Abstract
Background:
Various adjuncts have been used with lignocaine to decrease tourniquet pain and prolong post-operative analgesia during intravenous regional anesthesia (IVRA). Calcium-channel blockers potentiate the analgesic effect of local anesthetics. This study was designed to evaluate the efficacy of diltiazem as an adjunct to lignocaine in IVRA with respect to tourniquet tolerance, perioperative analgesia, and quality of anesthesia.
Methods:
In this prospective, randomized, and double-blind study, 40 patients (American Society for Anesthesiologists grade I/II) undergoing elective hand surgery under IVRA were assigned into two groups of 20 each and administered IVRA either with lignocaine 3 mg/kg (group Lignocaine (L)) or lignocaine 3 mg/kg plus diltiazem 0.2 mg/kg (group Lignocaine-Diltiazem (LD)) with normal saline (total volume-40 ml). Hemodynamic parameters, onset of the complete sensory blockade, motor blockade, and intraoperative (tourniquet pain) and post-operative Visual Analogue Scale scores, total intraoperative and consumption of post-operative fentanyl intraoperative were recorded.
Results:
Sensory block was established in 2.5±0.688 min in group LD verses 5.60±0.851 min in group L. Motor blockade was established in 8.65±0.933 min in group LD and 13.46±0.604 min in group L. The mean VAS scores >3 were attained early at 30 min (3.1±0.912) in group L. Patients in group L requested early rescue analgesic at 30±8.633 min compared with 49.64±7.958 min in group LD.
Conclusions:
Diltiazem as an adjunct to lignocaine provided enhanced intraoperative and post-operative analgesia without any significant side effects.
Keywords: Diltiazem, intravenous regional anesthesia, lignocaine
INTRODUCTION
Intravenous regional anesthesia (IVRA) is a simple, safe, and effective technique of providing anesthesia for short surgical procedures on the hand and forearm for an anticipated duration of 60-90 min.[1] It is an ideal technique for short operative procedures on extremities, performed on a day-care basis. Tourniquet pain and poor post-operative analgesia are common problems associated with IVRA.[2] Various adjuncts, e.g. opioid, nonsteroidal antiinflammatorry drugs, clonidine, dexmedetomidine, dexamethasone, muscle relaxants, neostigmine, ketamine, and magnesium, have been tried to hasten the onset, maintain adequate muscle relaxation, reduce tourniquet pain, and increase the duration of analgesia.
Calcium ions play a vital role in analgesia mediated by local anesthetics. Local anesthetics reduce the permeability of calcium and clinical investigations have shown that calcium-channel blockers potentiate the analgesic effect of local anesthetics.[3] Calcium-channel blocker acts primarily by vasodilatation and reduction of peripheral vascular resistance. Calcium-channel blockers along with local anesthetics not have been used in IVRA. This study was designed to evaluate the effectiveness of adding diltiazem as an adjunct to lignocaine in IVRA.
METHODS
After obtaining approval from the Institute Ethical committee and written informed consent, 40 ASA I-II patients scheduled for day-care upper-limb surgeries were included in this prospective, randomized, double-blind study [Figure 1]. Patients with a history of hypersensitivity to local anesthetics, peripheral vascular disease, sickle-cell trait, significant soft-tissue injury, severe cardiac disease, conduction abnormalities, and renal dysfunction were excluded from the study. All patients included for the study were explained about the procedure. Patients were subjected to a complete preanesthetic examination and were explained about the use of the Visual Analogue Scale (VAS). Patients were instructed to indicate pain in both the intraoperative period and the post-operative period by using VAS. Patients were randomized to one of the two groups of 20 each, based on a computer-generated random number list to receive lignocaine 3 mg/kg (group L) and lignocaine 3 mg/kg plus diltiazem 0.2 mg/kg (group LD) diluted to 40 ml with normal saline.
Figure 1.
