Abstract
Pulmonary arterial hypertension (PAH) is characterized by progressive increases in pulmonary vascular resistance, leading to right heart failure and death. Guidelines recommend customization of treatment, necessitating the development of effective strategies for transitioning patients among treatments. In this study, we characterized our experience with patient transitions from parenteral prostacyclin to inhaled iloprost. We retrospectively assessed records from 11 centers of 37 consecutive patients with PAH aged ≥ 18 years who were treated with intravenous (IV) or subcutaneous (SC) prostacyclin analogues and transitioned to inhaled iloprost. The transition period began on the first day of inhaled iloprost with the intent of discontinuing parenteral prostacyclin and ended on the first day on inhaled iloprost free of parenteral prostacyclin. Persistence was defined as the absence of (1) parenteral prostacyclin while remaining on inhaled iloprost during post-transition Days 1-90 and (2) no reinitiation of parenteral prostacyclin during post-transition Days 90-365. All patients were clinically stable before transitioning to inhaled iloprost. The mean age was 46.5 years, 70.3% were female, 51.4% had idiopathic PAH, and 43.0% were in New York Heart Association Functional Class III. Among patients with an overlapping transition, the mean transition period was 10.5 days. A transition dosing algorithm was used in 10 patients (27.0%). At one year, 78.4% of the patients remained persistent on inhaled iloprost and 81.1% were free of clinical worsening. In selected patients on background oral PAH therapy, transitioning from parenteral prostacyclin to inhaled iloprost appears safe and feasible and is associated with long-term success. Further study is needed to define the optimal patient selection criteria and transition algorithm.
Keywords: iloprost, persistence, prostacyclin, pulmonary arterial hypertension, transition, treatment strategy
Pulmonary arterial hypertension (PAH) is a life-threatening disease characterized by a progressive increase in pulmonary vascular resistance (PVR) leading to right ventricular failure and death.[1,2] Principle pathophysiologic changes associated with PAH include remodeling of the vascular wall, in situ thrombosis, and vasoconstriction.[1] PAH is defined hemodynamically as a mean pulmonary artery pressure (mPAP) > 25 mmHg, pulmonary capillary wedge pressure ≤ 15 mmHg, and PVR > 3 Wood units.[3]
Parenteral prostacyclin analogues (intravenous [IV] epoprostenol and treprostinil; subcutaneous [SC] treprostinil)[4,5,6,7,8] are accepted PAH therapies;[9,10,11] however, chronic treatment with these compounds may be limited by major adverse events (AEs) attributed to pump malfunctions, catheter-related infections, and vascular thrombosis with IV administration, and infusion site pain when administered subcutaneously. The short half-life of epoprostenol (two to three minutes) requires continuous IV infusion through a tunneled catheter, which may trigger systemic AEs or infusion site pain and erythema.[12] Abrupt cessation of the infusion can cause life-threatening rebound pulmonary hypertension (PH),[13] and the risk of catheter-related infections related to IV infusion may limit its appeal. Barst et al. demonstrated that approximately 10% of patients with PAH receiving IV epoprostenol developed a catheter line infection over an 84-day period.[5] Badesch et al. reported an incidence of 4% in patients receiving continuous IV epoprostenol for PH due to the scleroderma spectrum of diseases.[6] Treprostinil has a longer half-life than epoprostenol (three to five hours) and can be administered via SC or IV infusion. However, compared with IV epoprostenol, IV treprostinil has been associated with a higher rate of bloodstream infections in patients with PAH, which has been related to the pH of the diluent.[14,15,16] A recent analysis from the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL), an uncontrolled registry, showed that bloodstream infection rates were significantly greater in patients receiving IV treprostinil compared with those receiving IV epoprostenol (0.36 vs. 0.12 per 1,000 treatment days; P < 0.001).[17]
Iloprost, a prostacyclin analogue formulated for delivery via inhalation, was approved in the United States in December 2004 to treat patients with PAH. In the Aerosolized Iloprost Randomized (AIR) study, a significantly greater percentage of patients with severe PAH and chronic thromboembolic PH receiving iloprost (median dose = 30 μg/day or six treatments/day) had an improvement in New York Heart Association (NYHA) functional class (FC) and 6-Minute Walk Distance (6MWD) of ≥ 1 and ≥ 10%, respectively, compared with placebo, indicating that iloprost is an effective therapy in these patients.[12] Iloprost is delivered during distinct treatment periods necessitating 6-9 inhalations per day. This treatment schedule can be challenging for some patients and thus may limit compliance. However, because of the inhaled route of administration, iloprost allows for direct drug delivery[6,12,18] and has an AE profile that may be more favorable than prostanoids with continuous delivery,[4,5,6,7,8,12] potentially resulting in fewer systemic AEs while eliminating the risk of infection or site pain.
