Abstract
Background:
Postoperative nausea and vomiting (PONV) is a frequent complication of middle ear surgery. Ondansetron has been shown to be effective for early PONV and dexamethasone has been shown to be effective for late PONV. Therefore, a combination of dexamethasone and ondansetron is commonly used for middle ear surgery. This study was conducted to compare the combination of ondansetron and dexamethasone with ramosetron for early and late PONV up to 48 h after middle ear surgery.
Methods:
One hundred and twenty adults scheduled for middle ear surgery were allocated to receive either dexamethasone 8 mg and ondansetron 4 mg (n=60) or ramosetron 0.3 mg (n=60). General anesthesia with inhalation agents was used for all the patients. The incidence and severity of PONV, administration of rescue antiemetic, and the side effects of the antiemetic were documented during the first 48 h after surgery.
Results:
The incidence of nausea was significantly lower in the dexamethasone and ondansetron group compared to the ramosetron group between 2 and 24 h. The complete response, which is patients with no nausea or vomiting, was significantly more in dexamethasone and ondansetron group compared to ramosetron group between 2 and 24 h and between 24 and 48 h (76% vs. 56%, P=0.02, 93% vs. 81%, P=0.05, respectively). Overall, complete response was more in dexamethasone and ondansetron group compared to ramosetron group (71% vs. 40%, P=0.01).
Conclusion:
The combination of dexamethasone and ondansetron is superior to ramosetron for prevention of PONV after middle ear surgeries.
Keywords: Dexamethasone, middle ear surgery, ondansetron, postoperative nausea and vomiting, ramosetron
INTRODUCTION
Postoperative nausea and vomiting (PONV) is among the most common complications after anesthesia and surgery, with a relatively high incidence after middle ear surgery.[1] Since the middle ear surgery involves stimulation of the labyrinth, PONV is expected to be prolonged. Ondansetron provides significant reduction in early PONV.[2] Dexamethasone has been used mainly to reduce late PONV.[3,4,5] Therefore, a combination of dexamethasone and ondansetron is considered the optimum choice for prevention of PONV after middle ear surgery.[6,7] The newer 5-HT3 antagonist ramosetron, with long duration of action, has been found to be more effective than ondansetron in reducing the early as well as delayed PONV, when used in other surgeries.[8]
Therefore, we conducted this study to compare the combination of ondansetron and dexamethasone with ramosetron for prevention of early and late PONV up to 48 h after the middle ear surgery.
METHODS
Approval from the ethical committee of our hospital (SDM College of Medical Sciences, Hospital, Ref: 021: 2010) was obtained. Written informed consent was obtained from all the patients for this prospective, randomized, double-blind study. One hundred and twenty patients in the age group of 16-65 years, with American Society of Anesthesiologists (ASA) physical status classification I or II, undergoing middle ear surgery in our medical college hospital were included in the study. The patients who received other antiemetic medication or perioperative steroids as antiedema therapy for facial nerve damage were excluded from the study. Known risk factors for PONV, as suggested by the simplified risk score system of Apfel, were assessed.[9] This score system identifies high risk of PONV in a patient with four characteristics, namely, female gender, nonsmoking, the use of postoperative opioids, and prior history of motion sickness or PONV.
Tablet diazepam (10 mg, PO) was given as premedication the night before and on the morning of the surgery for anxiolysis. General anesthesia was induced with fentanyl (2-3 mcg/kg), propofol (2 mg/kg), and vecuronium (0.1 mg/kg) to facilitate endotracheal intubation. Anesthesia was maintained with isoflurane 1%-1.5% with nitrous oxide 60% in oxygen. The patients received intravenous diclofenac 75 mg infusion during the surgery. Ventilation was mechanically controlled and adjusted to maintain an end-tidal concentration of CO2 between 35 and 40 mmHg. To reduce the blood loss, anesthetic depth was adjusted to keep mean arterial pressure about 20%-30% below baseline. The patients’ heart rate, mean arterial pressure, and minimum anesthetic concentration (MAC) were noted every 30 min during surgery. Neuromuscular block was reversed with neostigmine and glycopyrrolate at the end of surgery. The total amount of reversal used in milliliters was noted (1 ml=neostigmine 0.5 mg and glycopyrrolate 0.1 mg). After the clinical assessment of adequacy of the reversal of neuromuscular block, trachea was extubated. Near the end of surgery, all the patients were given morphine 0.1 mg/kg intravenously for the postoperative analgesia.