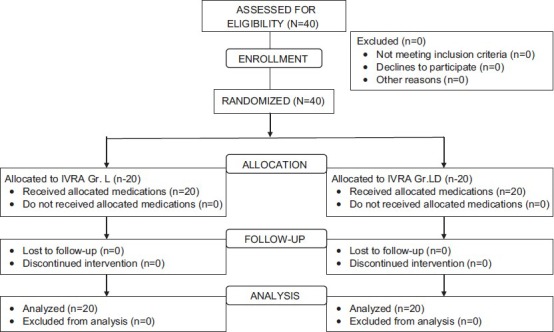
Randomization of patients in both group LD and group L
All patients were premedicated with Midazolam 0.5 mg/kg IV, 15 min before the start of surgery. An 18-gauge venous cannula was secured to gain venous access in the nonoperative limb. Standard monitoring was established for monitoring non-invasive blood pressure NIBP, respiratory rate, electrocardiograph ECG, and SPO2 (Datex-Ohmeda S/5, GE Health Care, Finland). A 20-gauge venous cannula was inserted into a distal vein on the dorsum of the operative hand. A double-cuffed tourniquet was applied, and the arm was exsanguinated by elevation and application of Esmarch bandage. In case of a foreign body, injury, or cystic swelling, only arterial compression and limited elevation were performed. Proximal cuff was inflated up to 250 mmHg. Circulatory isolation of the limb was detected by loss of radial pulse and loss of plethysmographic tracing from the index finger of the operative hand. Randomization of patients was carried out by a computer-generated random list. An independent anesthesiologist not involved in the study prepared the study drug solution in identical syringes. Another independent resident anesthesiologist blinded to the study drug and group allocation observed and recorded the data.
IVRA was established by slow injection of the study solution into the venous cannula over 90 s by a blinded, independent anesthesiologist. Ten minutes after the injection of the study solution, the distal tourniquet cuff was inflated to 250 mmHg while the proximal cuff was deflated. The tourniquet cuff was deflated after 60 min, or at the end of surgery.
Sensory blockade was tested at 30 s intervals with an alcohol swab and pin-prick test with a sterile 26 G hypodermic needle. Onset of complete sensory block (interval between the time of injection of study solution to loss of sensation in all dermatomes distal to the tourniquet) was recorded. Time for onset of the complete motor blockade (interval between the times of injection of the study solution until the time the patient was not able to move his fingers) was recorded. Hemodynamic parameters (pulse rate, NIBP, and SPO2) were recorded at 5 min intervals. Tourniquet pain scores were assessed at every 5-min interval from 0 to 75 min on VAS (0=no pain and 10=worst pain imaginable). Patients received fentanyl 0.5 μg/kg IV if the VAS score was less than three. Time for first analgesic use and total intraoperative fentanyl consumption was recorded. Pulse, BP, SPO2, and respiratory rate were monitored at 0, 5, 10, 15, 20, and 30 min and also at every 1 h interval up to 8 h following deflation of the tourniquet. VAS scores were measured simultaneously; if VAS scores were more than three, the patient received 0.5 μg/kg of fentanyl. Time for the first analgesic required after tourniquet deflation and total post-operative fentanyl consumption was recorded. Time to sensory recovery (time to sensory recovery was defined as the time interval between deflation and sensation in all dermatomes of the hand and forearm) and time to motor recovery (time from deflation to the time patient can move his fingers) were noted.
Adverse effects such as dizziness, hallucinations, tinnitus, postoperative nausea and vomiting (PONV), respiratory depression (<10/min), hypoxemia (SPO2 (90%)), hypotension (BP (20% below baseline)), and bradycardia (HR<60/min) were recorded. Patients received diclofenac 3 mg/kg orally at the end of 8 h after tourniquet deflation and continued twice daily for the next 2 days. Patients were discharged 8 h after tourniquet deflation and were followed up to 24 h telephonically for any severe pain, additional analgesic requirement, nausea vomiting, dizziness, and drowsiness. Patients were asked to rate their experience at the time of discharge on a – four point Rating Scale (1) Not satisfied, will not come to the same hospital for the same procedure, (2) Satisfied but would have preferred another technique, (3) Satisfied but would have preferred more analgesia, (4) Very satisfied. At the end of the procedure, the surgeons were asked to rate the experience on a four point rating scale (0) unsuccessful, (1) Poor, (2) Acceptable, (3) Perfect.