The transition of patients with PAH from parenteral prostacyclin therapy to inhaled iloprost has been previously investigated in small patient populations;[19,20,21,22] however, it is important to further understand factors that contribute to a successful transition to a parenteral-free treatment regimen.[23] We studied a retrospective cohort of patients with PAH (World Health Organization [WHO] Group I) who transitioned from a parenteral prostacyclin analogue to inhaled iloprost to (1) characterize the profile of transitioned patients; (2) determine the utilization of transition algorithms; (3) ascertain the rationale for transitioning patients; and (4) determine the duration and predictors of persistence with a parenteral-free treatment regimen following transition.
MATERIALS AND METHODS
Study design
We performed a retrospective cohort study of patients with PAH aged ≥ 18 years who were treated with IV or SC prostacyclin analogues. We identified 37 patients who attempted to transition to inhaled iloprost, either concurrently (overlapping) or within one day of discontinuing parenteral prostacyclin analogue therapy. Transition Day 1 was defined as the first day of inhaled iloprost therapy initiated with the intent of discontinuing parenteral prostacyclin. Post-transition Day 1 was defined as the first day on inhaled iloprost without parenteral prostacyclin therapy (Fig. 1). Patient data were collected through the earliest of the following time points: Post-transition Day 365; reinitiation of parenteral prostacyclin therapy; date of discontinuation of inhaled iloprost during post-transition Days 1-90 (therapy breaks ≤ 28 days were considered interruptions, not discontinuations); lung transplantation; atrial septostomy; death; and date of electronic case report form (eCRF) entry of medical chart data. All patients were monitored clinically throughout the study period.
Figure 1.
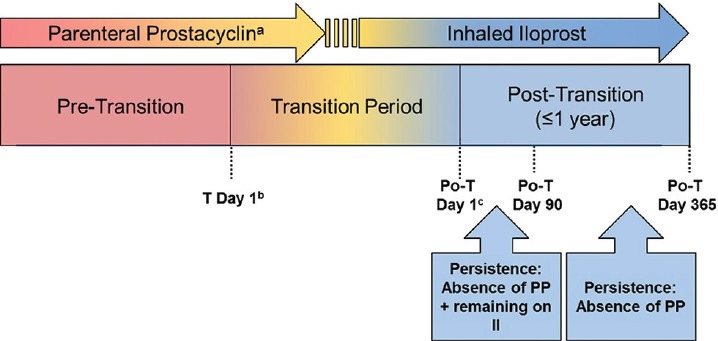
Study design. aParenteral prostacyclin comprises intravenous epoprostenol and intravenous/subcutaneous treprostinil. bTransition Day 1 is defined as the start day of inhaled iloprost with intent of discontinuing parenteral prostacyclin therapy. cPost-transition Day 1 is defined as the first day on inhaled iloprost and free of parenteral prostacyclin therapy. Depending on the clinical site and/or patient, there may be no period of concurrent (overlapping) administration of inhaled iloprost and parenteral prostacyclin therapy and, therefore, no transition period. In such cases, transition Day 1 is the same as post-transition Day 1. Po-T: post-transition; PP: parenteral prostacyclin; II: inhaled iloprost.
Patient population
Transition to inhaled iloprost had to occur after US Food and Drug Administration (FDA) approval (December 30, 2004) and before August 2009. Eligible patients must have transitioned to iloprost for ≥ 24 hours in the absence of parenteral therapy before August 2009. Patients had to be aged ≥ 18 years on transition Day 1 with a diagnosis of PAH (WHO Group I PH) within one of the following categories: Idiopathic PAH, heritable PAH, or PAH associated with another disease (APAH), including connective tissue disease; congenital heart disease, including congenital systemic-to-pulmonary shunts (unrepaired or at least two years post-surgical repair); human immunodeficiency virus; portal hypertension; or drugs and/or toxins. Patients provided signed informed consent if required by the study site institutional review board.
