Abstract
Background:
Serological safety is an integral part of overall safety for blood banks. Emphasis is on the use of routinue Red Blood Cell (RBC) antibody screen test, at set time intervals, to reduce risks related to alloantibodies. Also emphasis is on importance of issuing antigen negative blood to alloantibody positive patients. Effect of using leucodepleted blood on the rate of alloimmunization is highlighted. The concept of provision of phenotypically matched blood is suggested.
Materials and Methods:
Antibody screen test is important to select appropriate blood for transfusion. Repeat antibody screen testing, except if time interval between the earlier and subsequent transfusion was less than 72 hours, followed by antibody identification, if required, was performed in patients being treated with repeat multiple blood transfusions. Between February 2008 and June 2009, repeat samples of 306 multi-transfused patients were analyzed. Search for irregular antibodies and reading of results was conducted using RBC panels (three-cell panel of Column Agglutination Technology (CAT) and two cell panel of the Solid Phase Red Cell Adherence Technology (SPRCAT). Specificities of antibodies were investigated using appropriate panels, 11 cell panel of CAT and 16 cell panel of SPRCA. These technologies, detecting agglutination in columns and reactions in solid phase, evaluate the attachment of irregular incomplete antibody to antigen in the first phase of immunological reaction more directly and hence improve the reading of agglutination. Three to four log leuco reduced red blood cells were transfused to patients in the study using blood collection bags with integral filters.
Results:
Alloimmunization rate of 4.24% was detected from 306 multiply transfused patients tested and followed up. The Transfusion therapy may become significantly complicated.
Conclusion:
Red cell antibody screening and identification and subsequent issue of antigen negative blood have a significant role in improving blood safety. Centers that have incorporated antibody screen test and identification have ensured safe transfusion. Identified patients should be flagged in a database and information shared. Such patients can be given carry-on cards and educated about the names of the identified antibodies. Full red cell phenotyping of individuals, patients and donors, can be feasibility.
Keywords: Antibody screen Test, antibody identification, cell panel
Introduction
Red cell transfusions are a valuable health care resource especially for thalassemics, patients of myeloproliferative disorders, hematological disorders, end stage renal failure, patients of leukemia and organ transplant patients. Chronic red cell transfusions can cause unwanted complications called transfusion reactions in a patient.[1] Development of alloantibodies to red cell antigens is an important immune mediated delayed hemolytic transfusion reaction. It is a matter of great concern in multi-transfused patients and in patients who have had multiple pregnancies. Alloimmunization results from disparity between the donor and patient antigens. Prior exposure to donor antigens can lead to anamnestic or secondary response where even very small amounts of donor antigenic RBCs can elicit an alloimmune response resulting in increase in antibody production leading to red cell destruction since the patient is already immunized.[2] Alloimmunization occurs when incompatible antigens introduced in an immune-competent host evoke an immune response leading to irregular antibody formation. Alloimmunization against RBCs can result in delayed hemolytic transfusion reactions which can range from destruction of RBCs within hours or even minutes to decrease of survival by a few days or can cause hemolytic disease in newborns. Development of alloantibodies thus complicates and limits transfusion therapy, contributing not only to technical complications but also to morbidity and mortality.[1] A number of studies have shown the role of leucodepletion in preventing alloimmunization and autoimmunization.[3,4]
Materials and Methods
This study was conducted in the Department of Transfusion Medicine, Indraprastha Apollo Hospitals, New Delhi from February 2008 to June 2009. Patients receiving repeat multiple transfusions at Indraprastha Apollo Hospital, New Delhi ,and with diagnosis of thalassemics, patients of myeloproliferative disorders, hematological disorders, end stage renal failure, patients of leukemia and liver transplant patients whose follow up and regular antibody screen test was possible, were included in the study. In cases where detailed clinical history or data was not available or cases where regular antibody screen test and follow up was not possible due to unavoidable reasons, were excluded from the study. A detailed clinical and transfusion history was taken using a set performa which mentioned the name, identification number, age and sex, diagnosis, blood group, transfusions till date, transfusions in the study period, history of pregnancy, abortion, hydrops and recent anti D Immunoprophylaxis in case of females, any relevant drug history, result of serological testing like DAT and auto control, number of antibody screen tests in the study period with results and antibody identification results.
