Abstract
Purpose:
To analyze different factors affecting the outcome of patients undergoing Two Stage Esophagectomy (TSE) for the treatment of esophageal carcinoma (EC) while relating these factors to the length of stay on Intensive Care Unit (ILOS), mortality, and morbidity.
Methods:
Retrospective study of case-notes of 45 patients who underwent a TSE for resection of EC at a general district hospital in the United Kingdom (UK). These procedures were performed by the same surgical team and followed same approach, known as the Ivor-Lewis procedure.
Results:
The duration of One Lung Ventilation (OLV) during TSE was found to be critical for patient's outcome. Statistical analysis suggested a potentially strong effect of the duration of OLV (range: 90-320 minutes) on the ILOS (P=0.001). The ratio OLV: Total duration of surgery (TOT) was significantly different in early post-operative (PO) deaths (within 3 months) and late deaths after the third month (P=0.032). The POSSUM value (Physiological and Operative Severity Score for Enumeration of Mortality) correlated well with ILOS (P=0.05). Regression analysis showed a strong relationship between the two variables (P=0.03). An excellent to good quality of PO analgesia allowed for shorter ILOS (P=0.023).
Conclusions:
Duration of the OLV appears as an important factor in the outcome of patients. POSSUM value could help in planning the post-operative critical care need of patients undergoing TSE. A well managed post-operative pain allowed to reduce the ILOS.
Keywords: Intensive care unit, Intra-operative fluid therapy, length of stay, major surgery, one lung ventilation, post-operative analgesia, thoracic epidural analgesia, two-stage esophagectomy
INTRODUCTION
Esophageal carcinoma (EC) is a very taxing disease with over 480,000 cases of diagnosed in 2008 and over 40,000 deaths were linked to it during the same period.[1] It is the eighth of most common cancers worldwide.[1] Two types of EC are well known: Adenocarcinoma and squamous cell carcinoma. The relative incidence of each type depends on gender, geographical region, and particular risk factors such as smoking, heavy alcohol consumption, Barret's disease, and alimentary regimes.[2,3,4,5] In the UK, the prevalence of this disease reaches 9/100,000 and accounts for 2.2% of all malignancies.[6] Patients often present to surgery at advanced stage (T3 N1 Mx) reducing the chances of 5-year survival (80% if stage T1; 30% if stage T2; 18% if T3, and 4% for stage T4).[7] Treatment of EC is two-fold: Palliative and curative. Aiming to cure the local tumor, the surgery can be a one-stage, two-stage, or three-stage depending on anatomical location and extent of the disease. Minimally-invasive, laparoscopic, and thoracoscopic techniques have also been successfully implemented.[7] Patients are usually managed on the Intensive Care Unit (ICU) in the early post-operative (PO) period before being transferred to a surgical ward. Systemic algorithms have been proposed in order to reduce the need for ICU and expedite patient recovery.[8,9] Two-Stage Esophagectomy (TSE), also known as “Ivor-Lewis” procedure, is a commonly adopted technique for the resection of EC. TSE is a major surgical intervention and often presents a real challenge for the operating team. This is because of the fragile nature of patient to be operated, the requirement for both thoracotomy and laparotomy and the potential significant impact of PO complications on patient outcome. Careful PO preparation and anesthetic technique, experienced surgical staff and appropriate intensive care backup could favorably impact on the survival rate and PO quality of life of the patient, at least for the short-term. Analyzing these factors seems thus critical to improve mortality and morbidity following TSE.
METHODS
Since patients were not subjected to any intervention other than usual care, no written informed consent was necessary. Personal information and patient confidentiality were guaranteed. Anonymous data collection, as approved and facilitated by the Audit department of the institution, was conducted. The study thus complied with Helsinki Declaration of 1975 and its subsequent revisions as well as with national regulations in the United Kingdom (UK).
