocyte transmigration studies (Figure 2D). It is important to note that this transmigration was dependent on T lymphocyte and neutrophil β2-integrin (CD18) and on endothelial ICAM-1 as demonstrated by the diminished transmigration in the presence of relevant blocking antibodies (Figures 2E–2H) similar to primary ECs (Rao et al., 2007).
Maintenance of a Dynamic Barrier
A critical function of the vascular endothelium is the maintenance of a tight dynamic barrier to contain the blood’s plasma and cellular constituents. ECs actively rearrange junctional and cytoskeletal proteins to modulate their barrier function in response to multiple physiological stimuli (Dejana et al., 2001; Mehta and Malik, 2006). To characterize the barrier phenotype of iPSC-ECs, we examined the role of known EC permeability factors on the electrical resistance across a monolayer of iPSC-ECs, a real time measure of permeability. As seen in Figure 3A, histamine induced a transient increase in permeability, whereas VEGF induced a sustained increase in permeability, which was partially abrogated by pretreatment with a FAK inhibitor (Figure S3A), as demonstrated in primary endothelium (Chen et al., 2012). Prostaglandin E2 produced a decrease in permeability as did sphingosine-1-phosphate. Treatment of iPSC-ECs with the cAMP analog, 8-pCPT-2′O-Me-cAMP (O-Me) that selectively activates Epacs (Bos, 2006), induced a sustained decrease in permeability as reported in primary ECs (Cullere et al., 2005). Compared to HUVECs, the iPSC-ECs display a lower VEGF-induced permeability and slower response to O-Me (Figure S3B). We next documented the rearrangements of structural proteins that modulate barrier properties (Mehta and Malik, 2006). As seen in Figure 3B, we found that treatment with O-Me resulted in redistribution of VE-cadherin from a jagged to a more linear geometry and favored the generation of actin filaments at the cell periphery over longitudinal actin filaments, as seen in primary ECs (Cullere et al., 2005).
Figure 3.

Barrier Properties of iPSC-ECs
(A) Changes in transendothelial electrical resistance after treatment with 10 μM histamine, 100 ng/ml VEGF, 200 ng/ml prostaglandin E2, 0.5 μg/ml sphingosine-1-phosphate, or 100 μM O-Me. Data are represented as mean ± SD (n = 3) with the asterisks (∗) denoting different from control (p < 0.05). Arrows indicate time of addition of compound.
(B) VE-cadherin and actin after 24 hr treatment with 100 μM O-Me seen by immunofluorescence microscopy.
See also Figure S3.
Directing Phenotypic Plasticity to Disease-Protective and -Prone States
A promising application of iPSC-derived endothelium is the ability to study endothelium in pathophysiologically relevant states in a patient-specific manner. Therefore, we evaluated whether iPSC-ECs are able to adopt atherosclerosis-protective and atherosclerosis-prone phenotypes in vitro, which are critical to enable their use in studies of endothelial contributions to cardiovascular disease. Distinct local hemodynamic environments have been linked to the nonrandom predictable distribution of atherosclerotic lesions in human and animal studies (Chiu and Chien, 2011; Gimbrone and García-Cardeña, 2013). In fact, local biomechanical forces can strongly influence endothelial phenotype and induce a functional phenotype or one that is dysfunctional, an early step in atherogenesis (Davies, 2009; Gimbrone, 1999; Hahn and Schwartz, 2009). In particular, the specific shear stress patterns exerted on ECs at the sites of atheroprotection and atherosusceptibility in the human carotid artery induce atheroprotective and atheroprone phenotypes respectively in cultured ECs (Dai et al., 2004). In order to generate these biomechanically induced phenotypes in iPSC-ECs, we applied atheroprotective or atheroprone shear stress waveforms for 72 hr (Figure 4A). As seen in Figures 4B and 4C, the iPSC-ECs selectively responded to atheroprotective flow by aligning in the direction of flow, whereas those exposed to atheroprone flow did not. A key molecular descriptor of the endothelial atheroprotective versus atheroprone phenotype is expression of the transcription factors Kruppel-like factors 2 (KLF2) and 4 (KLF4), integrators of the flow-mediated vasoprotective phenotype (Dekker et al., 2006; Parmar et al., 2006; Villarreal et al., 2010). Thus, we measured the expression of these genes in iPSC-ECs exposed to atheroprotective or atheroprone shear stress waveforms and observed that the atheroprotective waveform differentially induced the expression of KLF2 and KLF4 (Figure 4D). Furthermore, we measured the expression of several other mechano-activated genes and downstream effectors of KLF2 and KLF4 and observed that atheroprotective shear stress upregulates NOS3 (eNOS), argininosuccinate synthase 1 (ASS1), and downregulates vWF. In contrast, atheroprone flow upregulated endothelin-1 (EDN1), a potent vasoconstrictor known to be differentially regulated by flow (Figure 4D).