Each time a blood requisition for these patients was received, a sample for antibody screen testing, 3 to 4 ml in Ethylene Diamine Tetraacetic Acid (EDTA) tube, was requested as a protocol. Only exception was where second transfusion was scheduled within 72 hours of the previous transfusion in such cases, repeat antibody screen testing was omitted. Patient’s plasma was then screened for the presence of any red cell antibodies on a commercial three-cell panel of the CAT (Diamed Switzerland) or using a two cell panel of the SPRCAT (Capture, Immunor Inc. Norcross, GA). All cases where antibody screen test was positive were subjected to antibody identification. Patients’ blood samples, four in EDTA tubes and one in plain tube, 3 to 4 ml each, were acquired for this purpose. These samples were investigated to identify the detected antibodies using commercial cell panels, 11 cell panel of CAT (Diamed, Switzerland) and 16 cell panel of SPRCA (Capture, Immucor Inc. Norcross, GA) as and when required. The criteria for antibody specificity were based on the recommendations of AABB.[5] Once a sample was found positive by antibody screen test and antibody further typed by antibody identification, the patient was issued corresponding antigen negative unit and his/her screen and identification results documented.
Three to four log leuco reduced red blood cells were transfused to the patients in the study using blood collection bags with integral filters (Optipure RC bag-baxter).
Results
A total of 306 patients who received regular repeat red cell transfusions were followed for a period of 17 months from February 2008 to June 2009. Of these antibody screen was positive in 20 cases and alloantibodies were identified in 13 cases. This yields an alloimmunization rate of 4.24% (13/306).
Patients included in the study ranged from 1 to 85 years in age with a mean age of 42.35 years and median age of 46 years. Among the alloimmunized cases, the age range was 9 to 61 years with a mean age of 41.62 years and median age of 44 years.
A total of 195 men and 111 women were included in the study and among the alloimmunized cases,seven were males and six were females, rate therefore being 3.6% in men and 5.4% in women.
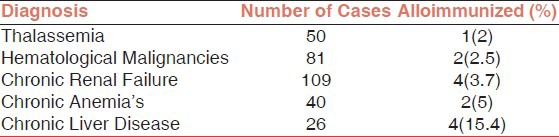
The total number of samples that were positive for irregular alloantibodies from the 109 cases of Chronic Renal failure studied was 4 (3.7%). Four out of 26 cases of Chronic Liver Disease developed alloantibodies (15.4%), two out of 40 patients of chronic anaemia developed alloantibodies (5%). Alloimmunization was recorded in one out of 50 (2%) thalassemics and 2 out of 81 (2.5%) patients with hematological malignancies [Table 1].
Table 1.
Rate of Alloimmunization according to Diagnosis
Maximum number of alloimmunizations followed 0-5transfusions in the study period, followed by 6-10 and then 11-15 transfusions.
A total of 22 antibodies were detected in 13 alloimmunized patients out of the total 306 multiply transfused patients. This yields an antibody prevalence of 4.24%, and included seven men and six women.