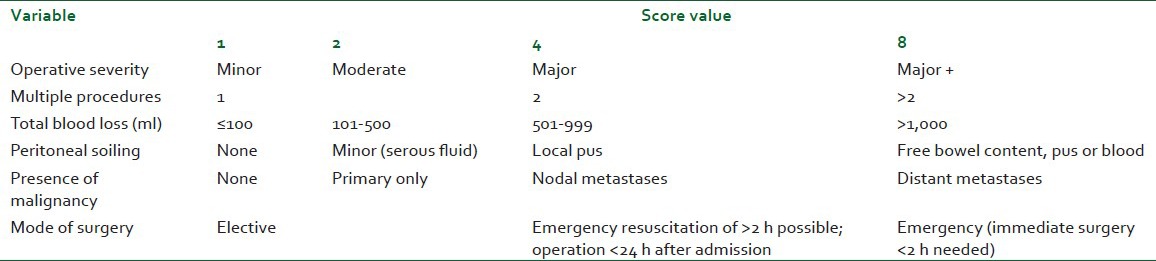
We identified 45 patients who underwent TSE at our district general hospital between 1999 and 2005. All had a confirmed EC and underwent the same surgical technique, two-stage esophagectomy, as main curative treatment. All were performed by the same surgical team as per the Ivor-Lewis approach. We attempted to identify relevant factors affecting their outcome throughout the peri-operative period. All patients had a general anesthetic-induced using Propofol, Fentanyl, and muscle relaxant. Airway was secured using a conventional single-lumen endotracheal cuffed tube and anesthesia maintained by inhaled agents (Sevoflurane or Isoflurane). Before starting the thoracic approach, patients had a fiber-optic guided bronchial blocker placed in the right main bronchus to allow deflation of the right lung. Patients were then placed in left lateral decubitus and the thoracic stage started. Thoracic Epidural Analgesia (TEA) was the main method of PO analgesia (see below). A thoracic epidural catheter was placed between T6 and T9 using loss of resistance technique. One patient had an intra-thecal catheter and one other was managed using patient controlled analgesia (PCA) with intravenous (IV) Morphine. An epidural infusion of a mixture of Bupivacaine 0.125% and Fentanyl 2 mcg/m was initiated before the reversal of general anesthesia and titrated to reach satisfactory analgesia while allowing a good cough effort. Continued on the ICU, the TEA was gradually weaned-off within the first week post-operatively. Data collected included: General demographic, pre-operative physiological status including POSSUM score [Tables 1 and 2][10] and the American Society of Anesthesiologists grades (ASA), anesthetic technique, and intra-operative cardiovascular optimization. Other intra-operative data was also explored: Length of the procedure Time on Table (TOT), duration of One Lung Ventilation (OLV) and estimated blood loss (EBL) in addition to the average rate of intra-operative fluid therapy (avIOF) and the corrected avIOF (avIOFc) calculated as the difference between avIOF and average EBL. PO data included: Length of stay on the ICU (ILOS), readmission due to requirement for mechanical ventilation (re-ventilation), method and quality of analgesia, morbidities and early and delayed mortalities.
Table 1.
