Abstract
Tapentadol is a novel, centrally acting analgesic with dual mechanism of action, combining mu-opioid receptor agonism with noradrenaline reuptake inhibition in the same molecule. It has an improved side effect profile when compared to opioids and nonsteroidal anti-inflammatory drugs. The dual mechanism of action makes Tapentadol a useful analgesic to treat acute, chronic, and neuropathic pain.
Keywords: Nonsteroidal anti-inflammatory drugs, opioids, tapentadol hydrochloride
INTRODUCTION
Pain is a disorder that everyone experiences and is often difficult to treat. Current drug treatment options for management of pain include opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), and paracetamol.[1] NSAIDs are limited by ceiling effect and are appropriate for relief of mild to moderate pain.[1] NSAIDs are contraindicated in patients with acid peptic disease, renal impairment, and bleeding tendency.[2] COX-2 inhibiting NSAIDs overcome some of these side effects,[3] but some of them increase the risk of cardiovascular side effects, including MI.[4] Opioids are considered as gold standard for treatment of moderate to severe pain. However, opioids are underutilised, as doctors may be reluctant to prescribe them and patients may be reluctant to take them due to potential risk of adverse effects, abuse, tolerance, withdrawal, and liability.[5] Suboptimal use of opioids can lead to unrelieved pain, which can lead to poor patient outcome and potentially life-threatening complications.[5] Analgesics having similar effectiveness with improved compliance in comparison to opioids are valuable additions to the analgesic armamentarium.[6] One strategy aimed at improving the compliance of mu-opioid receptor (MOR) agonists is to combine MOR agonism with monoamine reuptake inhibition. Tapentadol is a novel, next generation, centrally acting analgesic with dual mechanism of action that offers analgesic efficacy that is similar to that provided by a pure MOR agonist, but with an improved side-effect profile.[7] Literature search for the article was done using PubMed and Google Scholar using the terms “Tapentadol,” “extended release,” and “immediate release.”
CHEMISTRY
Tapentadol (3-((1R,2R)-3-(dimethylamino)-1-ethyl-2-methylpropyl) phenol hydrochloride) is a non-racemic molecule [Figure 1]. The molecular formula of Tapentadol is C14H23NO.HCl. It was first synthesized in USA and marketed by Ortho-McNeil-Janssen Pharmaceuticals; its trade name was Nucynta.
Figure 1.
Chemical structure of Tapentadol
PHARMACODYNAMICS
Tapentadol provides analgesia through two mechanisms of action, i.e., MOR agonism and noradrenaline reuptake inhibition (NRI).[8,9] It binds to MOR selectively, with a more than or equal to ten-fold affinity compared with delta- and kappa- opioid receptors. The affinity of Tapentadol for MOR is 44-fold lower than that of morphine.[10] NRI increases levels of noradrenaline (NA), which in turn leads to analgesia through activation of inhibitory alpha-2 receptors. This dual mechanism of action contributes to its opioid sparing effects.[5,11] Tapentadol is also a weak serotonin reuptake inhibitor, but this action does not contribute to its analgesic effect.[12] The analgesic potency of Tapentadol is only 2-3 times lower than that of morphine, despite its lower affinity of binding to MOR; this is due to additional noradrenergic mechanism of action of the drug.[10,13]
PHARMACOKINETICS
Absorption
Tapentadol is rapidly absorbed; oral bioavailability after single dose administration is 32% due to extensive first pass metabolism. Plasma steady-state concentration is achieved in 25-30 h when the drug is administered orally every 6 h.[13]
Distribution
Tapentadol is widely distributed throughout the body. Following intravenous (IV) administration, the volume of distribution is 540±98 L. Only 20% of the drug is bound to plasma proteins.
Metabolism and elimination
Tapentadol is extensively metabolised to inactive metabolites (97% of the administered dose). The major pathway of metabolism is conjugation with glucuronic acid to produce glucuronides; tapentadol-O-glucuronide is the major metabolite.[14,15] It is also metabolised to N-desmethyl tapentadol by CYP2C9 and CYP2C19 and to hydroxyl tapentadol by CYP2D6, which are further metabolised by conjugation. CYP enzymes do not play a major role in metabolism. None of the metabolites contribute to its analgesic action.[16] Tapentadol does not inhibit or induce the activity of any of the CYP isoforms.[14]
Tapentadol follows first-order elimination kinetics. Tapentadol and its metabolites are excreted almost exclusively (99%) via the kidneys. Half life is 4 h. The total clearance is 1530±177 ml/min.