Data collection
Patient demographic and clinical characteristics were collected from the most recent chart entries before transition Day 1. Informed consent dates, previous parenteral prostacyclin therapy and transition attempts, and reasons for the current transition were documented. Administration details for the most recent parenteral prostacyclin therapy before transition Day 1 and dates and reasons for any discontinuation of inhaled iloprost therapy during the post-transition period were documented (treatment interruptions were not recorded).
All investigational and FDA-approved PAH medications that were initiated, continued, or discontinued within 90 days before transition Day 1, during the transition period, or during the post-transition period were captured in the eCRF. The most recent 6MWD, NYHA FC before transition Day 1 (within one year), and hemodynamic parameters before transition Day 1 (within three years) were documented. The dates of lung transplantation, atrial septostomy, or death were recorded if they occurred within the transition or the post-transition period.
Exposure and outcome definitions and measurements
Persistence was defined and assessed as follows: (1) An absence of parenteral prostacyclin therapy while remaining on inhaled iloprost during post-transition Days 1-90 and (2) no reinitiation of parenteral prostacyclin therapy during post-transition Days 90-365. Persistence and survival were determined from post-transition Day 1. Persistent patients were followed to the earliest of the following dates: (1) Lung transplantation; (2) atrial septostomy; (3) death; (4) Day 365; and (5) eCRF entry. Clinical worsening following transition was defined as the earliest of four events occurring between post-transition Day 1 and Week 52: (1) Reinitiation of parenteral prostacyclin therapy due to clinical deterioration; (2) atrial septostomy; (3) lung transplantation; or (4) death. Time to clinical worsening was calculated from post-transition Day 1 to the date of onset of the first clinical worsening event. Patients who did not experience clinical worsening were followed to the date of eCRF entry or study Day 365.
Statistical analyses
Data analyses were stratified by etiology and baseline NYHA FC. Kaplan-Meier product-limit estimates were used to estimate the proportion of patients who persisted for one year following transition to inhaled iloprost, calculated from post-transition Day 1. Cox proportional hazards models were used to examine predictors of persistence as permitted by the sample size. P < 0.05 was considered significant.
RESULTS
Baseline characteristics and medications
Thirty-seven eligible patients on IV or SC prostacyclin analogues from 11 US centers were enrolled in the study. The patient cohort had a mean age of 46.5 years, PAH duration of 4.6 years, and was comprised of predominantly females (70.3%) and patients with idiopathic PAH (51.4%). The patient cohort had a median (min, max) duration from the date of right heart catheterization to transition Day 1 of 5.2 (0.0, 108.1) and 9.3 (0.0, 28.5) months in persistent and nonpersistent patients, respectively. The mean 6MWD, PVR, mPAP, and wedge pressure were 400.5 m, 9.3 Wood units, 49.0 mmHg, and 11.3 mmHg, respectively (Table 1). The most common pretransition parenteral prostacyclin was IV epoprostenol (48.6%), followed by SC treprostinil (43.2%) and IV treprostinil (8.1%; Table 2). PAH-specific medications utilized during the pre-transition, transition, and post-transition periods are shown in Figure 2.
Table 1.