Physiological score (to be scored at the time of surgery)
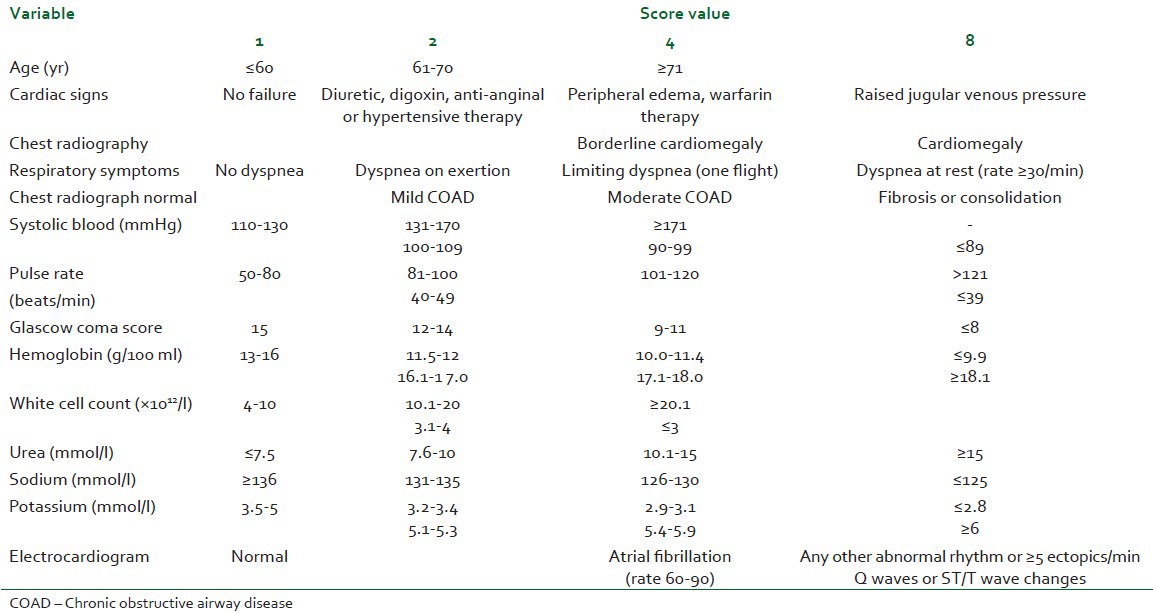
Table 2.
Operative severity score (Definitions of surgical procedures with regard to severity are guidelines; not all the procedures are listed and the closest should be selected)
RESULTS
Demographic data
Male:female ratio was 3:4. Average age was 64 years (range: 41-79). Tumor stage was known in 44 patients. Sixty-eight percent of patients presented to surgery at T3N1 stage (n=30), 16% were at T2Nx stage (n=8) and 14% at T1Nx (n=6).
Pre-and intra-operative data
The majority of patients were considered ASA II grade (67%; n=31) and ASA III (n=11; 25%) while a minority were considered as ASA I (n=8%; n=3). POSSUM mean score was 20. First and third quartiles were 16 and 22, consecutively. Patients were grouped into three arbitrary categories of POSSUM ranges: 11-15, considered low risk (n=10; 22%), 16-21, considered intermediate risk (n=20; 45%), and 22-30, considered high risk (n=15; 33%).
ILOS, PO complications, and OLV
The ILOS represented the total stay of immediate PO admission and any later readmission (excluding High Dependency Unit, HDU). Mean ILOS was 8.4 days (range: 2-62 days). The mean length of OLV was 150 minutes (range: 90-320 minutes). Patients discharge from the ICU was usually within 48 hours post-operatively provided they were vitally stable with satisfactory analgesia. The care was continued on surgical wards and occasionally on HDU. ILOS correlated positively with OLV (Spearsman's R=0.49, P=0.001). The linear regression model, in this case yielded a P value of 0.015 (F-ratio=6.58). The mean total duration of surgery (TOT) was 442 minutes (range: 240-600 minutes). The ILOS did not correlate with the TOT. All early deaths (within the first 3 month post-operatively) had a relatively more prolonged OLV compared to patients who died after the 3rd month (P=0.032, Pearson's Chi Square statistic: 8.813). The ratio OLV: TOT (mean=0.353; range: 0.25-0.75) more strongly correlated with the ILOS than OLV itself (P<0.001; Pearson's R statistic: 0.513; intercept: 0.345). Anastomosis leak seems also to be affected by this ratio (P=0.015; t statistic: −2.555; df: 37).