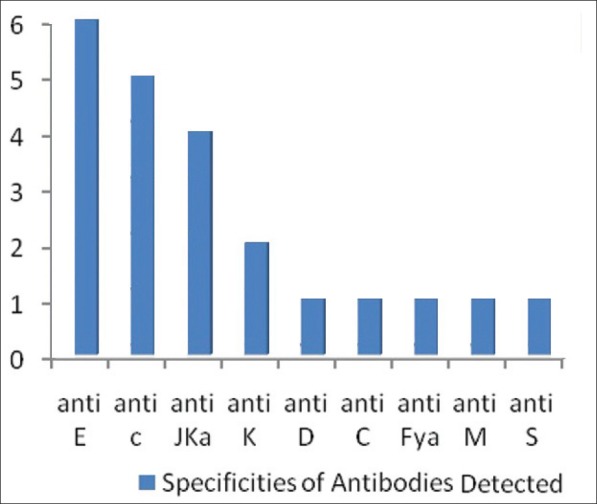
Antibodies identified in the samples reacting positive were as follows: Of 22 antibodies identified, the most frequent was anti E, which was detected in six out of 13 cases. Anti c was the second most frequent antibody, observed in five cases followed by anti Jka in four cases and anti K in two cases. Anti D, Anti C, Anti Fya, Anti M and Anti S were detected in one case each. A single alloantibody was detected in seven patients while 2-3, alloantibodies were detected in six patients [Table 2, Figure 1].
Table 2.
Details of Alloimmunized patients
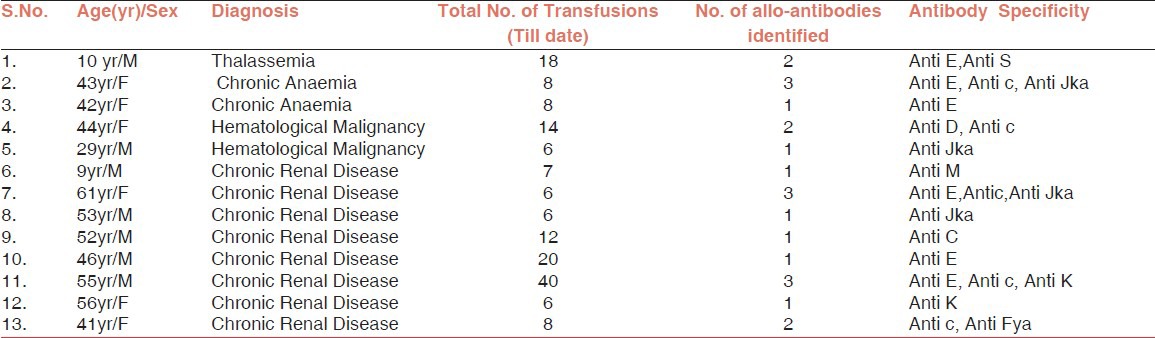
Figure 1.
Specificities of Antibodies Detected
Discussion
The development of red cell antibodies (allo-as well as auto-antibodies) occurs in a variable number of multiply transfused patients. In such circumstances, transfusion therapy may become significantly complicated. Effects of alloimmunization may include difficulty in finding compatible RBC units because of the presence of clinically significant RBC antibodies, transfusion reactions, or platelet refractoriness.[5] Present study is an effort to characterize blood group alloantibody formation in the patient population as only a few studies, mostly in non-Asian multiply transfused patients, have investigated the frequency and causes of red cell alloimmunization in the past.
Habibi and Lecolier[6] reported the incidence of red cell alloimmunization to be 1.72% in multi-transfused patients for hemodialysis. Blumberg et al,[7] studied patients with disorders that often lead to repetitive transfusions and the frequency of red cell antibodies was about 3% overall. In a study by Domen and Ramirez et al,[1] incidence of alloimmunization in chronic renal disease patients on hemodialysis was found to be 6.1%. In a study by Spanos et al,[8] to detect alloimmunization in patients of thalassemia and sickle cell/beta thalassemia, the immunization rate was 3.7%. Hmida et al[9] reported alloimmunization in 7.76% among the patients of thalassemia and sickle cell disease. Shukla and Chaudhary et al,[10] published a study where in they investigated the frequency of red cell alloimmunization in multi-transfused chronic renal failure patients undergoing hemodialysis. An alloimmunization rate of 9.8% was observed. Murao and Viana in 2005 conducted a study in patients with sickle cell disease to determine the frequency and risk factors for alloimmunization and found the frequency to be 9.9%.[11]
Incidence of alloantibodies in multiply transfused patients in the present study
The present study of 306 multiply transfused patients demonstrated the rate of alloimmunization of 4.24%.