Figure 4.

Acquisition of Atheroprotective and Atheroprone Endothelial Phenotypes
(A–C) A single period of atheroprotective and atheroprone shear stress waveforms (A). iPSC-ECs imaged by phase contrast after 72 hr of atheroprone (B) or atheroprotective flow (C; arrow indicates direction of flow).
(D) Relative (Rel.) gene expression after 72 hr of atheroprotective or atheroprone shear stress.
(E) Relative gene expression after 24 hr treatment with 10 μM simvastatin.
The asterisks (∗) indicate statistical significance with p < 0.05 (n = 4). All pooled data are represented as mean ± SD.
See also Figure S4.
In addition to biomechanical forces, ECs are responsive to pharmacological agents. In particular, our laboratory and others have documented that the endothelial atheroprotective phenotype and KLF2 expression can be evoked by statins, a class of HMG-CoA reductase inhibitors (Parmar et al., 2005; Sen-Banerjee et al., 2005). To illustrate that iPSC-ECs are a suitable substrate for personalized pharmacological studies, we examined whether iPSC-EC phenotype can be similarly modulated by statin treatment. As seen in Figure 4E, simvastatin upregulated KLF2 expression as well as downstream effectors NOS3, ASS1, thrombomodulin (THBD), integrin beta 4 (ITGB4), and prostaglandin-H2 D-isomerase (PTGDS). Simvastatin also downregulated proinflammatory ANGPT2 and EDN1. The induction of KLF2 and NOS3 in iPSC-ECs showed similar sensitivity to simvastatin concentration as primary ECs (Figure S4).
Discussion
In this study, we have generated vascular ECs from human iPSCs and characterized their humoral-, pharmacological-, and biomechanical-induced functional phenotypes, advancing iPSC-ECs as an experimental platform to study endothelial biology. The methodology to differentiate iPSCs into vascular endothelium was reproducible and robust across several iPSC lines created with different technologies. Previous studies have differentiated iPSCs to isolate ECs based on expression of CD31 or KDR (Choi et al., 2009; Homma et al., 2010; Li et al., 2011; Park et al., 2010; Rufaihah et al., 2011, 2013; Taura et al., 2009; White et al., 2013). In contrast, we isolated populations of ECs from the EBs based on VE-cadherin expression, a strong marker of endothelial identity, as we observed CD31+, and KDR+ cells subpopulations lacking VE-cadherin, and a CD31+ subpopulation expressing CD45.
After isolation and culture, iPSC-ECs displayed molecular markers of vascular endothelium and contained vWF-positive WPBs that can be rapidly exocytosed, a critical function for hemostasis. It is possible that the perinuclear localization observed in many of the WPBs may suggest an immature stage in WPB biogenesis (Valentijn et al., 2011; Zenner et al., 2007). iPSC-ECs are competent for the functional repertoire displayed by vascular endothelium critical in pathophysiological settings. Specifically, we demonstrated that proinflammatory stimuli induce an activated proinflammatory phenotype that included expression of leukocyte adhesion molecules, secretion of proinflammatory cytokines, and support for human leukocyte transmigration. We also observed that iPSC-EC monolayers display a dynamic permeability in response to a panel of physiological stimuli.
