Abstract
Purpose:
Changes of B-type natriuretic peptide (BNP) in sepsis and its utility in predicting intensive care unit outcomes remains a conflicting issue. To investigate the changes in plasma levels of BNP in patients with severe sepsis/septic shock and to study the association of BNP levels with the severity of the disease and prognosis of those patients.
Methods:
Thirty patients with severe sepsis or septic shock were enrolled in our study. BNP measurements and echocardiography were carried out on admission and on 4th and 7th days. Blood concentrations of BNP were measured by commercially available assays (Abbott methods). In-hospital mortality and length of stay were recorded multivariate analyses adjusted for acute physiology and chronic health evaluation score II (APACHE II score) was used for mortality prediction.
Results:
Twenty patients admitted with the diagnosis of severe sepsis and 10 patients with septic shock. The in-hospital mortality was 23.3% (7 patients). Admission BNP was significantly higher in the non-survivors 1123±236.08 versus 592.7±347.1 (P<0.001). By doing multivariate logestic regression, the predicatable variables for mortality was APACHE II score, BNP, and then EF.
Conclusion:
BNP concentrations were increased in patients with severe sepsis or septic shock and poor outcome was associated with high BNP levels; thus, it may serve as a useful laboratory marker to predict survival in these patients.
Keywords: Brain natriuretic peptide, mortality, severe sepsis, septic shock
INTRODUCTION
Worldwide, sepsis is one of the leading causes of morbidity and mortality. Patients are at high risk for irreversible organ failure and a lethal course. About 60,000 die from sepsis annually, and survivors have a reduced quality of life. It is presumed that demographic changes will lead to an increased incidence and overall mortality in the future. In addition, sepsis imposes a considerable economic burden to the society. Early and comprehensive treatment significantly improves outcome. An increased knowledge and awareness about the epidemiology, definitions, and therapy of sepsis might contribute to outcome improvement.[1]
BNP controversy
In the context of sepsis, the picture of brain natriuretic peptide (BNP) changes and its rule in prognosticating the mortality in patients with severe sepsis and septic shock is not congruent.
The concentrations of BNP were increased in patients with severe sepsis or septic shock regardless of the presence or absence of cardiac dysfunction. Neither the BNP levels for the first 3 days nor the daily changes in BNP provided prognostic value for in-hospital mortality and length of stay in this mixed group of patients, which included patients with chronic cardiac dysfunction.[2] However, in another study, the authors concluded that plasma BNP level is a valuable prognostic factor for severe sepsis and septic shock patients.[3]
Myocardial depression is well recognized as an early feature of human septic shock, causing absence of appropriate oxygen supply to the peripheral tissues and subsequent death. Early systolic dysfunction has been identified in these patients and it seems to be inversely related to mortality.[4,5]
BNP is a relatively inexpensive and simple test, which is now widely available in clinical practice. BNP is also a predictor of both echocardiographic parameters of ventricular dysfunction as well as clinical outcomes in patients with acute and chronic heart disease. BNP has also been hypothesized to be a marker for ventricular dysfunction in patients infected with human immunodeficiency virus.[6,7]
Hypothesis
We observed the increment on BNP levels in patients with severe sepsis and septic shock and hypothesized that BNP could have a beneficial rule in the emergency department and early in intensive care unit (ICU) for high risk stratification of critically ill patients with severe sepsis and septic shock.
Objectives
The objectives of this study were to investigate the plasma levels of BNP in patients with septic shock/severe sepsis and to study the association of BNP levels with hemodynamic and echocardiographic parameters, severity of the disease, and prognosis of those patients.
METHODS
Following approval of the Tawam/Johns Hopkins Hospital Ethics Committee, we prospectively included 30 patients in the study with a mean age of 49.8±16.7 years.
Inclusion criteria
Patients with severe sepsis or septic shock with age range of 19-72 years.
Exclusion criteria
Patients with pre-existing heart failure or chronic renal failure.