4.4% of patients (n=2) died within the first month of surgery, 2.2% within the first 3 months, and 6.7% (n=3) died after the 3rd month and within the 12 months post-operatively. All these deaths occurred in patients operated at stage tumor stage T3Nx and represented a mortality of 20% (n=6) in this group (n=30) and 13.3% of all patients (n=45). Respiratory complications such as chest infections, loss of space, pleural effusions, and retained secretions were observed in 54% of patients (n=24) with 18% (n=8) defined as “acute respiratory failure”. As a whole, respiratory complications did not correlate with the total ILOS (R=0.024; P=0.88), the duration of surgery (P=0.98) or the length of OLV period (P=0.94).
PO atrial fibrillation (AF) was observed in 27% of patients (n=12). The incidence of sepsis reached 20% (n=9). Anastomosis leak occurred in 9% of patients (n=4). Serious myocardial ischemic events were observed in 7% of patients (n=3). Variable mild gastrointestinal and central nervous system (CNS) symptoms were observed in 22% of patients (n=10).
Pre- and intra-operative status
POSSUM value [Tables 1 and 2] correlated with the ILOS (Spearsman's R=0.39, P=0.05). Regression analysis shows a relatively strong relationship between the two variables (P=0.03; F-ratio=5.33). ASA grade significantly correlated to the ILOS (Spearsman's R=0.39, P=0.007), linear regression model yields a significant P value at 0.017 (F-ratio=6.2). Age, on the opposite did not correlate with the actual ILOS (Spearsman's R=0.25, P=0.095).
Quality of PO analgesia
Depending on how often they required additional interventions to achieve satisfactory pain control, patients were grouped into three categories. In category “a”, 60% of patients (n=26), the TEA was adequate without requiring more than one “top-up bolus” dose as a rescue intervention, “b”, 16% of patients (n=7), up to three top-ups boluses were needed for adequate analgesia and “c”, 24% (n=10), more than three top-ups resiting the epidural catheter or complete change of mode of analgesia were required. Thus, “a” was considered as excellent, “b” very good to good and “c” poor analgesia. Patients who received an excellent to good epidural analgesia had shorter ILOS (P=0.023) but no statistical link could be made between the quality of analgesia and the incidence of PO adverse respiratory events (P=0.52).
Readmission to the ICU
As many as 25% of patients (n=11) had to be readmitted on the ICU for mechanical ventilation. However, this event itself did not lead to a longer overall ILOS of these patients (Mean 14.2; SD=12.8) versus those who did not require re-ventilation (Mean=6.5; SD=12.02). Despite clinical significance, statistical significance could not be confirmed (P=0.073). Moreover, the association between respiratory complications and re-ventilation requirement is found to be non-significant (P=0.063).
PO AF, respiratory events and avIOF
Although, as common as in 29% of patients (n=14), AF did not significantly increase the ILOS (Mean=11 vs 7.2 days; SD=13.3 vs 12.2; P=0.3). Nevertheless, the occurrence of respiratory complications was strongly associated with that of AF (P=0.011). The average rate of avIOF significantly affected the occurrence of respiratory complications (P=0.036) without an increase of the requirement for re-ventilation (P=0.07). No significant association was confirmed between avIOF (P=0.289) nor the ILOS (P=0.84). While the avIOFc, could have a significant effect on the ILOS. Thus, patients who received an avIOFc <0.6 l/hour were more likely to spend less than eight days ILOS (P=0.023; Pearson's Chi Square statistic=13.0).