Statistical analysis was performed using SPSS version 20.0 software (IBM Corporation, New York, US). For this study, we presumed that adding diltiazem would decrease tourniquet pain VAS by 20% compared with lignocaine alone. It was estimated that enrolment of 18 patients will be required to permit a type 1 error of α = 0.05 with a power of 80%. Forty patients were enrolled to compensate for any dropouts during the study. All the continuous data were subjected to a Shapio-Wilk test for normality. Demographic data, hemodynamic parameters, surgical duration, and time to onset and recovery of motor and sensory blocks were compared using the Student t-test. Non-parametric data VAS scores both intraoperative and post-operative, time to the first analgesia requirement following tourniquet deflation, fentanyl consumption intra-operatively and post-operatively, and patients’ satisfaction scores were compared using the Mann–Whiney U test. Categorical variables were analyzed using the Chi-square test. A P< 0.05 was considered statistically significant.
RESULTS
Demographic characteristics such as age, sex, and weight of patients, tourniquet time, and duration of surgery did not differ between the groups [Table 1]. There was no statistically significant difference between the groups with respect to hemodynamic parameters during the intraoperative period and after deflation of the tourniquet. During the intraoperative period, the mean VAS score of more than three was reached significantly earlier in the lignocaine group compared with the diltiazem group. The VAS scores in the lignocaine group were significantly higher than those in the diltiazem group at 10:40 min [Figure 2]. The VAS score after deflation of the tourniquet was significantly higher in the lignocaine group than in the diltiazem group at 15 min, 20 min, and 25 min intervals and at 4 h [Figure 3].
Table 1.
Patients’ demographic and surgical data for both the study groups
Figure 2.
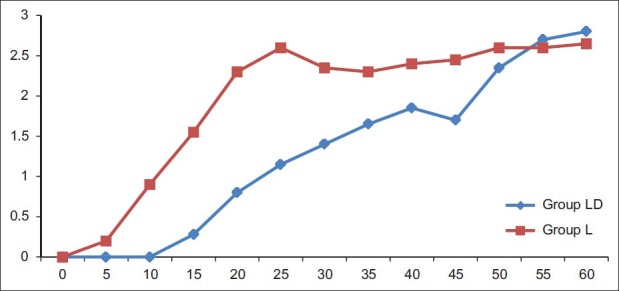
Intraoperative Visual analogue scale scores in both the groups LD‑Diltiazem group L‑Lignocaine group, *P<0.001, †P=0.017, ‡P=0.020
Figure 3.
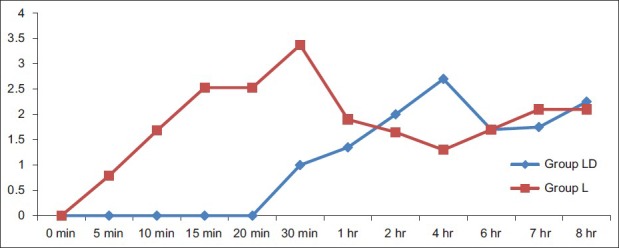
Post‑tourniquet deflation visual analogue scale scores in both the groups, LD‑Diltiazem group, L‑Lignocaine group, *P<0.001, †P=0.023, ‡P=0.015, §P=0.014
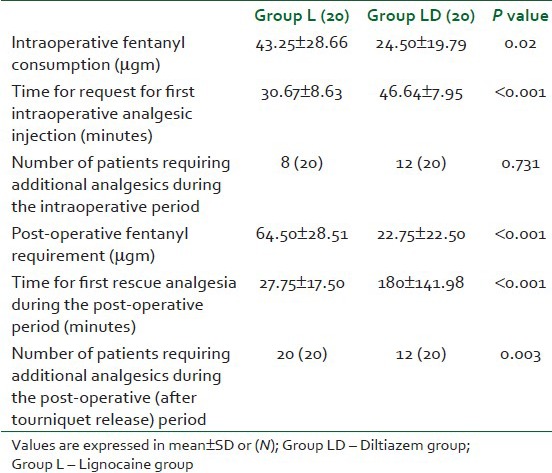
The times for complete sensory blockade and motor block were significantly shorter in the diltiazem group as shown in Table 2. There was no significant difference in time to sensory and motor recovery after tourniquet deflation between the two study groups [Table 1]. Patients in the lignocaine group first requested rescue analgesic significantly earlier than the patients in the diltiazem group during the intraoperative and post-operative periods; moreover, the amount of fentanyl required was significantly less in the diltiazem group in comparison to the lignocaine group [Table 3]. More patients in the lignocaine group required more analgesic during the intra-operative and post-operative periods [Table 3]. None of the patients required any additional analgesic during the study period in both the groups.