Patient characteristics, 6MWD, and hemodynamics
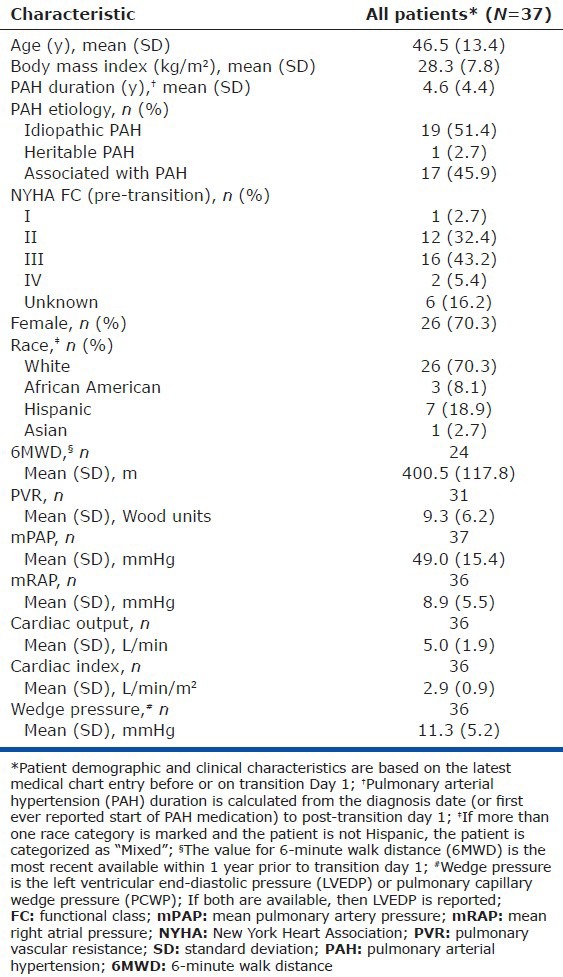
Table 2.
Parenteral prostacyclin pre-transition for all patients*
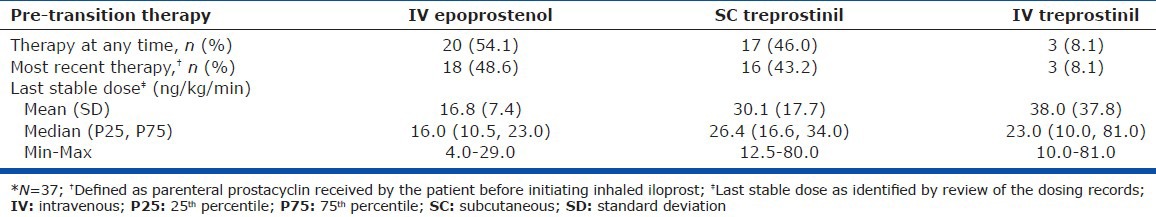
Figure 2.
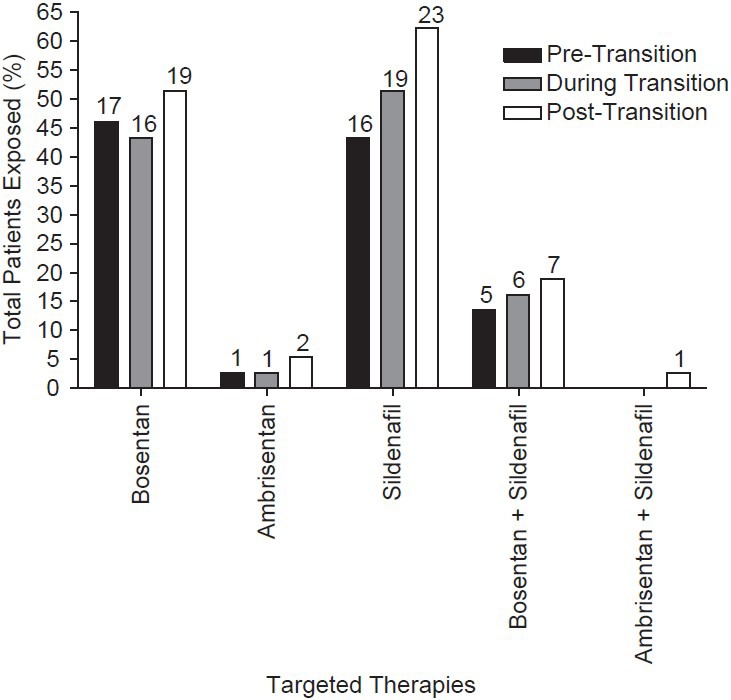
Pulmonary arterial hypertension-related medication usage for all patients. Pre-transition includes the medications patients were exposed to during the 90 days prior to transition (n= 37). During transition includes the medications patients were exposed to during the transition period (transition Day 1 through post-transition Day 0, n= 34) and patients with a non-overlapping transition period (n= 3). Post-transition includes the medications patients were exposed to during the 365 days after post-transition Day 1 (n= 37). During the transition and post-transition periods, many patients had PAH oral therapy modifications. Among persistent patients, four added an oral therapy during the transition period (one bosentan, three sildenafil), four added a therapy post-transition (one ambrisentan, three sildenafil), and one switched from bosentan to sildenafil during the post-transition period. Among non-persistent patients, one patient discontinued sildenafil therapy during the transition period and one patient added sildenafil during the post-transition period. All patients include charts from patients in the analysis population (i.e., those who satisfied all protocol criteria). Values above columns denote the number of patients.
