Abstract
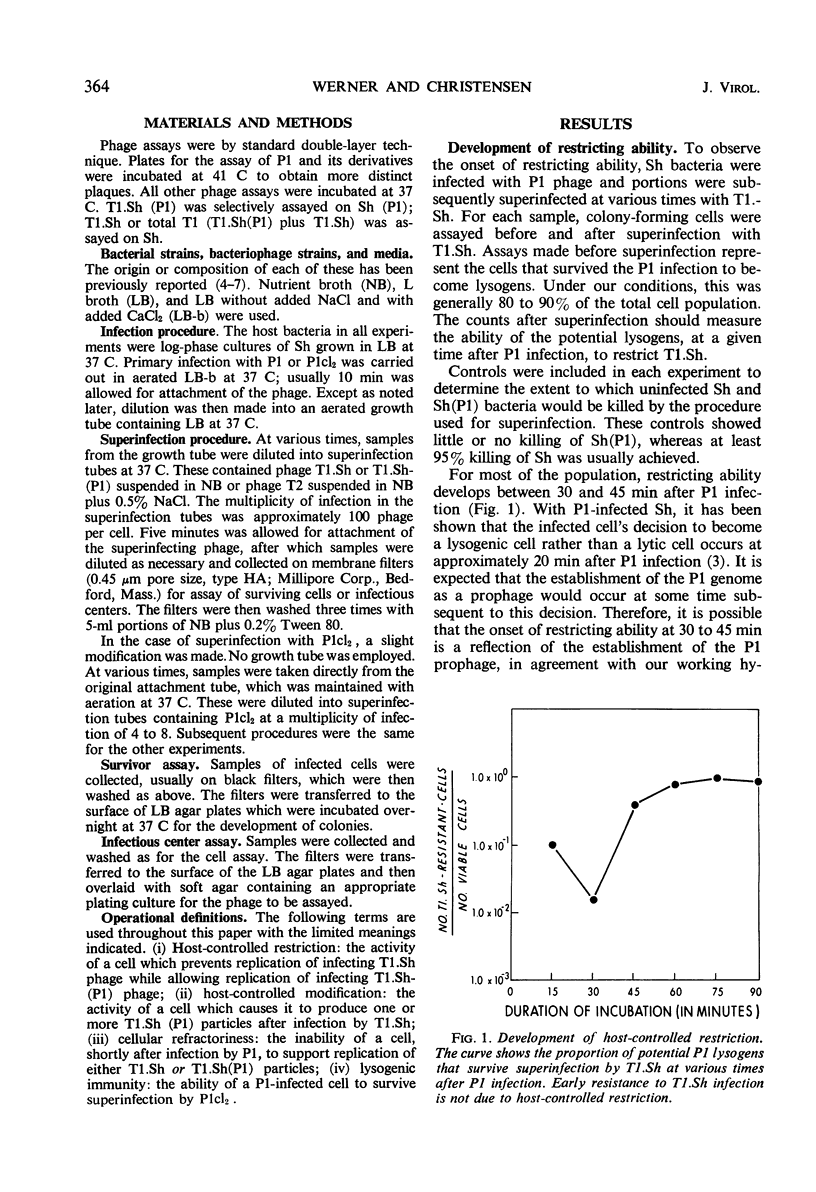
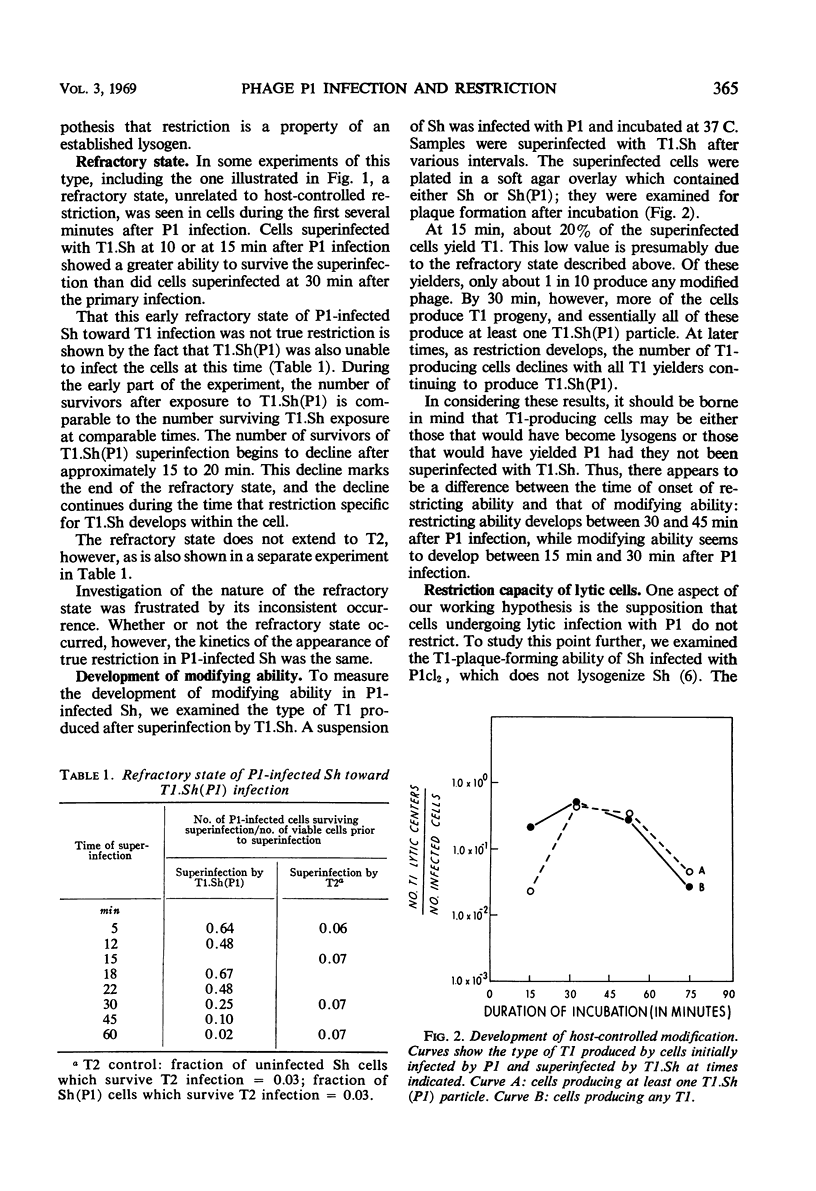
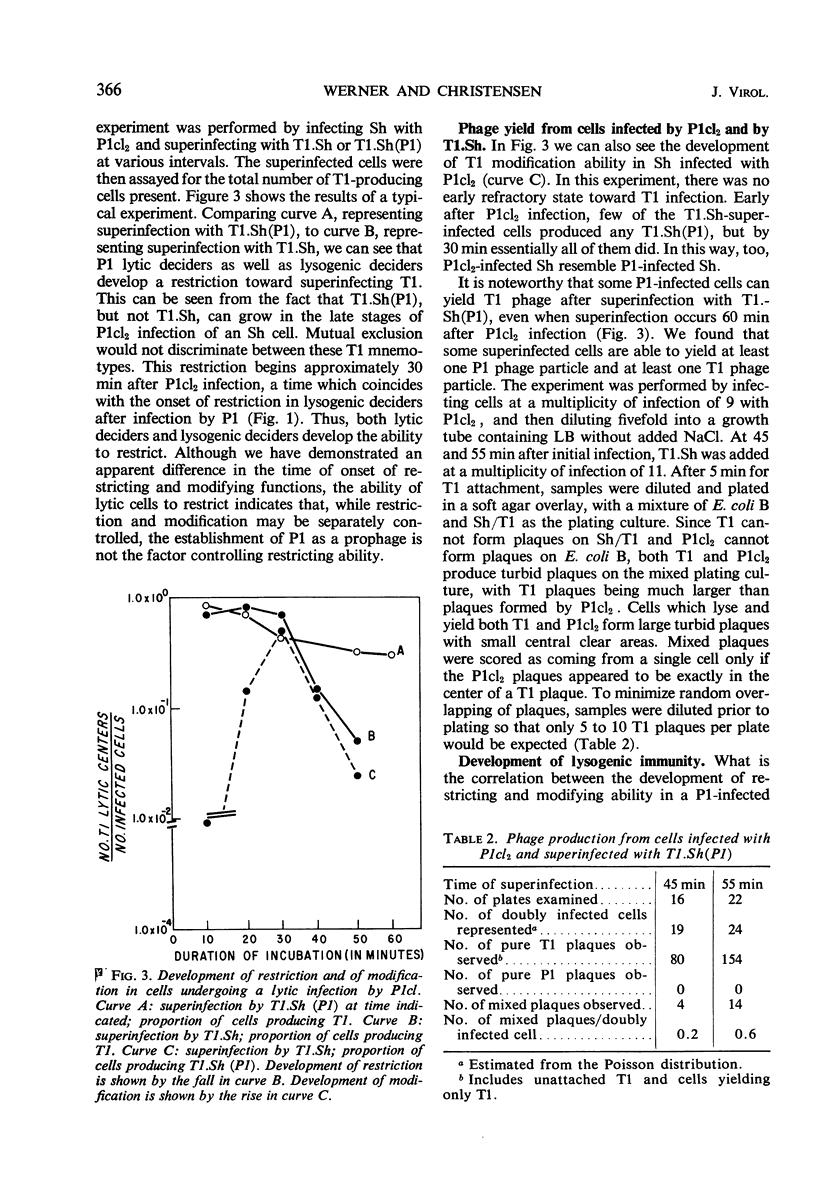
Shigella dysenteriae cells were infected with phage P1 or P1cl. The outcome of superinfection of these cells with phage T1.Sh or T1.Sh(P1) or P1cl was studied as a function of time after the initial infection. Cells undergoing either a lytic response or a lysogenic response to the primary infection develop the ability to specifically restrict T1.Sh between 30 and 45 min. Between 15 and 30 min, the cells seem to develop the ability to produce T1.Sh(P1) after infection by T1.Sh. However, reasons are given for believing that this apparent time difference is consistent with a simultaneous development of the two capacities (restriction and modification) within the cell. This development occurs between 30 and 45 min. Cells infected with P1cl and superinfected 45 or more min later with T1.Sh(P1) can yield both P1cl and T1. Cells infected with P1 become resistant