Human iPSC-ECs are a promising platform for studying endothelial contributions to cardiovascular diseases in the context of specific patients’ genetic backgrounds. We demonstrated that iPSC-ECs have the plasticity to acquire distinct flow-dependent phenotypes, namely atheroprotective and atheroprone phenotypes critical for atherosclerosis resistance and susceptibility. We observed that iPSC-ECs respond to atheroprotective shear stress by activating an atheroprotective gene expression program transcriptionally mediated by KLF2 and KLF4 expression, shown to integrate the flow-mediated endothelial atheroprotective functional phenotype by regulating leukocyte adhesion, redox state, and thrombotic function in cultured human ECs (Dekker et al., 2006; Lin et al., 2005; Parmar et al., 2006; SenBanerjee et al., 2004; Villarreal et al., 2010). The ability to direct the acquisition of flow-dependent functional and dysfunctional phenotypes using patient-specific endothelium should allow the study of specific genetic contributions to endothelial function and dysfunction in the context of human cardiovascular disease. Furthermore, we demonstrated that simvastatin activated an atheroprotective gene expression program and downregulated genes associated with an atheroprone phenotype in iPSC-ECs. This suggests that iPSC-ECs may be a suitable surrogate to assess the effects of drugs on the endothelia of specific patients.
The ability to generate human vascular endothelium from iPSCs is an enabling platform. Cell-based assays of endothelial function can be combined with ECs derived from iPSC lines produced from patients with genetic diseases, creating powerful model systems of human disease or associations where the pathological molecular mechanisms and their functional consequences are poorly understood (Adams and García-Cardeña, 2012; Grskovic et al., 2011; Kiskinis and Eggan, 2010). Extension of these disease iPSC-based assays to high throughput may enable the new therapeutic discovery for genetic diseases and permit the personalization of drug toxicity testing, an unmet need because vascular toxicity is a significant cause of attrition in drug development.
Experimental Procedures
Cell Culture
Four human iPSC lines were used in this study. Lines BJ1 and MSC1 were received from the laboratory of Dr. George Q. Daley (Children’s Hospital Boston), line BMC1 from the laboratory of Dr. Darrell N. Kotton (Boston University Medical Center), and line DH1F from the Harvard Stem Cell Institute.
Differentiation and Isolation of iPSC-ECs
Colonies of iPSCs were differentiated as EBs in suspension in ultralow adhesion plates (Corning) in differentiation medium for 10 days. EBs were dissociated into single cells with 2 mg/ml collagenase B (Roche) for 2 hr then Cell Dissociation Buffer (Invitrogen) for 15 min at 37°C shaking at 1,100 rpm. VE-cadherin+ cells were isolated by magnetic bead sorting (Miltenyi Biotec) using PE-conjugated anti-VE-cadherin antibody (BD Biosciences) and magnetic bead-conjugated anti-PE antibody (Miltenyi). Isolated iPSC-ECs were plated at 20,000 cells/cm2 on 50 μg/ml fibronectin (Sigma-Aldrich)-coated dishes in EC medium. Experiments were performed on passage one.
Please see Table S1 for a list of TaqMan probes used in this study and Table S2 for clones and vendors of primary antibodies.