Patient selection guidelines
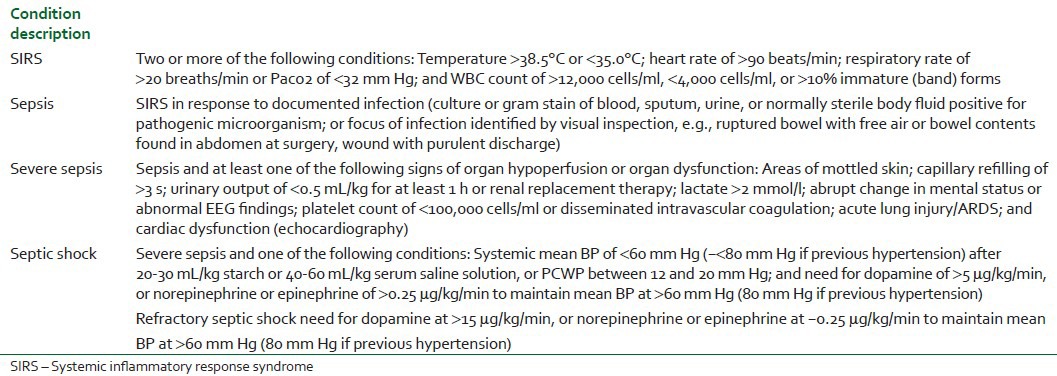
Sepsis has been defined as the presence of the systemic inflammatory response syndrome (SIRS) in response to a culture-proven infection.[8] However, SIRS can result not only from infection but also from a variety of conditions such as autoimmune disorders, vasculitis, thromboembolism, burns, or after a surgery. The severity of sepsis is graded according to the associated organ dysfunction and hemodynamic compromise. The original definitions have been revisited by a group of experts,[9] but, apart from expanding the list of signs and symptoms of sepsis, no relevant changes have been made. In a recently published review, Annane et al.[10] proposed a very practical modification of the definitions including exact hemodynamic definitions of septic shock. It is important to recognize that the original definitions relied only on the degree of vasodilatation, whereas in the modification by both the International Sepsis Definition Conference[9] and Annane et al.,[10] myocardial depression defined as low cardiac index or echocardiographic evidence of cardiac dysfunction has been included in the definition of severe sepsis [Table 1].
Table 1.
All patients were subjected to have the following:
BNP measurements
Plasma BNP concentrations were measured as previously described using the Triage BNP meter (Abbott Diagnostics, Germany).[11] The first BNP sample was taken on admission to the ICU (day 1). BNP levels were determined for each patient on admission and on 4th and 7th days.
For BNP determination (index tests), blood for measurement of natriuretic peptide concentrations was collected by venepuncture in Vacuette polyethylene terephthalate glycol EDTA tubes (Greiner Bio-One, Kremsmu˝nster, Austria) at the initial patient examination. Blood samples were centrifuged at 3500 g for 10 min at 4°C immediately after collection. BNP was analyzed within 4 h after blood withdrawal. BNP was assayed on an AxSYM analyser (Abbott Laboratories, Abbott Park, Illinois, USA). The AxSYM BNP assay is a fully automated microparticle enzyme immunoassay with two monoclonal mouse antibodies in a two-step sandwich format.[12]
The precision of the two methods was evaluated according to the National Committee for Clinical Laboratory Standards (NCCLS) guideline EP5-A.[13,14] Three pooled patient plasma samples were aliquoted into 40 tubes of 1.5 ml for each concentration and frozen at 270°C. We analyzed these samples in duplicate in two runs every day for 20 days on the two analyzers. Total imprecision was calculated by the NCCLS double run precision evaluation test.[13] Precision data of the two methods were as follows: The AxSYM BNP assay had a total coefficient of variance (CV) of 8.1% at a mean concentration of 108 ng/l (Pool 1), a total CV o1f 7.5% at a mean concentration of 524 ng/l (Pool 2), and a total CV of 10% at a mean concentration of 2117 ng/l (Pool 3).
Transthoracic echocardiography
The study was performed utilizing a General Electric Vivid i Sonos with a 2.5-MHz transducer. Two-dimensional and pulsed Doppler echocardiograms were obtained at rest with the patient placed in the left lateral position, to evaluate left ventricualr size and left ventricular systolic function. Echo parameters measured included the following dimensions: (i) Left ventricular end diastolic diameter (LVEDD), (ii) left ventricular end systolic diameter (LVESD), and (iii) ejection fraction (EF%). Measures were repeated on the 4th and 7th days of admission.