Factors involved in development of alloimmunization are complex and involve at least 3 main contributing elements: RBC antigenic difference between the blood donor and the recipient, Recipients immune status and Immuno-modulatory effect of the allogeneic blood transfusions on the recipient’s immune system.
Previous studies reporting low rate of alloimmunization (5 to 10%) include those by Chaudhary et al,[10] Blumberg et al,[7] and Hmida et al.[9] A high rate of approximately 20% was noted in studies by Spanos et al,[8] Michail-Merianou et al,[12] Singer et al,[13] and Ameen et al.[14]
Patients medical history
Clinical diagnosis of the study group may lead to a vulnerable immune status which may predispose to altered or increased immune response to various antigens. However, no significant correlation was demonstrated between the number of alloimmunized cases and patients diagnosis.
Immunomodulatory effect of allogeneic blood transfusions on recipient’s immune system
Alloimmunization reaction involves presentation of the donor antigens by antigen-presenting cells (APCs) i.e. monocytes, macrophages, dendritic cells, B-cells to recipient T-cells. The Th2 subset of CD4+T helper cells secrete interleukin IL-4, IL-5,IL-6 and IL10,activates B cells and initiates the antibody response. This is the direct pathway of allorecognition.[15,16] Alloimmunization from blood product by indirect pathway involves recognition of the alloantigen and activation of recipient CD4+ T cells by alloantigen presenting recipient APCs in combination with the additional signals generated by costimulatory cell surface structures. This process involves initial recognition of alloantigens by natural killer cells, which secrete interferon gamma. This cytokine in turn activates CD4+ Th2 cells which produce IL-2. This leads to proliferation and differentiation into effector-Th, memory-Th and suppressor Th cells.[17] Primary immunization with blood transfusion reflects the balance between clonal expansion and tolerogenic mechanisms (induced by Th2 subset of cells). Secondary response depends on the rest stimulation of memory cells. Repeated immunization eventually results in sustained clonal expansion and clinically significant antibody production.
Patients’ age at the start of transfusion
It has been observed that an earlier start of transfusion may impart immune tolerance in some patients.[8,12] Present study shows results in accordance to the earlier observations with only one patient developing alloimmunization after starting transfusions at an early age.
Specificity of RBC alloantibodies detected
The specificity of most alloantibodies detected in the present study was against Rh and Kell antigen systems due to their high immunogenicity, which is similar to previous reports of alloimmunization.[14] Anti E was detected in six patients, Anti c in five, Anti Jka in four, Anti K in two and Anti M, Anti S, Anti Fya, Anti C and Anti D in one patient each. Hence, the transfusion of blood matched for Rh and K antigens could prevent alloimmunization resulting in a significant difference in the alloimmunization rates, but the potential to form RBC alloantibodies to unmatched antigens will exist.[13]
Stringency levels have been constructed such that antigens have been ranked by decreasing relative immunization risk, in accordance with clinical observation [Table 3].[18] Level one matching requires a selected donor unit to be compatible with respect to the prospective recipients ABO and D types and to be antigen-negative with respect to alloantibodies identified in the recipients’ plasma. Increasing levels of stringency require further matching for C,c,E,e in addition at level two;Fya,Fyb,Jka,Jkb,S,and s at level three and k, M, N, Doa, Dob, Hy, Joa, and Lua and Lub at level four.
Table 3.
Antigen Matching Stringency Levels

Gender and alloimmunization
Females have been observed to be more prone to development of alloimmunization than males,[19] probably due to the fact that females, especially in developing countries, are anemic and pregnancy is an important risk factor for alloimmunization. In present study, six out of 13 alloimmunized patients were women, however gender was not a risk factor for alloimmunization (p = 0.557).