Table 2.
Block characteristics details in both the groups
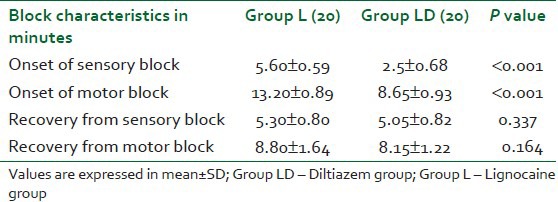
Table 3.
Analgesic requirement characteristics during the intraoperative and post-operative periods
None of the patients experienced tinnitus, seizures, hallucinations, or respiratory depression after deflation of the tourniquet. Eight patients in group LD and six patients in group L experienced transient dizziness after tourniquet deflation (P>0.05). Post-operative nausea and vomiting were observed in one patient in the diltiazem group and in two patients in the lignocaine group (P>0.05). However, none of these findings was statistically significant.
All the patients in the diltiazem group compared to only 65% patients in the lignocaine group were satisfied with the anesthesia technique but would have preferred more analgesia (P<0.001). There was no statistically significant difference in the surgeons’ satisfaction score (P=0.078).
DISCUSSION
In this study, addition of diltiazem hastens sensory and motor block onset time prolongs tourniquet time, and post-operative analgesia. Onset of sensory and motor block was faster in the diltiazem group than in the lignocaine group. Patients in the lignocaine group experienced tourniquet pain earlier compared to the diltiazem group.
Calcium ions also play a vital role in analgesia mediated by local anesthetics. Sensory neurons possess four types of voltage-dependent calcium channels: L, N, P, and T. Both L- and N-type calcium ion channels have been implicated in the release of neurotransmitters from peripheral neurons. The antinociceptive action of four Ca2+ channel blockers, nifedipine, nimodipine, verapamil, and diltiazem, was evaluated and in comparison to that of morphine using three algesiometric tests in mice and rats.[4] The findings suggest a pharmacological role of Ca2+ channel blockers in the modulation of antinociceptive under acute conditions.
The clinical efficacy of the calcium-channel blocker for pain relief has been documented in several studies. Local anesthetics reduce the calcium permeability and clinical investigations have shown that verapamil potentiates the analgesic effect of local anesthetics.[5] Omote et al.[6] interpreted that intrathecal calcium-channel blocker verapamil potentiates spinal anesthesia with local anesthetics. The primary mode of action of local anesthetics is through the sodium channel and axonal conduction blockade. Iwasaki et al.[5] concluded that the use of mixture of a local anesthetic and calcium-channel blocker potentiates lignocaine-induced sensory block at the level of peripheral nerves in rats. Ohmori et al.[7] studied the effect of adding calcium-channel blocker in procaine for local conduction block and concluded that sensory block by procaine of peripheral nerves is potentiated by the co-administration of a calcium-channel blocker. Omote et al.,[8] in a study conducted on rats, have suggested that calcium-channel blocking drugs synergistically potentiate the analgesic effects of morphine at the level of the spinal cord.
Diltiazem was chosen as the calcium channel blocker based on the following facts: (i) Diltiazem 0.1-0.2 mg has been used to treat supraventricular tachyarrhythmia and unstable angina. (ii) I.V. Diltiazem 0.09-0.23 mg/kg has been used to reduce abrupt circulatory changes in response to various surgical stimuli. (iii) Diltiazem 0.2 mg/kg is advocated to attenuate cardiovascular changes after tracheal intubation.[9]
The faster sensory and motor block onset times observed in the diltiazem group were an advantage in as it helped commence the surgery much earlier. In this study, we did not find any prolongation of the sensory and motor block recovery times after tourniquet deflation, thus helping the surgeon identify and correct any bleed from the surgical site and check for nerve integrity by observing the movement and sensation before completely closing the wound.