Other data collection
Baseline clinical variables including age, gender, cause of sepsis, and the admission APACHE II score were collected.[14] Other data collected included the requirements for mechanical ventilation (ventilation hours) and vasopressors, the length of stay in ICU (LOSICU) and in hospital (LOSHOS), and the patient's outcome (alive or dead).
Statistical analysis
The description of the data done in form of mean (±) SD for quantitative data and frequency and proportion for qualitative data. The analysis of the data was done to test statistical significant difference between groups. For quantitative date, student t test was used to compare between two groups. For qualitative data, Chi-square test was used and odds ratio was detected. Multivariate regression analysis was done for significant date in univariate analysis.[15] Primary outcome for the study was defined as ICU mortality. Clinical and echocardiographic data were entered into a database (Microsoft Excel 97, Redmond, WA, USA) and statistical analyses were performed (SPSS Inc. version 10.0.7 Chicago IL, USA).
P is significant if ≤0.05 at confidence interval of 95%.
RESULTS
Patients’ base line characteristics are presented in Table 2. All 30 patients stayed in the ICU for >48 h and a total of 18 male and 12 female were included in the study. Underlying cause of sepsis was pneumonia in 10 patients (33%), blood stream infection in 7 patients (23.3%), intrabdominal sepsis in 7 patients (23.7%), and UTI in 3 patients (10%); only 1 patient had central nervous system (CNS) infection. Twenty patients were admitted with the diagnosis of severe sepsis and 10 patients with that of septic shock. The mean length of hospital stay was 15.3±11.6 days, while the mean length of ICU stay was 8.2±5.1 days. Nineteen patients received mechanical ventilation; the mean length of mechanical ventilation was 151.23±91.2 h. Ten patients required norepinephrine and 2 also received vasopressin at admission. Seven patients required norepinephrine, with 5 also receiving vasopressin, at some stage in their ICU stays. The in-hospital mortality was 23.3% (7 patients).
Table 2.
Baseline patient characteristics
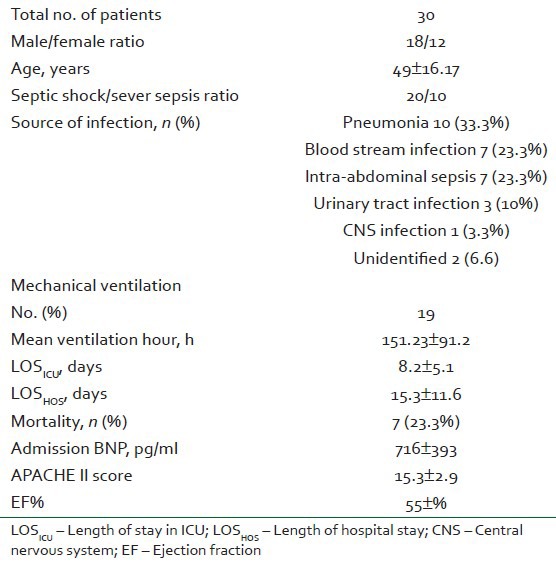
Table 3 showing that there was no statistical differences between septic shock and severe sepsis groups regarding the baseline LVEDD, LVESD, EF, LOSHOS, LOSICU, and LOV. The in-hospital mortality was significantly higher in septic shock group (P<0.05).
Table 3.
Comparison between the septic shock and severe sepsis groups
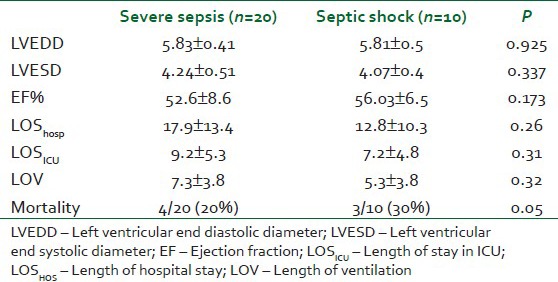
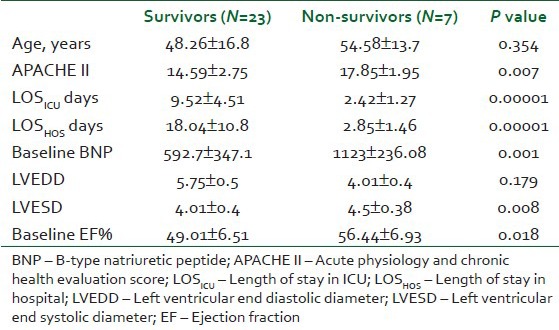
Table 4 showing the mean age was matched between the survivors and non-survivors groups. APACHE II score was significantly higher in the non-survivors (P=0.007). The mean length of ICU stay and length of hospitalization were significantly higher in the survivors (P=0.0001 and 0.0001, respectively).