Patients were randomly allocated to receive a combination of dexamethasone 8 mg (given at the beginning of surgery) and ondansetron 4 mg (given near the end of surgery) (group DO, n=60) or ramosetron 0.3 mg (near the end of surgery) (group R, n=60), by a computer-generated randomization table. Primary efficacy variables assessed were the incidence and severity of nausea and the incidence of vomiting in the first 48 h after the surgery. Secondary efficacy variables included the use of additional antiemetic as rescue, pain intensity, and medication-associated complications. These variables were assessed by an investigator who was blinded to the treatment group. Evaluations were performed in the first 2 h, 2-24 h, and 24-48 h postoperatively. Nausea was defined as subjectively unpleasant sensation associated with the urge to vomit. Vomiting was defined as the forceful expulsion of gastric contents. The severity of nausea was graded as: 0=none, 1=mild, 2=moderate, and 3=severe. The severity of postoperative pain was assessed by using a visual analog scale (VAS) that ranged from 0 (no pain) to 10 (worst pain imaginable). If the patient developed nausea or vomiting in the postoperative period, then prochloperazine 25 mg was given slowly intravenously as rescue antiemetic. If the patient's PONV persisted despite administering rescue antiemetic, the physician was allowed to give dexamethasone or ondansetron or any other antiemetic as per their discretion. All the patients received diclofenac tablets three times a day for the postoperative pain. If they complained of pain ≥5 on VAS, pethidine was used as a breakthrough analgesic. The patients were enquired about the common side effects of medication, namely, headache, dizziness, drowsiness, constipation, and flushing.
Our pilot study had shown the incidence of complete response (no PONV) as 72% in dexamethasone and ondansetron group, which were commonly used as antiemetics for middle ear surgery. Sample size calculation was done to get 20% improvement in the incidence of PONV, presuming an α error of 0.05 and to achieve a power of 0.8. The sample size calculation revealed that 57 patients were needed in each group. Statistical analyses were performed using SPSS ver. 20.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were compared using the chi-square test or fisher's exact test. Continuous variables were compared using independent t-test. Data are presented as mean ± standard deviation or as the number of patients and percentages. P value of less than 0.05 was considered statistically significant.
RESULTS
All the 120 patients completed the study protocol (no dropouts) and were analyzed for primary efficacy. The patient's characteristics, duration of surgery or anesthesia, incidence of motion sickness or history of PONV, and nonsmoking status were not significant between the two groups. The calculated simplified risk score of Apfel was also comparable between the groups [Table 1]. There was no significant difference in the measured mean arterial pressure, heart rate, and MAC values between the groups.
Table 1.
Patient characteristics, surgery and anesthetic data
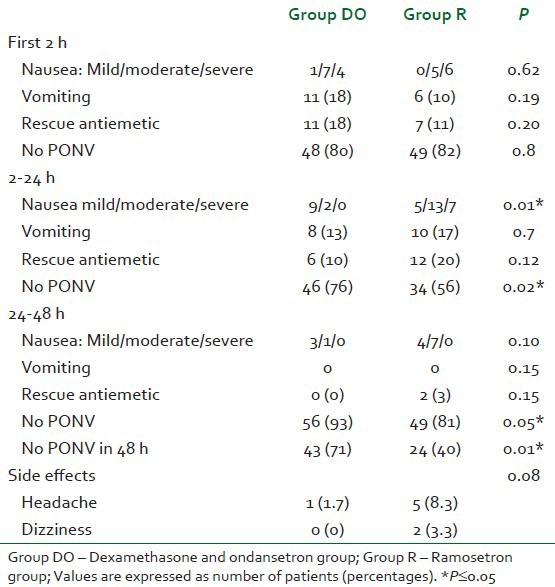
There was no significant difference in PONV in the first 2 h after the surgery. Between 2 and 24 h, the incidence of nausea was significantly lower in the dexamethasone and ondansetron group compared to the ramosetron group (P=0.01). The incidence of vomiting and use of rescue antiemetic was not different between the groups. The patients who never developed nausea or vomiting were considered to have had complete response. Between 2 and 24 h, higher number of patients in the dexamethasone and ondansetron group had complete response compared to the ramosetron group (76% vs. 56%, P=0.02). Between 24 and 48 h, even though the incidence of nausea was more in ramosetron group compared to combination therapy group, it did not reach statistical significance (11 vs. 4 patients). Between 24 and 48 h, the complete response was more in dexamethasone and ondansetron group compared to ramosetron group (93% vs. 81%, P=0.05). Overall, higher number of patients had a complete response in the dexamethasone and ondansetron group compared to ramosetron group (71% vs. 40%, P=0.01) [Table 2].
Table 2.