Diltiazem is a short-acting drug, and the prolonged duration observed in this study provides a probable explanation why we did not observe any significant difference in sensory block and motor block recovery times between the groups. Shorter onset of the sensory and motor blockade in the diltiazem group is in agreement with other studies in the literature. Reuben et al.[10] concluded that the calcium-channel blockers also block the sodium channel, in addition to calcium channels, in a dose-dependent manner and have local anesthetic action. The blockade of sodium and calcium channels apparently impairs membrane function to a greater extent than with either drug alone.
Local anesthetics reversibly block the conduction of nerve impulses by preventing increases in the permeability of nerve membrane to sodium ions. Calcium movement is essential for normal sensory processing and plays a role in axonal conduction and synaptic transmission. Direct inhibition of voltage-dependent calcium conductance and the consequent lowering of calcium ions of the peripheral nerves possibly caused the potentiation of sensory and motor block onset observed in this study. VAS scores in the lignocaine group were significantly higher than those in the diltiazem group at ten to 40 min (P<0.001).
These findings suggest that diltiazem, as an adjunct to lignocaine in IVRA, was effective in reducing tourniquet pain significantly compared to the control group. During the post-operative period, diltiazem provided better analgesia.
In conclusion, diltiazem as an adjuvant to lignocaine in IVRA was effective in hastening onset, reducing tourniquet pain, and increasing tourniquet tolerance, thereby contributing to better perioperative analgesia. Diltiazem provided post-operative analgesia for the longest duration. Overall quality of anesthesia and patient satisfaction was significantly better in the diltiazem group.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Brown EM, McGriff JT, Malinowski RW. Intravenous regional anaesthesia (Bier block): Review of 20 years’ experience. Can J Anaesth. 1989;36:307–10. doi: 10.1007/BF03010770. [DOI] [PubMed] [Google Scholar]
- 2.Johnson CN. Intravenous regional anesthesia: New approaches to an old technique. CRNA. 2000;11:57–61. [PubMed] [Google Scholar]
- 3.Smith FL, Davis RW, Carter R. Influence of Voltage-sensitive Ca (++) channel drugs on bupivacaine infiltration anesthesia in mice. Anesthesiology. 2001;95:1189–97. doi: 10.1097/00000542-200111000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Miranda HF, Bustamante D, Kramer V, Pelissier T, Saavedra H, Paeile C, et al. Antinociceptive effects of Ca2+ channel blockers. Eur J Pharmacol. 1992;217:137–41. doi: 10.1016/0014-2999(92)90833-p. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki H, Ohmori H, Omote K, Kawamata M, Sumita S, Yamauchi M, et al. Potentiation of local lignocaine-induced sensory block by calcium channel blockers in rats. Br J Anaesth. 1996;77:243–7. doi: 10.1093/bja/77.2.243. [DOI] [PubMed] [Google Scholar]
- 6.Omote K, Iwasaki H, Kawamata M, Satoh O, Namiki A. Effects of verapamil on spinal anesthesia with local anesthetics. Anesth Analg. 1995;80:444–8. doi: 10.1097/00000539-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Ohmori H, Iwasaki H, Omote K, Kawamata M, Kawamata T, Yamauchi M, et al. Potentiation of procaine-induced local sensory block by verapamil in rats. Masui. 1996;45:1100–4. [PubMed] [Google Scholar]
- 8.Omote K, Sonoda H, Kawamata M, Iwasaki H, Namiki A. Potentiation of antinociceptive effects of morphine by calcium-channel blockers at the level of the spinal cord. Anesthesiology. 1993;79:746–52. doi: 10.1097/00000542-199310000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Nishina K, Mikawa K, Maekawa N, Obara H. Attenuation of cardiovascular responses to tracheal extubation with diltiazem. Anesth Analg. 1995;80:1217–22. doi: 10.1097/00000539-199506000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Reuben SS, Reuben JP. Brachial plexus anesthesia with verapamil and/or morphine. Anesth Analg. 2000;91:379–83. doi: 10.1097/00000539-200008000-00027. [DOI] [PubMed] [Google Scholar]