DISCUSSION
We would not dispute that our study is limited by the relatively low number of subjects and its retrospective nature. However, the above results deserve further investigation and analysis as they raise few interesting issues. The following variables could be discussed as significant determinants of the short-term outcome of patients undergoing the TSE approach for EC resection:
Duration of OLV phase of the surgery
Patients may be exposed to relative hypoxia, hypercapnia, and increased acidemia, low cardiac output and unstable hemodynamic during OLV. This is due to the physiological changes occurring during the OLV which include hypoxic pulmonary vasoconstriction, hypercapnia, and decrease in cardiac output due to surgical manipulation.[11,12,13] This, in our view, could result in an increase of the anerobic metabolism and potentially compromise the short-term outcome. Such argument is supported by the absence of significant correlation between the ILOS and the incidence of respiratory complications as well as between the incidence of respiratory complications and the length of OLV as demonstrated above. Thus, the impact of OLV on the ILOS and the short-term mortality does not necessarily pass by ventilation abnormalities. Rather, it could well be a relatively high PO “oxygen debt” that imposed a longer ILOS and perhaps contributed to more intense inflammatory response. In line with this is the demonstrated absence of changes in lung isoform of nitric oxide (NO) synthase and vascular congestion indifferently during OLV or two-lung ventilation (TLV).[14] which leads us to believe that physiological local mechanisms are likely to be irrelevant in activating such inflammatory response. Plasma nitrite and endothelin levels were also similar in case of OLV or TLV as reported M. Lund et al.,[14] In addition, when inflammatory markers are evaluated, as in the work of Tsai et al., the terminal complement complex (TCC) shows a sustained and significantly higher increase-starting on day 2 post-operatively-in patients who received OLV.[15] In our study, two of the three patients who had serious PO cardiac events had a prolonged OLV duration (258 and 180 minutes). All patients who died within the first 3 months post-operatively had an OLV significantly over 150 minutes (P=0.032). Interestingly, the TOT did not itself have the same effect suggesting that the specific duration of the OLV was a critical factor. This is also made plausible with the strong correlation between ILOS and the OLV/TOT ratio. We can, therefore, assume that efforts to maintain a good perfusion and oxygen delivery-in particular during the thoracic stage-may reduce mortality and shortens the ILOS. In addition, it is likely to be beneficial to implement techniques that shorten the duration OLV or avoid it all together, limit the hypoxia and hypercapnia and achieve optimal hemodynamic. Data obtained from studies on the effects of protective lung strategies during the thoracic stage are encouraging in this respect and show a decrease in the pro-inflammatory mediators.[16,17]
POSSUM value
POSSUM is calculated based on intra-operative physiological status and operative surgical data. The POSSUM had been validated as a reasonably good tool in morbidity and mortality predictive models.[18] The correlation and linear regression model between POSSUM value and ILOS suggest that the numeric value of this score may help in planning of clinical area for PO admission. It is of course tempting to be able to avoid ICU all together. Nevertheless, considering the risks of unplanned readmissions to the ICU, it seems more reasonable to take steps to predict ahead those most probable readmissions or to avoid bypassing the ICU for those at high risk of prolonged ILOS.[9] Our results suggest that the POSSUM score is a valuable tool for the right decision and in particular, if combined with the duration of OLV and ASA grade.
Quality of post operative analgesia
Our results demonstrate that pain control directly impacted on ILOS despite having no real effect on the occurrence of respiratory complications. The reduction of stay on ICU may not fully be dependent on respiratory effects of TEA. We believe that role of TEA extends to its potential to improve circulatory and metabolic performance, at least regionally. TEA would allow better oxygenation efficiency in the early PO period by easing ventilation and reducing stress.[19]
Post operative AF
AF was common (29% of patients). Similar incidence of this arrhythmia has been reported by other authors.[20] Our results showed a significant relationship between the AF and respiratory complications (P=0.011). However, occurrence of the AF, per-se, did not lead to an increased ILOS. We were not able to identify a definite physiological mechanism to explain the link between respiratory complications and the PO AF but this could be biased by the size of our sample. For instance, the duration of OLV did not correlate with the occurrence of AF. A trend that was also found by Sudish et al.[20] Whether the mechanism is a surgical trauma to the vagus nerve is questionable. AF is also observed in non thoracic major surgery-not involving trauma to the vagus.[21,22]
Intra-operative fluid therapy
The impact of IOF on the incidence of anastomosis leak, respiratory complications, and cardiovascular complications has always been a hot topic in major gastro-intestinal surgery.[23,24,25] In our patients spending less than 8 days on ICU, a strong relationship was observed between the ILOS and the avIOFc therapy. No definite numerical correlation, however, was established with the crude avIOF. Equally, the avIOFc was significantly linked to the incidence of respiratory events but not to cases of anastomosis breakdown. A goal-directed fluid therapy in such major cases appears paramount. We believe that continuous cardiac output and oxygen delivery monitoring is essential in achieving balanced fluid therapy particularly during procedures requiring OLV.