Incidence and severity of nausea and vomiting and requirements for rescue antiemetic treatment
Incidences of side effects were not different between the groups. There was no significant difference in the pain scores between the groups [Table 3].
Table 3.
Pain scores

DISCUSSION
We noted that the incidence of nausea was less with the dexamethasone and ondansetron combination therapy group compared to ramosetron group after the first 2 h. Also, the overall number of patients with no PONV was higher in the combination group than in the ramosetron group.
PONV is a very common complication after middle ear surgeries, with an incidence up to 80%, when no antiemetics are used.[1] Ondansetron has been shown to be an effective antiemetic in reducing the PONV after middle ear surgery.[2] Studies have shown that ondansetron is more effective in preventing early but not late PONV, whereas dexamethasone was found to have more pronounced action in the late postoperative period.[3,4,5] This may be due to the shorter duration of action of ondansetron (4 h) in contrast to the prolonged duration of action of dexamethasone. Thus, the combination of ondansetron and dexamethasone can decrease the incidence of both early and late nausea and vomiting. Due to the stimulation of the labyrinth, PONV continues for longer duration in middle ear surgeries. Previous studies have shown that the combination of dexamethasone and ondansetron is more effective than ondansetron alone after middle ear surgery.[6,7] Thus, the combination of ondansetron and dexamethasone can decrease the incidence of both early and late nausea and vomiting. Therefore, combination of dexamethasone and ondansetron is considered as the prophylaxis of choice in reducing PONV after middle ear surgery and is commonly practiced in our institute.
Ramosetron is a newer 5-HT3 receptor antagonist which is more potent and has a longer duration of antiemetic action than the older agents. This has been attributed to the higher binding affinity and slower rate of dissociation from the target receptor of ramosetron compared to ondansetron. The elimination half-life of ramosetron is also longer than that of ondansetron (9 h vs. 3.5 h).[10] Many of the recent studies have shown that ramosetron is more effective than ondansetron in preventing PONV for the patients undergoing various other surgeries.[8,11,12,13] Furthermore, antiemetic efficacy of combination of ramosetron and dexamethasone was found to be similar to that of ondansetron and dexamethasone, when used as prophylaxis for the spine surgeries.[14] Addition of dexamethasone did not provide any advantage to ramosetron. Authors concluded that although the addition of corticosteroids could theoretically obviate the inferiority of the 5-HT3 antagonists, the more potent affinity for the 5-HT3 receptor would not lead to more synergistic pharmacodynamic results, if drugs from different classes partially involve common antiemetic mechanisms. Thus addition of steroid may not benefit ramosetron. Therefore, this study was carried out to compare the combination of ondansetron and dexamethasone with ramosetron for early and late PONV up to 48 h after surgery.
We noted that the combination of two antiemetics, ondansetron and dexamethasone, had better efficacy than the single agent when used as a prophylaxis against PONV. This is similar to the conclusion of other meta-analyses, which state that the combination of antiemetics is superior to single agent used for PONV prophylaxis.[15,16] It is recommended that the drugs with different mechanisms of action should be used in combination to optimize the efficacy. Etiology of PONV after middle ear surgeries is multifactorial. There are abundant 5-HT3 receptors present in the vicinity of trigeminal nerve and vestibular labyrinth; hence, 5-HT3 receptor antagonists are efficacious in middle ear surgeries. Dexamethasone may act by serotonin inhibition in the gut through prostaglandin antagonism. It can significantly decrease the tissue inflammation, and thus reduces the ascending impulse to the vomiting center. It also improves the action of other antiemetic drugs by sensitizing the pharmacologic receptors to the other antiemetics.[17]
Therefore, the combinations of dexamethasone and 5-HT3 antagonist have an additive effect in reducing the PONV.
The limitation of this study was we did not have combination ramosetron and dexamethasone group for comparison. Further studies on the combination of ramosetron and dexamethasone are needed. The results of our study may be applicable to all the surgeries with expected long duration of nausea and vomiting.