Table 4.
Comparison between the survivors and the non-survivors groups
BNP changes
To investigate the value of BNP as a marker of systolic myocardial dysfunction, we performed serial measurements of BNP plasma levels in these septic patients. Admission BNP concentrations were elevated in the studied group 716±393 pg/ml [Table 1]. BNP was significantly higher in the non-survivors (P<0.0001) [Figure 1]. BNP remain significantly higher on the 4th and 7th days (P=0.01 and 0.001, respectively, in the non-survivors). BNP tend to decrease throughout the course in survivors (592 in day 1, 318 in day 4, and 93 pg/ml in day 7).
Figure 1.
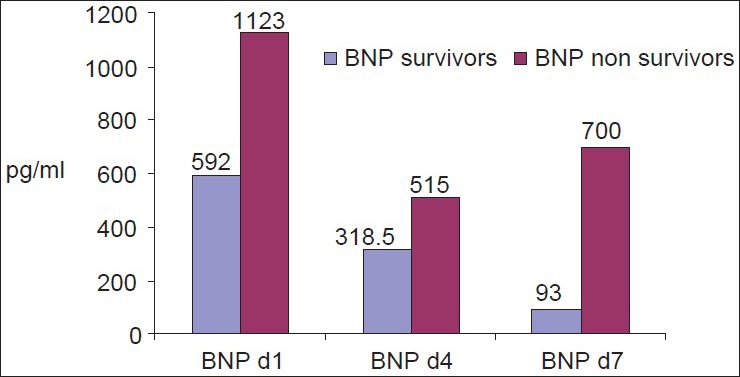
Admission and BNP changes among the studied group through the course in survivors and non-survivors
Echocardiographic changes
Left ventricular function
The LV systolic function was significantly higher in the non-survivors (P=0.018). Also, the LVEDD did not show similar changes and there was a significant difference in the LOSICU and LOSHOS between the survivors and non-survivors (P=0.0001 and 0.001, respectively). Daily changes in the EF were noted in Figure 2. EF tended to improve and remains significantly higher in the survivors.
Figure 2.
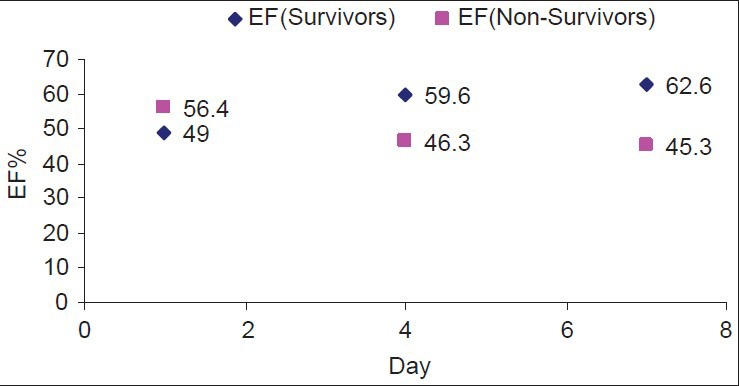
EF changes through the ICU course in survivors and nonsurvivors
The LV systolic function was significantly higher in the non-survivors (P=0.018). Also, the LVEDD did not show similar changes and there was a significant difference in the LOSICU and LOSHOS between the survivors and non-survivors (P=0.0001 and 0.001, respectively). Daily changes in the EF were noted in Figure 1. EF tended to improve and remains significantly higher in the survivors.
Mortality prediction
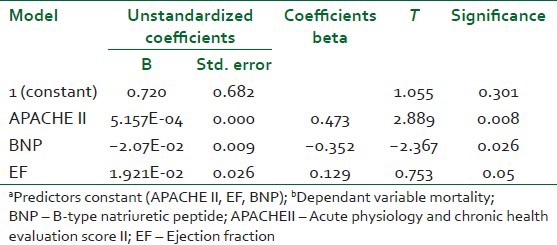
By doing univariate analysis and exluding the non-prognostic values, multivariate logestic regression, the predicatable variables is APACHE II score, BNP, and then EF [Table 5].