CONCLUSIONS
The main factors that seem to affect outcome and critical care resources requirement for the patients undergoing TSE include the duration of the OLV stage, numerical value of POSSUM, quality of PO analgesia, and avIOFc. In practice, approaches and techniques that would achieve optimization of these factors could well improve the outcome and reduce critical care resources required for patients undergoing TSO.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D, et al. Global Cancer Statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Engel LS, Chow WH, Vaughan TL, Ghammon MD, Risch HA, Stanford JL, et al. Population Attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1404–13. doi: 10.1093/jnci/djg047. [DOI] [PubMed] [Google Scholar]
- 3.Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental Causes of esophageal cancer. Gastroenterol Clin North Am. 2009;38:27–57. doi: 10.1016/j.gtc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gholipour C, Shalchi RA, Abbasi M. A histopathological study of esophageal cancer on the western side of the caspian littoral from 1994 to 2003. Dis Esophagus. 2008;21:322–7. doi: 10.1111/j.1442-2050.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- 5.Tran GD, Sun XD, Abnet CC, Fan JH, Dawsey SM, Dong ZW, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in china. Int J Cancer. 2005;113:456–63. doi: 10.1002/ijc.20616. [DOI] [PubMed] [Google Scholar]
- 6.Park DP, Welch CA, Harrison DA, Palser TR, Cromwell DA, Gao F, et al. Outcomes following oesophagectomy in patients with oesophageal cancer: A secondary analysis of the icnarc case, mix programme database. Crit Care. 2009;13(Suppl 2):S1. doi: 10.1186/cc7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen NT, Follette DM, Wolfe BM, Schneider PD, Roberts P, Goodnight JE., Jr Comparison of minimally invasive esophagectomy with transthoracic and transhiatal esophagectomy. Arch Surg. 2000;135:920–5. doi: 10.1001/archsurg.135.8.920. [DOI] [PubMed] [Google Scholar]
- 8.Munitiz V, Martinez-de-Haro LF, Ortiz A, Reuiz-de-Angulo D, Pastor P, Parrilla P. Effectiveness of a written clinical pathway for enhanced recovery after transthoracic (Ivor Lewis) oesophagectomy. Br J Surg. 2010;97:714–8. doi: 10.1002/bjs.6942. [DOI] [PubMed] [Google Scholar]
- 9.Cerfolio RJ, Bryant AS, Bass CS, Alexander JR, Bartolucci AA, et al. Fast tracking after ivor lewis esophagogastrectomy. Chest. 2004;126:1187–94. doi: 10.1378/chest.126.4.1187. [DOI] [PubMed] [Google Scholar]
- 10.Copeland GP, Jones D, Walters M. Possum: A scoring system for surgical audit. Br J Surg. 1991;78:356–60. doi: 10.1002/bjs.1800780327. [DOI] [PubMed] [Google Scholar]
- 11.Alexander NG, Justiaan S. Hypoxaemia during one-lung anaesthesia. BJA: Continuing Education in Anaesthesia, Critical Care & Pain. 2010;10:117–22. [Google Scholar]
- 12.Sutton CJ, Naguib A, Sprenker CJ, Camporesi EM. One-lung ventilation in infants and small children: Blood gas values. J Anesth. 2012;26:670–4. doi: 10.1007/s00540-012-1413-7. [DOI] [PubMed] [Google Scholar]