Positive direct antiglobulin test and alloimmunization
Nine of the thirteen patients (69.23%) had a positive direct antiglobulin test (DAT) without evidence of autoimmune hemolytic anemia and the DAT did not interfere in finding compatible blood. Postive DAT may indicate alloantibodies in a recipients circulation, reacting with antigens on recently transfused donor red cells. Also elevated IgG or complement have been noted on red cells of patients with sickle cell disease, β-thalassemia, renal disease, multiple myeloma, autoimmune disorders(including SLE).[20,21]
Effect of using leucodepleted blood
Another important aspect that has emerged is the role of contaminating leucocytes of the allogeneic blood transfusion in causing immunomodulatory effects in the recipient. Contaminating leucocytes down regulate T-helper cell type 1(Th1) immune response and drive the recipient towards a T-helper cell type 2(Th2) responses. Such skewing towards type 2 immunity may enhance alloantibody formation.[22] Leucodeplection also removes donor APCs, abrogating the direct pathway of alloimmunization by donor-recipient T cell interaction. Donor leucocytes are known to readily express activation and co-stimulatory molecules upon recognition of recipient antigens.[16] Besides this, both autologous and allogeneic non-leucodepleted blood components release soluble bioactive mediators during storage which mediate some of the Transfusion Related Immunomodulation effects, and the Prestorage leucodepletion has been shown to prevent some deleterious effects.[23] Majority of the patients in the present study had a long-term exposure to leucoreduced blood because of collection in optipure RC bags with integral filters.
Number of transfusions received
The risk of developing alloimmunization was not very clearly associated with the number of transfusions received, maximum number of cases, seven, followed 0-5 transfusions, followed by three cases developing alloantibodies after 6-10 transfusions. Some of the earlier studies have found a strong correlation between the number of blood units transfused and alloantibody formation[24,25] while other studies have found no relationship between the number of transfusions and alloimmunization rate.[14,26,32]
Monitoring of RBC alloantibody after each Transfusion Episode
Monitoring of patients for RBC antibodies after transfusion and repeating this after each transfusion episode[27] ie 72 hours after the first transfusion ensures that the transitory antibodies are not missed.
Newer techniques of antibody identification
Antibody screening was performed using column agglutination technology with the gel cards and solid phase red cell adherence technology. This increased the sensitivity of detection as antibodies present in low titres could also be detected as has been proven earlier.[28]
Applicability of local cell panels
The screening cells used for screening alloantibodies, presently, have to be procured from abroad which incurs high cost when used routinely. These cells have short shelf life, get damaged in transportation and are expensive when imported to meet the needs for typing and screening large patient population. These problems can be done away with by using indigenously prepared cell panels[4] or screen cells and panels manufactured by some Nationalized Blood Transfusion Centers could be another alternative.