In this study, we noted that significantly more patients were free of PONV in the dexamethasone and ondansetron combination group than the patients receiving monotherapy with ramosetron. Thus, the combination of dexamethasone and ondansetron is superior to ramosetron for prevention of PONV after middle ear surgery. Therefore, we recommend combination of dexamethasone and ondansetron for prophylaxis for PONV in middle ear surgeries.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Honkavaara P, Saarinvaara L, Klemola UK. Prevention of nausea and vomiting with transdermal hyocine in adults after middle ear surgery during general anaesthesia. Br J Anaesth. 1994;73:763–6. doi: 10.1093/bja/73.6.763. [DOI] [PubMed] [Google Scholar]
- 2.Honkavaara P. Effect of ondansetron on nausea and vomiting after middle ear surgery during general anaesthesia. Br J Anaesth. 1996;76:316–8. doi: 10.1093/bja/76.2.316. [DOI] [PubMed] [Google Scholar]
- 3.Olaondo LL, Carrascosa F, Pueyo FJ, Monedero P, Busto N, Sáez A. Combination of ondansetron and dexamethasone in the prophylaxis of postoperative nausea and vomiting. Br J Anaesth. 1996;76:835–40. doi: 10.1093/bja/76.6.835. [DOI] [PubMed] [Google Scholar]
- 4.Rajeeva V, Bharadwaj N, Batra YK, Dhaliwal LK. Comparison of ondansetron with ondansetron and dexamethasone in preventing PONV in diagnostic laparoscopy. Can J Anaesth. 1999;46:40–4. doi: 10.1007/BF03012512. [DOI] [PubMed] [Google Scholar]
- 5.Subramanium B, Madan R, Sadhasivam S, Sennaraj B, Tamilselvan P, Rajeshwari S, et al. Dexamethasone is a cost-effective alternative to ondansetron in preventing PONV after pediatric strabismus surgery. Br J Anaesth. 2001;86:84–9. doi: 10.1093/bja/86.1.84. [DOI] [PubMed] [Google Scholar]
- 6.Panda NB, Bharadwaj N, Kapoor P, Chari P, Panda NK. Prevention of nausea and vomiting after middle ear surgery: Combination of ondansetron and dexamethasone is the right choice. J Otolaryngol. 2004;33:88–92. doi: 10.2310/7070.2004.02091. [DOI] [PubMed] [Google Scholar]
- 7.Usmani H, Quadir A, Siddiqui RA, Sharma SC. Ondansetron and dexamethasone in middle ear procedures. Indian J Otolaryngol Head Neck Surg. 2003;55:97–9. doi: 10.1007/BF02974613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JW, Park HJ, Choi J, Park SJ, Kang H, Kim EG. Comparison of ramosetron's and ondansetron's preventive anti-emetic effects in highly susceptible patients undergoing abdominal hysterectomy. Korean J Anesthesiol. 2011;61:488–92. doi: 10.4097/kjae.2011.61.6.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: Conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Gan TJ. Selective serotonin 5-HT3 receptor antagonists for postoperative nausea and vomiting: Are they all the same? CNS Drugs. 2005;19:225–38. doi: 10.2165/00023210-200519030-00004. [DOI] [PubMed] [Google Scholar]
- 11.Hahm TS, Ko JS, Choi SJ, Gwak MS. Comparison of the prophylactic anti-emetic efficacy of ramosetron and ondansetron in patients at high-risk for postoperative nausea and vomiting after total knee replacement. Anaesthesia. 2010;65:500–4. doi: 10.1111/j.1365-2044.2010.06310.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim SI, Kim SC, Baek YH, Ok SY, Kim SH. Comparison of ramosetron with ondansetron for prevention of postoperative nausea and vomiting in patients undergoing gynaecological surgery. Br J Anaesth. 2009;103:549–53. doi: 10.1093/bja/aep209. [DOI] [PubMed] [Google Scholar]
- 13.Kim WO, Koo BN, Kim YK, Kil HK. Ramosetron for the prevention of postoperative nausea and vomiting (PONV): A meta-analysis. Korean J Anesthesiol. 2011;61:405–12. doi: 10.4097/kjae.2011.61.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi YS, Shim JK, Ahn SH, Kwak YL. Efficacy comparison of ramosetron with ondansetron on preventing nausea and vomiting in high-risk patients following spine surgery with a single bolus of dexamethasone as an adjunct. Korean J Anesthesiol. 2012;62:543–7. doi: 10.4097/kjae.2012.62.6.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gan T, Meyer TA, Apfel CC, Chung F, Davis PJ, Habib AS, et al. Society for ambulatory anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007;105:1615–28. doi: 10.1213/01.ane.0000295230.55439.f4. [DOI] [PubMed] [Google Scholar]
- 16.Habib AS, Gan TJ. Combination therapy for postoperative nausea and vomiting - a more effective prophylaxis? Ambul Surg. 2001;9:59–71. doi: 10.1016/s0966-6532(01)00103-2. [DOI] [PubMed] [Google Scholar]
- 17.Sagar S. The current role of antiemetic drugs in oncology: A recent revolution in patient symptom control. Cancer Treat Rev. 1991;18:95–135. doi: 10.1016/0305-7372(91)90009-o. [DOI] [PubMed] [Google Scholar]