Dosage and formulation
Tapentadol is available as 50, 75, and 100 mg oral tablets. Maximum dosage is 600-700 mg in divided doses every 4-6 h.[17] In India, it is marketed under the trade names Vorth TP (Glenmark Pharmaceuticals), Tydol (Ranbaxy Laboratories Ltd.), and Dolproxyvon (Wockhardt Ltd.). It can be administered with or without food. Tapentadol is available as immediate-release (IR) and extended-release (ER) formulations. A study has been published regarding the conversion of Tapentadol IR to ER, which suggests that a direct milligram to milligram conversion on a total daily dose basis is appropriate.[18] The IV formulation is presently under clinical trials and its use is not yet approved.
Special group
Safety of tapentadol in pregnant, lactating women, and pediatric patients <18 years of age is not yet established. It is not recommended for use in patients with severe renal impairment. Tapentadol should be used with caution and reduced dosage in patients with moderate hepatic impairment and is not recommended for use in severe hepatic impairment.[19] It is advisable to start with lower range of recommended doses in elderly patients.
Drug interactions
Tapentadol has low protein binding, displacement reactions are unlikely.[13] Tapentadol has a low potential for pharmacokinetic drug-drug interactions.[11] No significant change is plasma concentration of tapentadol was observed when it was administered with paracetamol, naproxen, or aspirin.[20] There are no drug interactions between Tapentadol and metoclopramide and between omeprazole and probenecid.[21,22,23] Patients receiving other opioids, general anesthetics, phenothiazines, sedative-hypnotics, or other CNS depressants like alcohol along with Tapentadol may have additional CNS depression that may manifest as respiratory depression, hypotension, or profound sedation.[24]
Contraindications
Patients with impaired pulmonary function (acute bronchial asthma, significant respiratory depression) in unmonitored settings or in the absence of resuscitative equipment
Paralytic ileus
Current or recent (within 14 days) use of MAOIs due to potential increase in NA levels, which may cause adverse cardiovascular events[24]
Indications
Moderate to severe acute pain
Chronic pain
Neuropathic pain
The efficacy of Tapentadol for relief of moderate to severe acute pain has been evaluated in three randomized, double blind, phase three studies.[6,25,26] In one of the postoperative pain study, patients received Tapentadol 50 or 75 mg, oxycodone 10 mg, or placebo every 4-6 h for 72 h following bunionectomy;[25] in the other postoperative pain study, patients received Tapentadol 50, 75 or 100 mg, oxycodone 15 mg, or placebo every 4-6 h for 72 h post-bunionectomy.[6] In both studies, improvements in pain intensity were observed with Tapentadol that were similar to that observed with oxycodone based on pain intensity measurements on the numerical rating scale (NRS). In a study on postsurgical dental pain, patients undergoing third molar extraction were randomized to receive single doses of Tapentadol (50, 75, 100, or 200 mg), morphine 60 mg, ibuprofen 400 mg, or placebo. The results of the study showed that single doses of Tapentadol 75 mg or higher effectively reduced pain in a dose-related fashion and were well-tolerated relative to morphine.[26]
Efficacy for short-term use in chronic inflammatory pain was examined in patients with end-stage degenerative joint disease over a period of 10 days, while awaiting knee or hip replacement surgery in a double-blind placebo controlled trial; Tapentadol 50 mg and 75 mg and oxycodone 10 mg produced significant reductions in pain intensity as compared with placebo and Tapentadol was not inferior to oxycodone.[27] In a recent analysis of pooled data from three phase three studies in patients with chronic osteoarthritis knee or low back pain evaluating the efficacy and tolerability of Tapentadol in comparison with placebo and oxycodone showed that Tapentadol (100-250 mg, twice daily) provided efficacy that was similar to that by oxycodone (20-50 mg, twice daily) with a superior gastrointestinal tolerability profile and fewer treatment discontinuations.[28,29] A phase three trial in patients with painful diabetic peripheral neuropathy showed the efficacy of Tapentadol in neuropathic pain.[30] NRI action of Tapentadol makes it especially useful in neuropathic pain.[5]
COMPARISON WITH TRAMADOL
Tramadol is the other analgesic with dual mechanism of action. Hence, it would be pertinent to compare both the drugs. Tapentadol has several advantages in comparison with Tramadol.[31] The comparison of both the drugs is summarised in Table 1.
Table 1.