An additional advantage of cells from the local ethnic groups would be a better detection of antibodies in local population while imported cells may miss certain antibodies against antigens in local population.[4] There are certain antigens that are predominantly found in the Asian population. One such antigen is the Mi11 phenotype of the Miltenberger subsystem (or GP Mur), this antigen being relatively common in Southeast Asia, especially along the south-east coast of China and Taiwan.[29] There is a possibility that such antigens may also be present in the Indian population. Since the screening cells are made from donors of mainly Caucasian descent, they will be lacking such antigens and in that case the antibodies, if present, will not be picked up during the antibody screening. This may be a hindrance in implementing type and screen in countries such as India. In the Peoples’ Republic of China, they have included red cells expressing Mi(a) and Di(a) because of the relatively high frequency of both these antibodies and antigens in their population.[30,31]
Provision of extended red cell phenotying and use of phenotype-matched blood units
The benefits of extended red cell phenotying to minimize alloimmunization have been debated in literature.[32] but cross matching for Rh and Kell systems, obtained after doing extended red cell phenotyping of patients and donors, from the time of initial transfusion, has been reported to lead to significant decrease in the alloimmunization incidence rate. Definitive advantages of RBC phenotying include identification of the RBC antigenic profile among regular repeat donors for the ease of availability of compatible blood for multiply transfused patients.[32]
A study was conducted by Hong Kong government[33] as a part of planning to introduce an electronic smart identity card for all seven million citizens in 2003. Red cell phenotype was determined for 407 donor blood units and 493 patients for whom an antibody screen had been ordered. Since the genes encoding all major blood group antigens have been identified and cloned, it is possible to determine red cell phenotype accurately by molecular techniques with full automation.[34,35]
Conclusion
Alloimmunization increases the time required for crossmatching and may delay treatment, in addition to increasing the chances of transfusion reaction. To avoid the effects of alloimmunization, routine RBC antibody screening at set time intervals after transfusion i.e. repeat antibody screen of the patient if the time interval between two transfusions is more than 72 hours, should be ensured. After antibody screen and identification, corresponding antigen negative blood should be given to the patient. The indigenous development of local cell panels would be a better option to ensure adequate supplies of reagent red cells and introduction of type and screen policy for all the patients. Universal type and screen policy would help in finding the prevalence of alloantibodies in general patient and donor population. Patients with previously identified alloantibodies can be flagged in a database and the information shared between institutions and shared with the patient.
Patients from ethnic minorities who receive multiple transfusions, phenotyping for commonly involved antigens and using antigen negative units can be of much help. A more cost-effective approach is to match the ethnic origin of donors and recipients to the extent possible and then reserve phenotyped matched transfusion for patients who develop one or more alloantibodies.
Alloimmunized patients benefit from leucodepleted RBCs since leuco reduction results in decreased stimulation of Th2 lymphocytes associated with transfusions.
Reducing alloimmunization definitely ensures safer transfusion.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Domen RE, Ramirez G. Red cell alloimmunization in chronic renal failure patients undergoing hemodialysis. Nephron. 1988;48:284–5. doi: 10.1159/000184943. [DOI] [PubMed] [Google Scholar]