Transition to inhaled iloprost
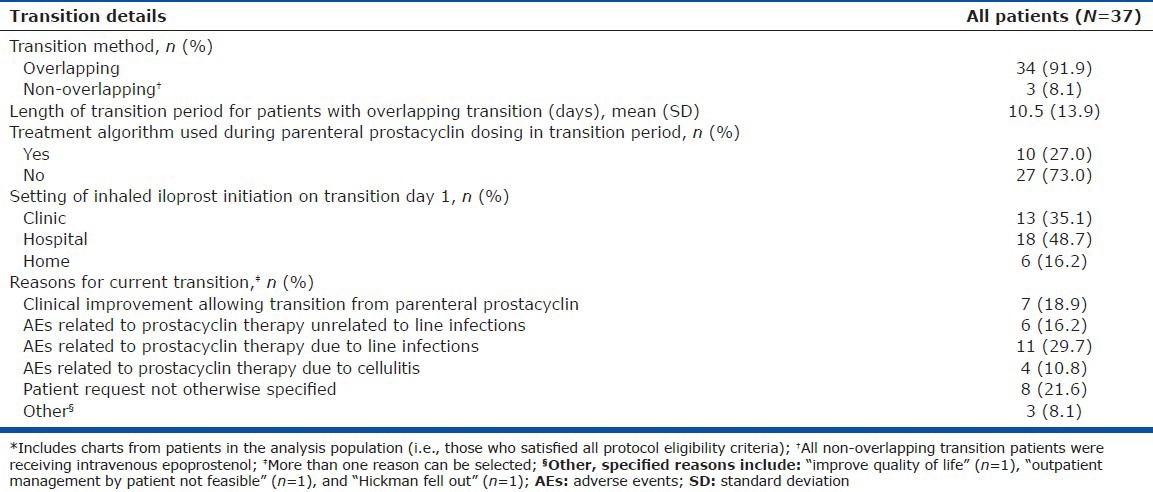
Details of the patient transitions to inhaled iloprost, including whether the transition was overlapping, the transition duration, and whether a treatment algorithm was used, are shown in Table 3. The majority of patients (91.9%) had an overlapping transition with a mean transition period of 10.5 ± 13.9 days. All three of the patients who underwent a nonoverlapping transition had received IV epoprostenol. Reasons for transitioning included the following: Clinical improvement permitting transition from a parenteral prostacyclin (18.9%); AEs associated with prostacyclin therapy that were not due to infections (16.2%); AEs associated with IV prostacyclin therapy that were due to line infections (29.7%); AEs associated with SC prostacyclin therapy that were due to cellulitis (10.8%); or site pain (18.9%). PAH-related oral medication usage during transition included endothelin receptor antagonists (ERAs; 54.1%), phosphodiesterase type-5 (PDE-5) inhibitors (51.4%), and calcium channel blockers (10.8%). Ten patient charts (27.0%) indicated that a transition dosing algorithm was used. All seven patients who demonstrated clinical improvement before transitioning remained persistent with a parenteral-free treatment regimen post-transition.
Table 3.