Table 5.
Comparison of logistic regression models
DISCUSSION
The main objective of this study was to determine if BNP data would add prognostication to existing clinical variables in ICU patients with severe sepsis and septic shock and if it could be a helpful tool in the emergency department.
The findings of this study can be summarized as follows: (i) Admission BNP differ between the survivors and the non-surviviors, (ii) Admission APACHE II score, EF, and BNP showed significant changes between survivors and non-survivors, and (iii) BNP could be used as an independent mortality predictor in severe sepsis and septic shock.
BNP changes
The relationship between BNP and sepsis is complex. The cardiac ventricles are the main source of circulating BNP in humans. The stimulus for BNP release is ventricular wall stretch as a result of either volume expansion or pressure overload.[15] BNP levels are elevated in patients with symptomatic left ventricular dysfunction and correlate with filling pressures.[16]
Admission BNP concentrations were elevated early in the studied group 716±393 pg/ml on admission, BNP were significantly higher in the non-survivors (P<0.0001). BNP remain significantly higher in non-survivors on the 4th and 7th days (P=0.01 and 0.001, respectively, in the non-survivors) [Figure 1]. The high elevation of BNP in our studied group could alert the physician to the severity of the disease. Our results were concomitant with Mclean et al., the authors found that BNP concentrations were increased in patients with severe sepsis or septic shock regardless of the presence or absence of cardiac dysfunction.[17]
However, Pirracchio et al.[18] did a study of over 32 patients and they concluded that high levels of BNP might be related to an alteration in BNP clearance. During sepsis, high BNP levels were not predictive of fluid non-responsiveness. Nevertheless, in fluid non-responders, acute ventricular stretching can result in further BNP release. In another study by Issa et al.,[19] they report on an inverse association between positive end-expiratory pressure and BNP levels in patients with severe sepsis and septic shock. BNP and creatinine levels should be taken into consideration when analyzing BNP levels in this setting. We excluded patients with chronic renal failure in our studied group.
Rivers et al.[20] did a study over of 252 patients and confirmed elevation in BNP levels, which are significantly associated with organ and myocardial dysfunction, global tissue hypoxia, and mortality. Serial BNP levels may be a useful adjunct in the early detection, stratification, treatment, and prognostication of high-risk patients with severe sepsis and septic shock. We observed early high elevation of BNP level in our group, which was related to the severity of illness.
Mortality prediction
Systolic myocardial dysfunction is present in 44% of patient with severe sepsis or septic shock. BNP seems useful to detect myocardial dysfunction, and high plasma levels appear to be associated with poor outcome of sepsis.[21] Also, Post et al.[22] found that plasma BNP concentration represents a reliable marker for identification of patients developing sepsis-induced myocardial depression. In addition, BNP concentration on day 5 may be used as a prognostic marker to identify patients with an elevated risk for an adverse outcome.
Despite initial recovery from critical illness requiring ICU admission, many patients remain at risk of subsequent deterioration and death. This may result in readmission to ICU or death on another ward or during the ICU readmission. Early identification of patients at the highest risk would allow resources to be targeted appropriately and prevent avoidable morbidity and mortality. ICU readmission rates have been advocated as a marker of ICU quality on the basis that early readmissions (within 48 h) may indicate premature discharge or discharge to an inappropriate clinical area.[23,24]
In a study by Parker et al.,[25] patients were grouped according to their mortality and patients showing left ventricular dilation and depression of LVEF had a good prognosis. Paradoxically, many studies using echocardiography showed that an impaired LVEF is associated with a poor prognosis.[21,26] This might be explained by the fact that, in patients with septic shock, the measurement of LVEF alone does not sufficiently characterize the underlying hemodynamic pattern and that the outcome depends on parameters other than LVEF.
While in a study done by Ritter et al.,[27] the authors concluded that cardiac index and cardiac function index (CFI) both provide prognostic information in patients with severe sepsis or septic shock. In another study by Sawchwk et al.,[28] the authors mention that TTE does not improve prediction of outcome over APACHE II in medical-surgical intensive care. We tried to have our own conclusions in the preceding dilemma.