- 2.Makroo RN. Compendium of Transfusion Medicine: Blood Transfusion React. 2009;2:337. [Google Scholar]
- 3.Ghio M, Contini P, Mazzei C, Brenci S, Barberis G, Filaci G, et al. Soluble HLA Class I, HLA Class II, and Fas ligand in blood components: A possible key to explain the immunomodulatory effects of allogeneic blood transfusions. Blood. 1999;93:1770–7. [PubMed] [Google Scholar]
- 4.Salamat N, Bhatti FA, Yaqub M, Hafeez M, Hussain A, Ziaullah Indigenous Development of Antibody Screening Cell Panels at Armed Forces Institute of Transfusion. J Pak Med Asso. 2005;55:439–43. [PubMed] [Google Scholar]
- 5.Brecher E. 12th ed. Chap 18,19. United States: American Association of Blood Banks; 2009. Technical Manual; pp. 389–91. 407. [Google Scholar]
- 6.Habibi B, Lecolier B. Inconvenience of planned prophylaxis of transfusion-induced alloimmunization to red cells in chronic renal insufficiency. Transfus Immunohematol. 1983;26:267–77. doi: 10.1016/s0338-4535(83)80031-2. [DOI] [PubMed] [Google Scholar]
- 7.Blumberg N, Peck K, Ross K, Avila E. Immune response to chronic red blood cell transfusion. Vox Sang. 1983;44:212–7. doi: 10.1111/j.1423-0410.1983.tb01886.x. [DOI] [PubMed] [Google Scholar]
- 8.Spanos T, Karageorga M, Ladis V, Peristeri J, Hatziliami A, Kattamis C. Red cell alloantibodies in patients with Thalassemia. Vox Sang. 1990;58:50–5. doi: 10.1111/j.1423-0410.1990.tb02055.x. [DOI] [PubMed] [Google Scholar]
- 9.Hmida S, Mojaat N, Maamar M, Bejaoui M, Mediouni M, Boukef K. [PubMed] [Google Scholar]
- 10.Risk factors for alloimmunization by patients with sickle cell disease. Transfus Clin Biol. 1994;1:27–34. [Google Scholar]
- 11.Shukla JS, Chaudhary RK. Red cell alloimmunization in multi-transfused chronic renal failure patients undergoing hemodialysis. Indian J Pathol Microbiol. 1999;42:299–302. [PubMed] [Google Scholar]
- 12.Murao M, Viana MB. Risk factors for alloimmunization by patients with sickle cell disease. Braz J Biol Med Res. 2005;38:675–82. doi: 10.1590/s0100-879x2005000500004. [DOI] [PubMed] [Google Scholar]
- 13.Michail-Merianou V, Pamphili-Panousopoulou L, Piperi-Lowes L, Pelegrinis E, Karaklis A. Alloimmunization to red cell antigens in Thalassemia: Comparative study of usual versus Better-Match Transfusion. Vox Sang. 1987;52:95–8. doi: 10.1111/j.1423-0410.1987.tb02999.x. [DOI] [PubMed] [Google Scholar]
- 14.Saied DA, Kaddah AM, Badr Eldin RM, Mohaseb SS. Alloimmunization and erythrocyte autoimmunization in transfusion dependent thalassemic patients of predominantly asian descent. Blood. 2000;96:3369–73. [PubMed] [Google Scholar]
- 15.Ameen R, Al-Shemmari S, Al-Humood S, Chowdhury RI, Al-Eyaadi O, Al-Bashir A. RBC alloimmunization and autoimmunization among transfusion-dependent Arab thalassemia patients. Transfusion. 2003;43:1604–10. doi: 10.1046/j.1537-2995.2003.00549.x. [DOI] [PubMed] [Google Scholar]
- 16.Adibzadeh M, Bornhak S, Friccius H, Halder T, Kalbacher H, Li K, et al. The role of endogenous peptides in the direct pathway of all ore activity to human MNC class II molecules expressed on CHO cells. Immunol Rev. 1996;154:155–73. doi: 10.1111/j.1600-065x.1996.tb00933.x. [DOI] [PubMed] [Google Scholar]
- 17.Fast LD. Recipient elimination of allogeneic lymphoid cells: Donor CD4+cells are effective allogeneic-presentation cells. Blood. 2000;96:1144–9. [PubMed] [Google Scholar]
- 18.Blumberg N, Heal JM. The transfusion immunomodulation theory: The Th1/Th paradigm and an analogy with pregnancy as a unifying mechanism. Semin Hemat. 1996;33:329–40. [PubMed] [Google Scholar]
- 19.Klapper E, Zhang Y, Figueroa P, Ness P, Stubbs J, Abumuhor I, et al. Toward extended phenotype matching a new operational paradigm for the transfusion service. Transfusion. 2010;50:536–46. doi: 10.1111/j.1537-2995.2009.02462.x. [DOI] [PubMed] [Google Scholar]