to infection by P1cl within 5 to 10 min. It is argued that this early immunity is not necessarily different in mechanism from true lysogenic immunity.
Full text
PDF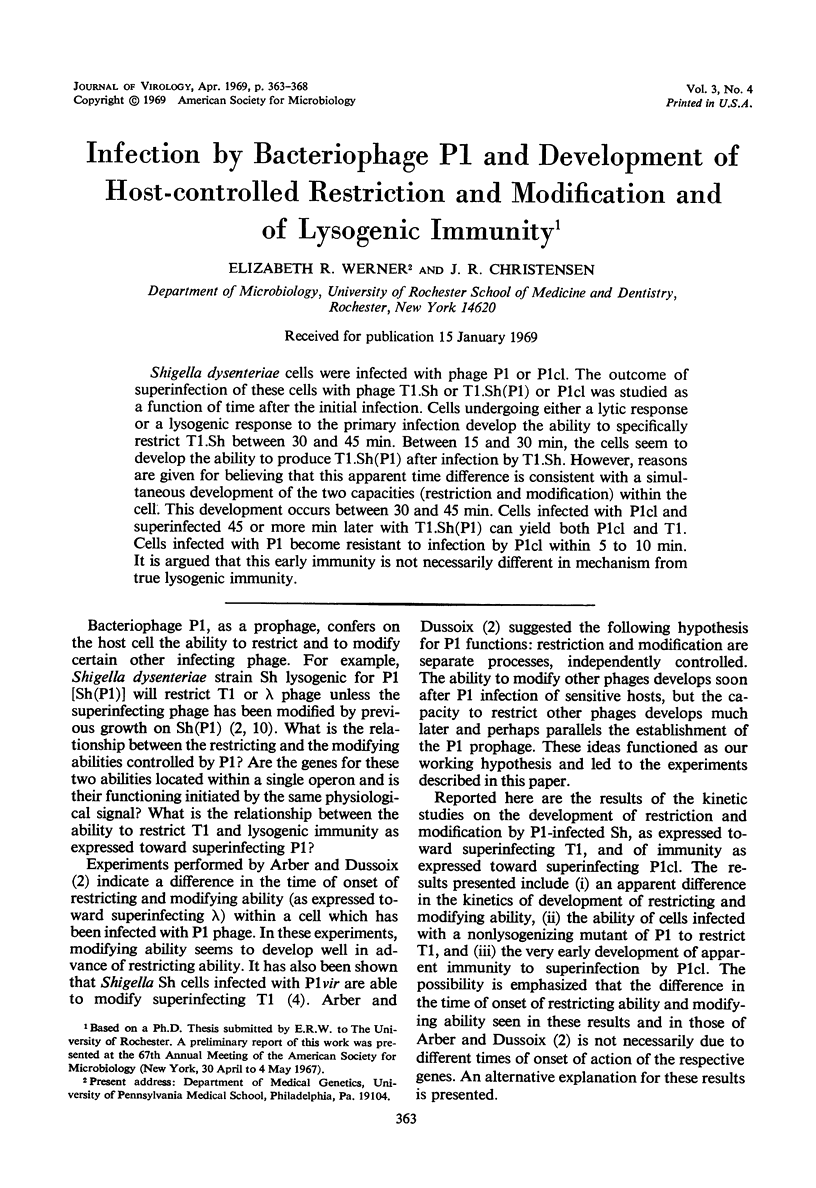

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W., DUSSOIX D. Host specificity of DNA produced by Escherichia coli. I. Host controlled modification of bacteriophage lambda. J Mol Biol. 1962 Jul;5:18–36. doi: 10.1016/s0022-2836(62)80058-8. [DOI] [PubMed] [Google Scholar]
- ARBER W. HOST SPECIFICITY OF DNA PRODUCED BY ESCHERICHIA COLI V . THE ROLE OF METHIONINE IN THE PRODUCTION OF HOST SPECIFICITY. J Mol Biol. 1965 Feb;11:247–256. doi: 10.1016/s0022-2836(65)80055-9. [DOI] [PubMed] [Google Scholar]
- BERTANI G., NICE S. J. Studies on lysogenesis. II. The effect of temperature on the lysogenization of Shigella dysenteriae with phage P1. J Bacteriol. 1954 Feb;67(2):202–209. doi: 10.1128/jb.67.2.202-209.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTENSEN J. R. On the process of host-controlled modification of bacteriophage. Virology. 