Comparison of tapentadol and tramadol
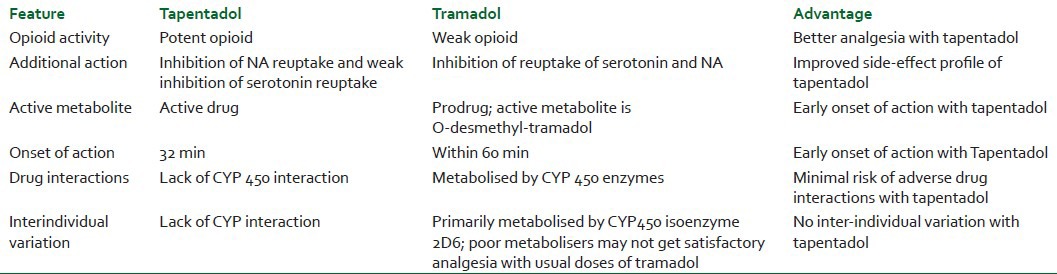
Lesser incidence of nausea and vomiting has been reported with Tapentadol as compared to that by tramadol because Tapentadol is a weak inhibitor of serotonin reuptake, whereas tramadol is a potent inhibitor.
LIMITATIONS OF TAPENTADOL IN COMPARISON TO TRAMADOL
At present IV/IM formulation of Tapentadol is not approved for use, hence in situations where the patient is fasting Tapentadol cannot be used. Tramadol can be administered through various routes like oral IV, IM, suppository, and epidural, whereas Tapentadol can only be administered oral. Tapentadol is more expensive when compared to tramadol or other opioids. A cost-effectiveness analysis comparing Tapentadol and oxycodone for acute pain suggested that increased direct drug costs are offset by reductions in expenses incurred due to treatment of adverse effects, treatment discontinuation, and conversion to alternate opioids.[32]
SAFETY AND TOLERABILITY
Opioids are commonly associated with side effects like nausea, vomiting, constipation, dizziness, headache, somnolence, and pruritis. These adverse effects may be dose limiting and are often severe enough for patients to discontinue therapy.[33,34,35] The tolerability of Tapentadol has been evaluated in trials in which patients with acute pain received multiple oral doses. The most common adverse effects noted with Tapentadol were nausea, vomiting, constipation, dizziness, somnolence, headache, and pruritis;[6,25,27,28,29] these effects are typical of a MOR agonist. The incidence of these adverse effects has been reported to be lesser with Tapentadol in comparison to that by opioids like morphine and oxycodone; this can possibly be due to lower affinity of Tapentadol for MOR in comparison to other opioids.[26] Tapentadol does not cause significant respiratory depression. Studies have shown that abrupt discontinuation of the drug after long-term therapy does not cause opioid-like withdrawal symptoms and, even if they do occur, these symptoms are mild. Long-term therapy was not associated with clinically significant changes in laboratory values (including hepatic and renal parameters), vital signs, or Electrocardiography findings.[24,29]
CONCLUSION
Tapentadol is the first FDA approved (November 2008)[36] centrally acting analgesic with MOR agonist and NRI actions. This dual mode of action is responsible for its opioid sparing effect, which contributes to reduction in some of the typical opioid related adverse effects and may result in improved compliance.[28] This combination of potent analgesia and tolerability may provide a substantial improvement over current pain relief regimens and would be a valuable addition to the analgesic armamentarium.[8] Tapentadol has not yet been studied in cancer pain. Given the limited number of published studies, the side-effect profile, and pharmacoeconomic impact will need to be confirmed in further studies.