- 20.Garvatty G. Autoantibodies induced by blood transfusion. Transfusion. 2004;44:5–9. doi: 10.1111/j.0041-1132.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- 21.Toy PT, Chin CA, Reid ME, Burns MA. Factors associated with positive direct antiglobulin tests in pretransfusion patients: A case control study. Vox Sang. 1985;49:215–20. doi: 10.1111/j.1423-0410.1985.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 22.Clark JA, Tanley PC, Wallas CH. Evaluation of patients with positive direct antiglobulin tests and nonreactive eluates discovered during pretransfusion testing. Immunohematology. 1992;8:9–12. [PubMed] [Google Scholar]
- 23.Blumberg N, Heal JM. The Transfusion Modulation Theory. In: Anderson KC, Ness PM, editors. Scientific Basis of Transfusion Medicine. 2nd ed. Philadelphia PA: W. B. Saunders; 2000. pp. 427–43. [Google Scholar]
- 24.Jensen LS, Kissmeyer-Nielsen P, Wolff B, Qvist N. Randomised comparison of leucocyte –depleted versus buffy-coat-poor blood transfusion and complications after colorectal surgery. Lancet. 1996;348:841–5. doi: 10.1016/S0140-6736(96)06168-5. [DOI] [PubMed] [Google Scholar]
- 25.Vishinski E, Earles A, Johnson R, Hoag M, Williams A, Lubin B. Aloimmunization in sickle cell anaemia and transfusion of racially unmatched blooding Engle. J Med. 1990;322:1617–21. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 26.Rosse WF, Gallagher D, Kinney TR, et al. Transfusion and alloimmunization in sickle cell disease. Blood. 1990;76:1431–7. [PubMed] [Google Scholar]
- 27.Choudhary R, Sukla JS, Pradhan D, Pahi J. Red Cell Alloimmunization in Multiply Transfused Patients. Indian J Hematol Blood Transfusion. 1997;15(Suppl 4):10–2. [Google Scholar]
- 28.Schonewille H, Haak HL, van Zijl AM. Alloimmunization after blood transfusion in patients with hematologic and oncologic diseases. Transfusion. 1999;39:763–71. doi: 10.1046/j.1537-2995.1999.39070763.x. [DOI] [PubMed] [Google Scholar]
- 29.Bromilow IM, Adams KE, Hope J, Eggington JA, Duguid JK. Evaluation of the ID-gel test for antibody screening and identification. Transfusion Med. 1991;1:159–61. doi: 10.1111/j.1365-3148.1991.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 30.Prathibha R, Lopez CG, Usin FM. The prevalence of GP Murand anti-“Mia”in a tertiary hospital in Peninsula Malaysia. Malaysian J Path. 2002;24:95–8. [PubMed] [Google Scholar]
- 31.Lin CK, Mak KH, Cheng G, Lao TT, Tang MH, Yuen CM, et al. Serologic characteristicsand clinical significance of Miltenberger antibodies among Chinese patients in Hong Kong. Vox Sang. 1998;74:59–60. doi: 10.1046/j.1423-0410.1998.7410059.x. [DOI] [PubMed] [Google Scholar]
- 32.Cheng G, Hui CH, Lam CK, Hal SY, Wong L, Mak KH, et al. Haemolytic transfusion reactions due to Mi-antibodies;need to include Miltenberger III positive cells in pre-transfusion antibodies screening in Hong Kong. Clin Lab Haematol. 1995;17:183–4. [PubMed] [Google Scholar]
- 33.Blumberg N, Ross K, Avila E, Peck K. Should Chronic Transfusions be matched for antigens other than ABO and Rho(D)? Vox Sang. 1984;47:205–8. doi: 10.1111/j.1423-0410.1984.tb01587.x. [DOI] [PubMed] [Google Scholar]
- 34.Lau FY, Wong R, Chan NP, Chui CH, Ng E, Ng MH, et al. Provision of phenotype-matched blood units: No need for pre-transfusion antibody screening. [Last accessed on 2012 May 20];Haematologica. 2001 86:742–8. Available from: http://www.haematologica.it/2001_07/0742.htm . [PubMed] [Google Scholar]
- 35.Olsson ML, Hansson C, Avent ND, Akesson IE, Green CA, Daniels GL. A clinically applicable method for determining the three major alleles at the Duffy(FY) blood group locus using polymerase chain reaction with allele-specific primers. Transfusion. 1998;38:168–73. doi: 10.1046/j.1537-2995.1998.38298193099.x. [DOI] [PubMed] [Google Scholar]