Transition details for all patients*
Persistence during the post-transition period
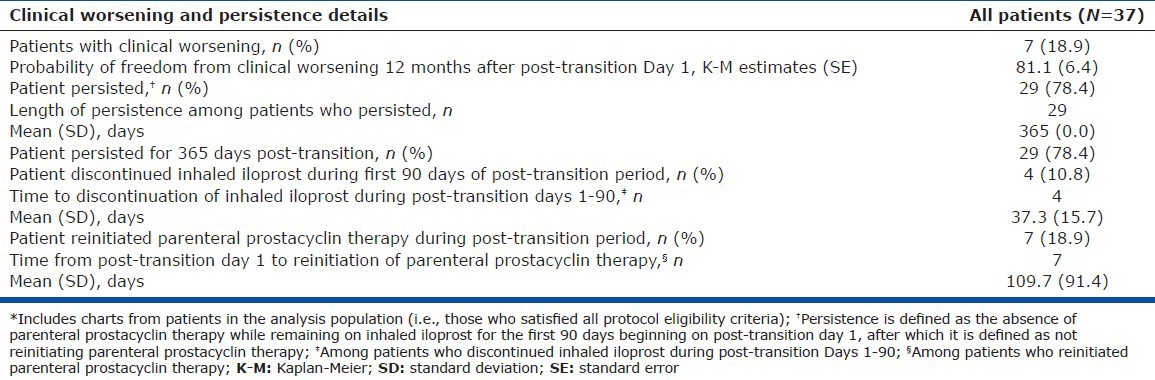
The Kaplan-Meier estimated probability ± standard error of being free from clinical worsening at one year was 81.1 ± 6.4%. Of the whole cohort, seven patients (18.9%) demonstrated clinical worsening (Table 4). The one patient death was a female aged 72 years with NYHA FC III symptoms who died 10 months after reinitiating parenteral therapy.
Table 4.
Clinical worsening and persistence details during the post-transition period in all patients*
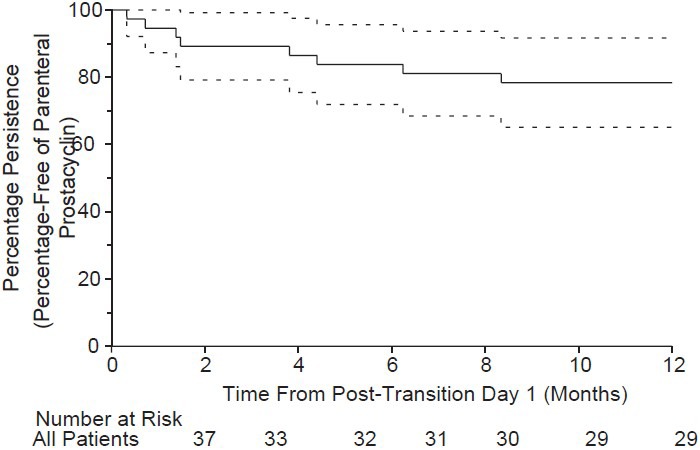
Among the entire cohort, 29 patients (78.4%) were persistent and 27 patients remained on inhaled iloprost continuously for one year post-transition. Of the 29 patients who persisted, two patients discontinued inhaled iloprost 90 days post-transition, one patient added an ERA, three patients added a PDE-5 inhibitor during the post-transition period, and one patient switched from an ERA to a PDE-5 inhibitor during the post-transition period. Eight patients (21.6%) were nonpersistent (Fig. 3); four (10.8%) discontinued inhaled iloprost during the first 90 days of the post-transition period (mean time to discontinuation, 37.3 ± 15.7 days; Table 4), one added a PDE-5 inhibitor, and one discontinued PDE-5 inhibitor therapy during the post-transition period. Reasons for discontinuation of inhaled iloprost during the post-transition period included clinical deterioration (n = 7), AEs (n = 1), and other (noncompliance, n = 1; enrolled in an oral prostacyclin drug study, n = 1).
Figure 3.
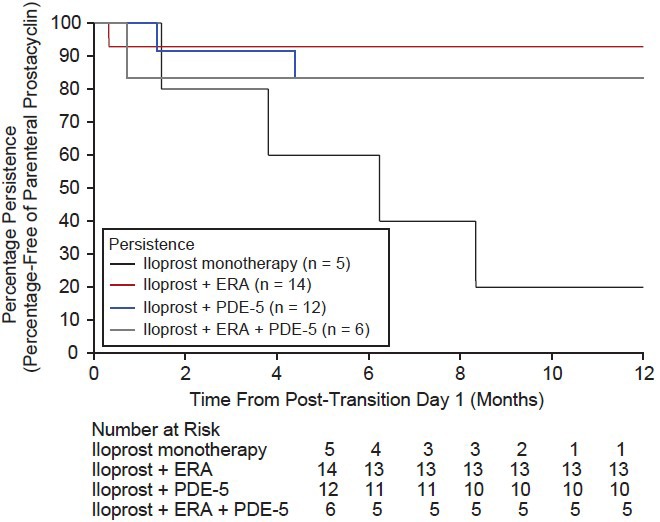
One-year persistence was observed in 29 patients (78.4%); these patients remained on inhaled iloprost continuously for 1 year post-transition. Four patients (10.8%) discontinued inhaled iloprost during the first 90 days of the post-transition period (mean time to discontinuation = 37.3 ± 15.7 days). Reasons for discontinuation during the post-transition period included clinical deterioration (n= 7), adverse events (n= 1), and other (non-compliance [n= 1] and joined oral prostacyclin drug study [n= 1]).