1961 Jan;13:40–43. doi: 10.1016/0042-6822(61)90029-0. [DOI] [PubMed] [Google Scholar]
- CHRISTENSEN J. R. The fate of genes from restricted T1 in lysogenic Shigella dysenteriae, Sh (P1). Virology. 1962 Feb;16:133–139. doi: 10.1016/0042-6822(62)90288-x. [DOI] [PubMed] [Google Scholar]
- Christensen J. R. Multiplication of bacteriophage P1 mutants in Shigella dysenteriae strain Sh(P1). J Virol. 1968 Dec;2(12):1408–1417. doi: 10.1128/jvi.2.12.1408-1417.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREXLER H., CHRISTENSEN J. R. Genetic crosses between restricted and unrestricted phage T1 in lysogenic and non-lysogenic hosts. Virology. 1961 Jan;13:31–39. doi: 10.1016/0042-6822(61)90028-9. [DOI] [PubMed] [Google Scholar]
- FUKASAWA T. THE COURSE OF INFECTION WITH ABNORMAL BACTERIOPHAGE T4 CONTAINING NON-GLUCOSYLATED DNA ON ESCHERICHIA COLI STRAINS. J Mol Biol. 1964 Aug;9:525–536. doi: 10.1016/s0022-2836(64)80224-2. [DOI] [PubMed] [Google Scholar]
- Huppert J., Blum-Emerique L., Breugnon M. M. Mixed infection of Escherichia coli by RNA and DNA bacteriphages. I. Inhibition of RNA phage multiplication by superinfection with DNA phages. Virology. 1967 Oct;33(2):307–316. doi: 10.1016/0042-6822(67)90149-3. [DOI] [PubMed] [Google Scholar]
- LEDERBERG S. Suppression of the multiplication of heterologous bacteriophages in lysogenic bacteria. Virology. 1957 Jun;3(3):496–513. doi: 10.1016/0042-6822(57)90006-5. [DOI] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Molholt B., Fraser D. Reversal of restriction for host modified T2 and T4 DNA upon conversion of non-permissive Escherichia coli to spheroplasts. Biochem Biophys Res Commun. 1965 May 18;19(5):571–575. doi: 10.1016/0006-291x(65)90376-1. [DOI] [PubMed] [Google Scholar]
- ZINDER N. D. Lysogenization and superinfection immunity in Salmonella. Virology. 1958 Apr;5(2):291–326. doi: 10.1016/0042-6822(58)90025-4. [DOI] [PubMed] [Google Scholar]