BNP provided prognostic value for in-hospital mortality and length of stay in this mixed group of patients, which included patients with chronic cardiac dysfunction.[29] We excluded patients with heart failure patients to limit the changes in BNP induced by the disease.
In a study by Kandil et al.,[30] the authors confirmed the relationship between BNP level elevation and severity of sepsis independent of congestive heart failure. It also supports the utility of BNP level as a marker for mortality in septic shock. Also, in a study by Rivers et al.,[20] they found that patients with severe sepsis and septic shock often have elevated BNP levels, which are significantly associated with organ and myocardial dysfunction, global tissue hypoxia, and mortality. Serial BNP levels may be a useful adjunct in the early detection, stratification, treatment, and prognostication of high-risk patients.
By doing univariate analysis, exluding the non-prognostic values, multivariate logestic regression, predicatable variables of APACHE II score, BNP, and then EF, our data confirmed the prognostic value of BNP; it may serve as a diagnostic and prognostic tool at the emrgency department for high risk sratifiaction of critically ill patients with severe sepis and septic shock, BNP is relatively simple and unexpensive test in comparison with the APACHE II score. Also, BNP is diagnostic for myocardial dysfunction, which may necessiate support and exlude echocardiography [Table 5].
Cardiac function
The phenomena of sepsis-related cardiomyopathy had been described in many trials.[25,31] Parker et al.,[26] were the first to describe left ventricular hypokinesis in septic shock, in which patients with severely impaired LVEF and adequate LV stroke output could be maintained through acute LV dilatation.[32]
Our data showed that the LV systolic function was significantly higher in the non-survivors (P=0.018). Also, the LVEDD did not show similar changes and there was a significant difference in the LOSICU and LOSHOS between the survivors and non-survivors (P=0.0001 and 0.001, respectively). Daily changes in the EF were noted in Figure 2 and EF tends to improve and remains significantly higher in the survivor as left ventricular performance may return to the previous baseline following recovery from septic shock. Improvement in contractility may result from resolution of sepsis-induced cardiomyopathy.
Our data was supported by the study of Charpentier et al.,[21] who evaluated the cardiac performance in patients with sepsis by echocardiography found an LVEF (using TTE) or a left ventricular fractional area contraction (LVFAC) using TEE of <50% in approximately 50% of patients with severe sepsis and septic shock. However, the typical pattern of left ventricular dilation in combination with an impaired LVEF was found in only one study by Ver Elst et al.,[29] whereas in the study of Charpentier et al.,[21] ventricular dimensions were normal despite low LVEF. In a study by McLeans et al.,[2] 7 patients (18% of the cohort) displayed reversible cardiac dysfunction (RCD), which was characterized by an initially reduced LVEF (<55%) with subsequent normalization of LVEF (i.e., LVEF >55%).
Limitations
It is known that BNP levels are determined by the interplay of a number of confounding factors. For example, fluid loading can stimulate BNP release by ventricular wall stretch, which could not be adjusted. Second, the relatively small sample size may reduce the power of some analyses (comparisons). Nonetheless, our results are relevant because of the similar BNP levels found between the severe sepsis and septic shock groups (P=0.8). Third, the interpretations of cardiac function might be affected by the use of β1-agonists such as norepinephrine.[33] The use of inotropes in these patients might improve the cardiac function and lead to an overestimation of cardiac variables such as LVEF. Finally, BNP can be increased in decompensated heart failure, pulmonary hypertension, pulmonary embolus, coronary artery disease, acute respiratory distress syndrome, chronic obstructive pulmonary disease, renal failure, liver cirrhosis, subarachnoid hemorrhage, hyperthyroidism, and a host of other diseases.[34]
CONCLUSION
This study demonstrated that BNP levels were increased in patients with severe sepsis or septic shock and the increment in BNP level was related to the severity of illness. In septic patients, BNP levels could not be used as an independent predictor of mortality.