Acknowledgments
We wish to thank Kay Case and Vannessa Davis for the isolation of human umbilical cord ECs and Odelya Hartung for instruction in human iPSC culture. This work was supported by grants RO1AG032443, PO1HL076540, PO1HL036028, and RO1HL089940 from the National Institutes of Health. W.J.A. is a recipient of a Ruth L. Kirchstein National Research Service Award F31AG037249 from the National Institutes of Health.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information
References
- Adams W.J., García-Cardeña G. Novel stem cell-based drug discovery platforms for cardiovascular disease. J. Biomol. Screen. 2012;17:1117–1127. doi: 10.1177/1087057112454741. [DOI] [PubMed] [Google Scholar]
- Bos J.L. Epac proteins: multi-purpose cAMP targets. Trends Biochem. Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Chen X.L., Nam J.O., Jean C., Lawson C., Walsh C.T., Goka E., Lim S.T., Tomar A., Tancioni I., Uryu S. VEGF-induced vascular permeability is mediated by FAK. Dev. Cell. 2012;22:146–157. doi: 10.1016/j.devcel.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu J.J., Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.D., Yu J., Smuga-Otto K., Salvagiotto G., Rehrauer W., Vodyanik M., Thomson J., Slukvin I. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullere X., Shaw S.K., Andersson L., Hirahashi J., Luscinskas F.W., Mayadas T.N. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105:1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- Dai G., Kaazempur-Mofrad M.R., Natarajan S., Zhang Y., Vaughn S., Blackman B.R., Kamm R.D., García-Cardeña G., Gimbrone M.A., Jr. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc. Natl. Acad. Sci. USA. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P.F. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat. Clin. Pract. Cardiovasc. Med. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Spagnuolo R., Bazzoni G. Interendothelial junctions and their role in the control of angiogenesis, vascular permeability and leukocyte transmigration. Thromb. Haemost. 2001;86:308–315. [PubMed] [Google Scholar]
- Dekker R.J., Boon R.A., Rondaij M.G., Kragt A., Volger O.L., Elderkamp Y.W., Meijers J.C., Voorberg J., Pannekoek H., Horrevoets A.J. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107:4354–4363. doi: 10.1182/blood-2005-08-3465. [DOI] [PubMed] [Google Scholar]
- Doukas J., Pober J.S. IFN-gamma enhances endothelial activation induced by tumor necrosis factor but not IL-1. J. Immunol. 1990;145:1727–1733. [PubMed] [Google Scholar]
- Gimbrone M.A., Jr. Endothelial dysfunction, hemodynamic forces, and atherosclerosis. Thromb. Haemost. 1999;82:722–726. [PubMed] [Google Scholar]
- Gimbrone M.A., Jr., García-Cardeña G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc. Pathol. 2013;22:9–15. doi: 10.1016/j.carpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M.A., Jr., Topper J.N., Nagel T., Anderson K.R., Garcia-Cardeña G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann. N Y Acad. Sci. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. [DOI] [PubMed] [Google Scholar]
- Grskovic M., Javaherian A., Strulovici B., Daley G.Q. Induced pluripotent stem cells—opportunities for disease modelling and drug discovery. Nat. Rev. Drug Discov. 2011;10:915–929. doi: 10.1038/nrd3577. [DOI] [PubMed] [Google Scholar]
- Hahn C., Schwartz M.A. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Homma K., Sone M., Taura D., Yamahara K., Suzuki Y., Takahashi K., Sonoyama T., Inuzuka M., Fukunaga Y., Tamura N. Sirt1 plays an important role in mediating greater functionality of human ES/iPS-derived vascular endothelial cells. Atherosclerosis. 2010;212:42–47. doi: 10.1016/j.atherosclerosis.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Kiskinis E., Eggan K. Progress toward the clinical application of patient-specific pluripotent stem cells. J. Clin. Invest. 2010;120:51–59. doi: 10.1172/JCI40553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Hu S., Ghosh Z., Han Z., Wu J.C. Functional characterization and expression profiling of human induced pluripotent stem cell- and embryonic stem cell-derived endothelial cells. Stem Cells Dev. 2011;20:1701–1710. doi: 10.1089/scd.2010.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z., Kumar A., SenBanerjee S., Staniszewski K., Parmar K., Vaughan D.E., Gimbrone M.A., Jr., Balasubramanian V., García-Cardeña G., Jain M.K. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ. Res. 2005;96:e48–e57. doi: 10.1161/01.RES.0000159707.05637.a1. [DOI] [PubMed] [Google Scholar]