ACKNOWLEDGMENT
We would like to thank Dr. T Sivashanmugam, Associate Professor (Department of Anaesthesiology and Critical Care, Mahatma Gandhi Medical College, Pillaiyarkuppam, Pondicherry 607402) for his help towards preparation of the manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Brown AK, Christo PJ, Wu CL. Strategies for postoperative pain management. Best Pract Res Clin Anaesthesiol. 2004;18:703–17. doi: 10.1016/j.bpa.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Power I, Barratt S. Analgesic agents for the postoperative period: Nonopioids. Surg Clin North Am. 1999;79:275–95. doi: 10.1016/s0039-6109(05)70383-2. [DOI] [PubMed] [Google Scholar]
- 3.Stephens J, Laskin B, Pashos C, Pena B, Wong J. The burden of acute postoperative pain and the potential role of the COX-2-specific inhibitors. Rheumatology (Oxford) 2003;42:40–52. doi: 10.1093/rheumatology/keg497. [DOI] [PubMed] [Google Scholar]
- 4.McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: A systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296:1633–44. doi: 10.1001/jama.296.13.jrv60011. [DOI] [PubMed] [Google Scholar]
- 5.Hatrick CT. Tapentadol immediate release for the relief of moderate-to-severe acute pain. Expert Opin Pharmacother. 2009;10:2687–96. doi: 10.1517/14656560903313734. [DOI] [PubMed] [Google Scholar]
- 6.Daniels S, Casson E, Stegmann JU, Oh C, Okamoto A, Rauschkolb C, et al. A randomized, double-blind, placebo-controlled phase 3 study of the relative efficacy and tolerability of tapentadol IR and oxycodone IR for acute pain. Curr Med Res Opin. 2009;25:1551–61. doi: 10.1185/03007990902952825. [DOI] [PubMed] [Google Scholar]
- 7.Tzschentke TM, Christoph T, Kögel B, Schiene K, Hennies HH, Englberger W, et al. (1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): A novel opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther. 2007;323:265–76. doi: 10.1124/jpet.107.126052. [DOI] [PubMed] [Google Scholar]
- 8.Afilalo M, Stegmann JU, Upmalis D. Tapentadol immediate release: A new treatment option for acute pain management. J Pain Res. 2010;3:1–9. doi: 10.2147/jpr.s4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bee LA, Bannister K, Rahman W, Dickenson AH. Mu-opioid and noradrenergic alpha (2)-adrenoreceptor contributions to the effects of tapentadol on spinal electrophysiological measures of nociception in nerve-injured rats. Pain. 2011;152:131–9. doi: 10.1016/j.pain.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Tzschentke TM, Jahnel U, Kogel B, Christoph T, Englberger W, De Vry J, et al. Tapentadol hydrochloride: A next generation, centrally acting analgesic with two mechanisms of action in a single molecule. Drugs Today (Barc) 2009;45:483–96. doi: 10.1358/dot.2009.45.7.1395291. [DOI] [PubMed] [Google Scholar]
- 11.Hartrick CT, Rozek RJ. Tapentadol in pain management. A µ-opioid receptor agonist and noradrenaline reuptake inhibitor. CNS Drugs. 2011;25:359–70. doi: 10.2165/11589080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Cristoph T, De Vry J, Jahnel U, Tzschentke TM. Anti-allodynic activity of tapentadol in a rat model of neuropathic pain depends on opioid and noradrenergic, but no serotonergic, mechanisms. Eur J Pain. 2009;13:205. [Google Scholar]
- 13.Tzschentke TM, De Vry J, Terliden R. Tapentadol hydrochloride: Analgesic mu-opioid receptor agonist noradrenaline reuptake inhibitor. Drugs Future. 2006;31:1053–61. [Google Scholar]
- 14.Kneip C, Terlinden R, Beier H, Chen G. Investigations into drug-drug interaction potential of tapentadol in human liver microsomes and fresh human hepatocytes. Drug Metab Lett. 2008;2:67–75. doi: 10.2174/187231208783478434. [DOI] [PubMed] [Google Scholar]
- 15.Terlinden R, Kogel BY, Englberger W, Tzschentke TM. In vitro and in vivo characterization of tapentadol metabolites. Methods Find Exp Clin Pharmacol. 2010;32:31–8. doi: 10.1358/mf.2010.32.1.1434165. [DOI] [PubMed] [Google Scholar]
- 16.Terliden R, Ossig J, Fliegert F, Lange C, Göhler K. Absorption, metabolism, and excretion of 14C-labeled tapentadol HCl in healthy male subjects. Eur J Drug Metab Pharmacokinet. 2007;32:163–9. doi: 10.1007/BF03190478. [DOI] [PubMed] [Google Scholar]
- 17.Harsoor SS. Emerging concepts in post-operative pain management. Indian J Anaesth. 2011;55:101–3. doi: 10.4103/0019-5049.79872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etropolski MS, Okamoto A, Shapiro DY, Rauschkolb C. Dose conversion between tapentadol immediate and extended release for low back pain. Pain Physician. 2010;13:61–70. [PubMed] [Google Scholar]