Footnotes
Source of Support: This work was supported by Tawam Hospital “In affiliation with John Hopkins University”, AlAin, UAE. Special thanks to all members of Critical Care Medicine and Clinical Pathology Departments for great help throughout the work
Conflict of Interest: None declared
REFERENCES
- 1.Moerer O, Quintel M. (Sepsis in adult patients-definitions, epidemiology and economic aspects) (790-4, 796-8).Internist (Berl) 2009;50:788. doi: 10.1007/s00108-008-2285-7. [DOI] [PubMed] [Google Scholar]
- 2.McLean AS, Huang SJ, Hyams S, Poh G, Nalos M, Pandit R, et al. Prognostic values of B-type natriuretic peptide in severe sepsis and septic shock. Crit Care Med. 2007;35:1019–26. doi: 10.1097/01.CCM.0000259469.24364.31. [DOI] [PubMed] [Google Scholar]
- 3.Zhao HY, An YZ, Liu F. (Prognostic values of B-type natriuretic peptide in severe sepsis and septic shock) 2009;21:293–5. [PubMed] [Google Scholar]
- 4.Yoshimura M, Yasue H, Okumura K, Ogawa H, Jougasaki M, Mukoyama M, et al. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation. 1993;87:464–9. doi: 10.1161/01.cir.87.2.464. [DOI] [PubMed] [Google Scholar]
- 5.Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. doi: 10.1161/01.cir.90.1.195. [DOI] [PubMed] [Google Scholar]
- 6.Carrillo-Jimenez R, Lamas GA, Hennekens CH. Plasma level of brain natriuretic peptide: A potential marker for HIV-related cardiomyopathy. J Cardiovascular Pharmacol Ther. 2002;7:135–7. doi: 10.1177/107424840200700302. [DOI] [PubMed] [Google Scholar]
- 7.Carrillo-Jimenez R, Treadwell TL, Goldfine H, Buenano A, Lamas GA, Hennekens CH. Brain natriuretic peptide and HIV-related cardiomyopathy. AIDS Read. 2002;12:501–3. 508. [PubMed] [Google Scholar]
- 8.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 9.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 10.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 11.Mueller T, Gegenhuber A, Poelz W, Haltmayer M. Comparison of the Biomedica NTproBNP enzyme immunoassay and the Roche NT-proBNP chemiluminescence immunoassay: Implications for the prediction of symptomatic and asymptomatic structural heart disease. Clin Chem. 2003;49(6 Pt 1):976–9. doi: 10.1373/49.6.976. [DOI] [PubMed] [Google Scholar]
- 12.Mueller T, Gegenhuber A, Poelz W, Haltmayer M. Preliminary evaluation of the AxSYM B-type natriuretic peptide (BNP) assay and comparison with the ADVIA Centaur BNP assay. Clin Chem. 2004;50:1104–6. doi: 10.1373/clinchem.2004.031484. [DOI] [PubMed] [Google Scholar]
- 13.NCCLS document EP5-A. Wayne: NCCLS; 1999. National Committee for Clinical Laboratory Standards. Evaluation of precision performance of clinical chemistry devices: Approved guideline. [Google Scholar]
- 14.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. Apache II: A severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 15.Munro BH. fourth edition. University of Pennsilvania: Boston collage Lipincott U.S.A; 2001. Statistical methods for health care research; pp. 1–412. [Google Scholar]
- 16.Suttner SW, Boldt J. Natriuretic peptide system. Physiology and clinical utility. Curr Opin Crit Care. 2004;10:336–41. doi: 10.1097/01.ccx.0000135513.26376.4f. [DOI] [PubMed] [Google Scholar]
- 17.Maisel AS, McCord J, Nowak RM, Hollander JE, Wu AH, Duc P, et al. Bedside B-type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the Breathing Not Properly Multinational study. J Am Coll Cardiol. 2003;41:2010–7. doi: 10.1016/s0735-1097(03)00405-4. [DOI] [PubMed] [Google Scholar]
- 18.Pirracchio R, Deye N, Lukaszewicz AC, Mebazaa A, Cholley B, Matéo J, et al. Impaired plasma B-type natriuretic peptide clearance in human septic shock. Crit Care Med. 2008;36:2542–6. doi: 10.1097/CCM.0b013e318183f067. [DOI] [PubMed] [Google Scholar]