- Mehta D., Malik A.B. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Park S.W., Jun Koh Y., Jeon J., Cho Y.H., Jang M.J., Kang Y., Kim M.J., Choi C., Sook Cho Y., Chung H.M. Efficient differentiation of human pluripotent stem cells into functional CD34+ progenitor cells by combined modulation of the MEK/ERK and BMP4 signaling pathways. Blood. 2010;116:5762–5772. doi: 10.1182/blood-2010-04-280719. [DOI] [PubMed] [Google Scholar]
- Parmar K.M., Nambudiri V., Dai G., Larman H.B., Gimbrone M.A., Jr., García-Cardeña G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J. Biol. Chem. 2005;280:26714–26719. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- Parmar K.M., Larman H.B., Dai G., Zhang Y., Wang E.T., Moorthy S.N., Kratz J.R., Lin Z., Jain M.K., Gimbrone M.A., Jr., García-Cardeña G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J. Clin. Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R.M., Yang L., Garcia-Cardena G., Luscinskas F.W. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ. Res. 2007;101:234–247. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- Rufaihah A.J., Huang N.F., Jamé S., Lee J.C., Nguyen H.N., Byers B., De A., Okogbaa J., Rollins M., Reijo-Pera R. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler. Thromb. Vasc. Biol. 2011;31:e72–e79. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufaihah A.J., Huang N.F., Kim J., Herold J., Volz K.S., Park T.S., Lee J.C., Zambidis E.T., Reijo-Pera R., Cooke J.P. Human induced pluripotent stem cell-derived endothelial cells exhibit functional heterogeneity. Am. J. Transl. Res. 2013;5:21–35. [PMC free article] [PubMed] [Google Scholar]
- SenBanerjee S., Lin Z., Atkins G.B., Greif D.M., Rao R.M., Kumar A., Feinberg M.W., Chen Z., Simon D.I., Luscinskas F.W. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J. Exp. Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen-Banerjee S., Mir S., Lin Z., Hamik A., Atkins G.B., Das H., Banerjee P., Kumar A., Jain M.K. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720–726. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]
- Soldner F., Jaenisch R. Medicine. iPSC disease modeling. Science. 2012;338:1155–1156. doi: 10.1126/science.1227682. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Taura D., Sone M., Homma K., Oyamada N., Takahashi K., Tamura N., Yamanaka S., Nakao K. Induction and isolation of vascular cells from human induced pluripotent stem cells—brief report. Arterioscler. Thromb. Vasc. Biol. 2009;29:1100–1103. doi: 10.1161/ATVBAHA.108.182162. [DOI] [PubMed] [Google Scholar]
- Valentijn K.M., Sadler J.E., Valentijn J.A., Voorberg J., Eikenboom J. Functional architecture of Weibel-Palade bodies. Blood. 2011;117:5033–5043. doi: 10.1182/blood-2010-09-267492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal G., Jr., Zhang Y., Larman H.B., Gracia-Sancho J., Koo A., García-Cardeña G. Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem. Biophys. Res. Commun. 2010;391:984–989. doi: 10.1016/j.bbrc.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D.D., Frenette P.S. The vessel wall and its interactions. Blood. 2008;111:5271–5281. doi: 10.1182/blood-2008-01-078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M.P., Rufaihah A.J., Liu L., Ghebremariam Y.T., Ivey K.N., Cooke J.P., Srivastava D. Limited gene expression variation in human embryonic stem cell and induced pluripotent stem cell-derived endothelial cells. Stem Cells. 2013;31:92–103. doi: 10.1002/stem.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenner H.L., Collinson L.M., Michaux G., Cutler D.F. High-pressure freezing provides insights into Weibel-Palade body biogenesis. J. Cell Sci. 2007;120:2117–2125. doi: 10.1242/jcs.007781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