- 19.Xu XS, Smit JW, Lin R, Stuyckens K, Terlinden R, Nandy P. Population pharmacokinetics of tapentadol immediate release (IR) in healthy subjects and patients with moderate or severe pain. Clin Pharmacokinet. 2010;49:671–82. doi: 10.2165/11535390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Smit JW, Oh C, Rengelshausen J, Terlinden R, Ravenstijn PG, Wang SS, et al. Effects of acetaminophen, naproxen and acetylsalicylic acid on tapentadol pharmacokinetics: Results of two randomized, open-label, crossover drug-drug interaction studies. Pharmacotherapy. 2010;30:25–34. doi: 10.1592/phco.30.1.25. [DOI] [PubMed] [Google Scholar]
- 21.Mangold B, Oh C, Jaeger D, Terlinden R, Upmalis D. The pharmacokinetics of tapentadol are not affected by omeprazole: Results of a two-way crossover drug-interaction study in healthy subjects. Pain Pract. 2007;7:55. [Google Scholar]
- 22.Smit JW, Oh C, Rengelshausen J, Terlinden R, Ravenstijn PG, Wang SS. Effects of metoclopramide on tapentadol pharmacokinetics: Results of an open-label, cross-over, drug-drug interaction study. J Clin Pharmacol. 2009;49:1104. [Google Scholar]
- 23.Smit JW, Oh C, Lannie C. Effects of probenecid on tapentadol immediate release pharmacokinetics: Results of an open-label, crossover, drug-drug interaction study. J Clin Pharmacol. 2009;49:1104. [Google Scholar]
- 24.Raritan (NJ): Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2008. Nucynta (tapentadol) immediate-release oral tablets. US prescribing information. [Google Scholar]
- 25.Daniels SE, Upmalis D, Okamoto A, Lange C, Haeussler J. A randomized, double-blind, phase 3 study comparing multiple doses of tapentadol IR, oxycodone IR and placebo for postoperative (bunionectomy) pain. Curr Med Res Opin. 2009;25:765–76. doi: 10.1185/03007990902728183. [DOI] [PubMed] [Google Scholar]
- 26.Kleinert R, Lange C, Steup A, Black P, Goldberg J, Desjardins P. Single dose analgesic efficacy of Tapentadol in postsurgical dental pain: The results of a randomized, double blind, placebo-controlled study. Anesth Analg. 2008;107:2048–55. doi: 10.1213/ane.0b013e31818881ca. [DOI] [PubMed] [Google Scholar]
- 27.Hartrick C, Van Hove I, Stegmann JU, Oh C, Upmalis D. Efficacy and tolerability of tapentadol immediate release and oxycodone HCl immediate release in patients awaiting primary joint replacement surgery for end-stage joint disease: A 10-day, phase three, randomized, double-blind, active and placebo-controlled study. Clin Ther. 2009;31:260–71. doi: 10.1016/j.clinthera.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Lange B, Kuperwasser B, Okamoto A, Steup A, Häufel T, Ashworth J, et al. Efficacy and safety of tapentadol prolonged release for chronic osteoarthritis pain and low back pain. Adv Ther. 2010;27:381–99. doi: 10.1007/s12325-010-0036-3. [DOI] [PubMed] [Google Scholar]
- 29.Hale M, Upmalis D, Okamoto A, Lange C, Rauschkolb C. Tolerability of tapentadol immediate release in patients with lower back pain or osteoarthritis of the hip or knee over 90 days: A randomized, double blind study. Curr Med Res Opin. 2009;25:1095–104. doi: 10.1185/03007990902816970. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz S, Etropolski M, Shapiro DY, Okamoto A, Lange R, Haeussler J, et al. Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: Results of a randomized placebo controlled trial. Curr Med Res Opin. 2011;27:151–62. doi: 10.1185/03007995.2010.537589. [DOI] [PubMed] [Google Scholar]
- 31.Guay DR. Is tapentadol an advance on tramadol? Consult Pharm. 2009;24:833–40. doi: 10.4140/tcp.n.2009.833. [DOI] [PubMed] [Google Scholar]
- 32.Kwong WJ, Ozer-Stillman I, Miller JD, Haber NA, Russell MW, Kavanagh S. Cost-effectiveness analysis of tapentadol immediate release for the treatment of acute pain. Clin Ther. 2010;32:1768–81. doi: 10.1016/j.clinthera.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 33.McNicol E, Horowicz-Mehler N, Fisk RA, Bennett K, Gialeli-Goudas M, Chew PW, et al. Management of opioid side effects in cancer-related and chronic non cancer pain: A systematic review. J Pain. 2003;4:231–56. doi: 10.1016/s1526-5900(03)00556-x. [DOI] [PubMed] [Google Scholar]
- 34.Bell TJ, Panchal SJ, Miaskowski C, Bolge SC, Milanova T, Williamson R. The prevalence, severity and impact of opioid-induced bowel dysfunction: Results of a US and European Patient Survey. Pain Med. 2009;10:35–42. doi: 10.1111/j.1526-4637.2008.00495.x. [DOI] [PubMed] [Google Scholar]
- 35.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105–20. [PubMed] [Google Scholar]
- 36.Ellis, Jarosh F. Leading Epileptologist Susan S. Spencer, MD, Dies. Neurol Today. 2009;9:7. [Google Scholar]