- 13.Westbrook JL, Sykes MK. Peroperative arterial hypoxaemia. The interaction between intrapulmonary shunt and cardiac output. A computer model. Anaesthesia. 1992;47:307–10. doi: 10.1111/j.1365-2044.1992.tb02170.x. [DOI] [PubMed] [Google Scholar]
- 14.Lund M, Ny L, Malmström RE, Lundberg JO, Ost A, Bjornstedt M, et al. Nitric oxide and endothelin-1 release after one-lung ventilation during thoracoabdominal esophagectomy. Dis Esophagus. 2012 doi: 10.1111/j.1442-2050.2012.01388.x. DOI: 10.1111/j. 1442-2050.2012.01388.x. [DOI] [PubMed] [Google Scholar]
- 15.Tsai JA, Lund M, Lundell L, Nilsson-Ekdahi K. One-lung ventilation during thoracoabdominal esophagectomy elicits complement activation. J Surg Res. 2009;152:331–7. doi: 10.1016/j.jss.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 16.Buise M, van Bommel J, van Genderen M, Tilanus H, van Zundert A, Gommers D. Two-lung high-frequency jet ventilation as an alternative ventilation technique during transthoracic esophagectomy. J Cardiothorac Vasc Anesth. 2009;23:509–12. doi: 10.1053/j.jvca.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Michelet P, D’journo XB, Roch A, Doddoli C, Marin V, Papazian L, et al. Protective ventilation influences systemic inflammation after esophagectomy: A randomized controlled study. Anesthesiology. 2006;105:911–9. doi: 10.1097/00000542-200611000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Copeland GP, Jones D, Walters M. POSSUM: A Scoring System For Surgical Audit. Br J Surg. 1991;78:355–60. doi: 10.1002/bjs.1800780327. [DOI] [PubMed] [Google Scholar]
- 19.Michelet P, Roch A, D’Journo XB, Blayac D, Papzianh L, Thomas P. Effect of thoracic epidural analgesia on gastric blood flow after oesophagectomy. Acta Anaesthesiol Scand. 2007;51:587–94. doi: 10.1111/j.1399-6576.2007.01290.x. [DOI] [PubMed] [Google Scholar]
- 20.Sudish C Murthy, Law S, Whooley BP, Alexandrou A, Chu KM, Wong J. Atrial fibrillation after esophagectomy is a marker for postoperative morbidity and mortality. J Thorac Cardiovasc Surg. 2003;126:1162–7. doi: 10.1016/s0022-5223(03)00974-7. [DOI] [PubMed] [Google Scholar]
- 21.Brathwaite D, Weissman C. The new onset of atrial arrhythmias following major noncardiothoracic surgery is associated with increased mortality. Chest. 1998;114:462–8. doi: 10.1378/chest.114.2.462. [DOI] [PubMed] [Google Scholar]
- 22.Amar D. Postoperative atrial fibrillation. Heart Dis. 2002;4:117–23. doi: 10.1097/00132580-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Joshi GP. Intraoperative fluid restriction improves outcome after major elective gastrointestinal surgery. Anesth Analg. 2005;101:601–5. doi: 10.1213/01.ANE.0000159171.26521.31. [DOI] [PubMed] [Google Scholar]
- 24.Kita T, Mammoto T, Kishi Y. Fluid management and postoperative respiratory disturbances in patients with transthoracic esophagectomy for carcinoma. J Clin Anesth. 2002;14:252–6. doi: 10.1016/s0952-8180(02)00352-5. [DOI] [PubMed] [Google Scholar]
- 25.Neal JM, Wilcox RT, Allen HW, Low De. Near-total esophagectomy: The influence of standardized multimodal management and intraoperative fluid restriction. Reg Anesth Pain Med. 2003;28:328–4. doi: 10.1016/s1098-7339(03)00197-4. [DOI] [PubMed] [Google Scholar]