- 19.Issa VS, Taniguchi LU, Park M, Cruz LM, Bocchi EA, Velasco IT, et al. Positive end-expiratory pressure and renal function influence B-type natriuretic peptide in patients with severe sepsis and septic shock. Arq Bras Cardiol. 2008;91:107–12. doi: 10.1590/s0066-782x2008001400008. [DOI] [PubMed] [Google Scholar]
- 20.Rivers EP, McCord J, Otero R, Jacobsen G, Loomba M. Clinical utility of B-type natriuretic peptide in early severe sepsis and septic shock. J Intensive Care Med. 2007;22:363–73. doi: 10.1177/0885066607307523. [DOI] [PubMed] [Google Scholar]
- 21.Charpentier J, Luyt CE, Fulla Y, Vinsonneau C, Cariou A, Grabar S, et al. Brain natriuretic peptide: A marker of myocardial dysfunction and prognosis during severe sepsis. Crit Care Med. 2004;32:660–5. doi: 10.1097/01.ccm.0000114827.93410.d8. [DOI] [PubMed] [Google Scholar]
- 22.Post F, Weilemann LS, Messow CM, Sinning C, Münzel T. B-type natriuretic peptide as a marker for sepsis-induced myocardial depression in intensive care patients. Crit Care Med. 2008;36:3108–9. doi: 10.1097/CCM.0b013e31818b9153. [DOI] [PubMed] [Google Scholar]
- 23.Anaheim CA Society of Critical Care Medicine Quality Indicators Committee. Candidate Critical Care Quality Indicators. Society of Critical Care Medicine. 1995 [Google Scholar]
- 24.Metnitz PG, Fieux F, Jordan B, Lang T, Moreno R, Le Gall JR, et al. Critically ill patients readmitted to intensive care units-lessons to learn? Intensive Care Med. 2003;29:241–8. doi: 10.1007/s00134-002-1584-z. [DOI] [PubMed] [Google Scholar]
- 25.Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100:483–90. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- 26.Parker MM, McCarthy KE, Ognibene FP, Parrillo JE. Right ventricular dysfunction and dilatation, similar to left ventricular changes, characterize the cardiac depression of septic shock in humans. Chest. 1990;97:126–31. doi: 10.1378/chest.97.1.126. [DOI] [PubMed] [Google Scholar]
- 27.Ritter S, Rudiger A, Maggiorini M. Crit Care. Vol. 12. Belgium: 28th International Symposium on Intensive Care and Emergency Medicine Brussels; 2008. Low cardiac function index predicts ICU mortality in patients with severe sepsis or septic shock; p. 410. 18-21 March 2008. Doi: 101186/cc6631. [Google Scholar]
- 28.Sawchuk CW, Wong DT, Kavanagh BP, Siu SC. Transthoracic echocardiography does not improve prediction of outcome over APACHE II in medical-surgical intensive care. Can J Anesth. 2003;50:305–10. doi: 10.1007/BF03017803. [DOI] [PubMed] [Google Scholar]
- 29.ver Elst KM, Spapen HD, Nguyen DN, Garbar C, Huyghens LP, Gorus FK. Cardiac troponin I and T are biological markers of left ventricular dysfunction in septic shock. Clin Chem. 2000;46:650–7. [PubMed] [Google Scholar]
- 30.Kandil E, Burack J, Sawas A, Bibawy H, Schwartzman A, Zenilman ME, et al. B-type natriuretic peptide: A biomarker for the diagnosis and risk stratification of patients with septic shock. Arch Surg. 2008;143:242–6. doi: 10.1001/archsurg.2007.69. [DOI] [PubMed] [Google Scholar]
- 31.Fonarow GC, Peacock WF, Horwich TB, Phillips CO, Givertz MM, Lopatin M, et al. Usefulness of B-type natriuretic peptide and cardiac troponin levels to predict in-hospital mortality from ADHERE. Am J Cardiol. 2008;101:231–7. doi: 10.1016/j.amjcard.2007.07.066. [DOI] [PubMed] [Google Scholar]
- 32.Omland T. Advances in congestive heart failure management in the intensive care unit: B-type natriuretic peptides in evaluation of acute heart failure. Crit Care Med. 2008;36:S17–27. doi: 10.1097/01.CCM.0000296266.74913.85. [DOI] [PubMed] [Google Scholar]
- 33.LeDoux D, Astiz ME, Carpati CM, Rackow EC. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med. 2000;28:2729–32. doi: 10.1097/00003246-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Phua J, Lim TK, Lee KH. B-type natriuretic peptide: Issues for the intensivist and pulmonologist. Crit Care Med. 2005;33:2094–13. doi: 10.1097/01.ccm.0000178351.03327.9f. [DOI] [PubMed] [Google Scholar]