Comparison of persistent and nonpersistent patients revealed no differences in 6MWD, FC, or time since last right heart catheterization; however, significant differences were observed regarding the use of specific oral PH therapies. Persistent patients were more likely to use ERA or PDE-5 inhibitor therapy at any time during the transition (P = 0.027; Table 5) and were more likely to be receiving ERA or PDE-5 inhibitor therapy at the end of the transition (P = 0.012; Fig. 4 and Table 5). Cox proportional hazards models revealed a significant association of persistence with oral therapy use compared with no oral therapy (ERA vs. no orals [P = 0.019]; PDE-5 vs. no orals [P = 0.044]; any orals vs. no orals [P = 0.004]; Fig. 5.
Table 5.
6MWD and PAH medication usage for all patients,* stratified by persistence
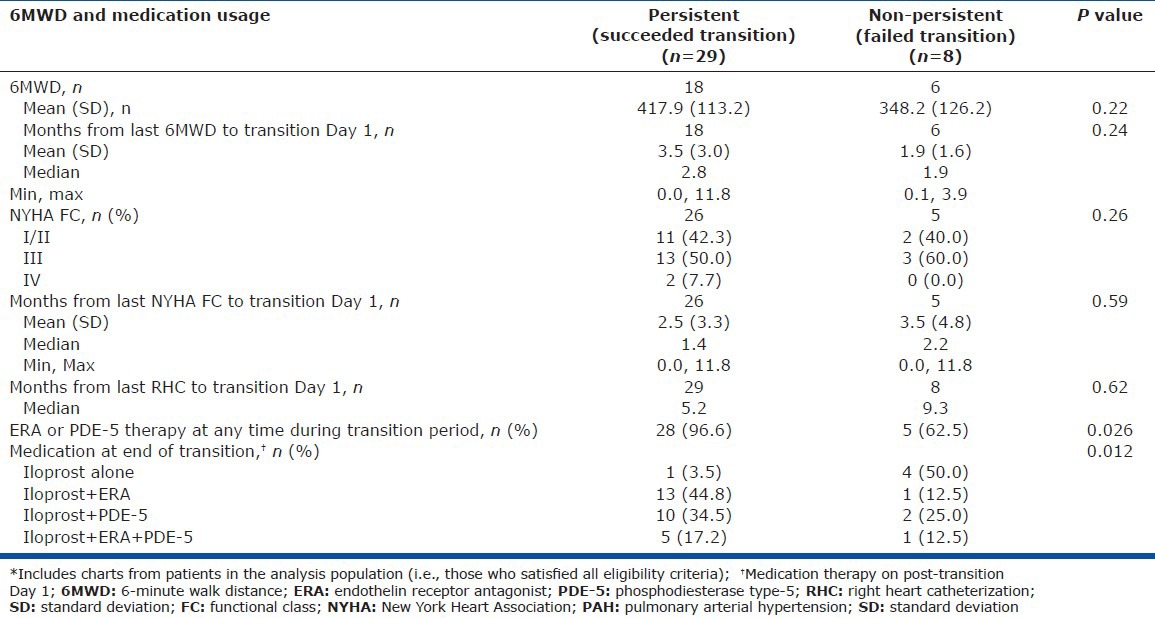
Figure 4.
One-year estimates of persistence from post-transition Day 1 in all patients were as follows: iloprost monotherapy, 20.0 ± 17.9%; iloprost + ERA, 92.9 ± 6.9%; iloprost + PDE-5, 83.3 ± 10.8%; iloprost + ERA + PDE-5, 83.3 ± 15.2%. Log-rank P value, 0.009. ERA: endothelin receptor antagonist; PDE-5: phosphodiesterase type-5.
Figure 5.
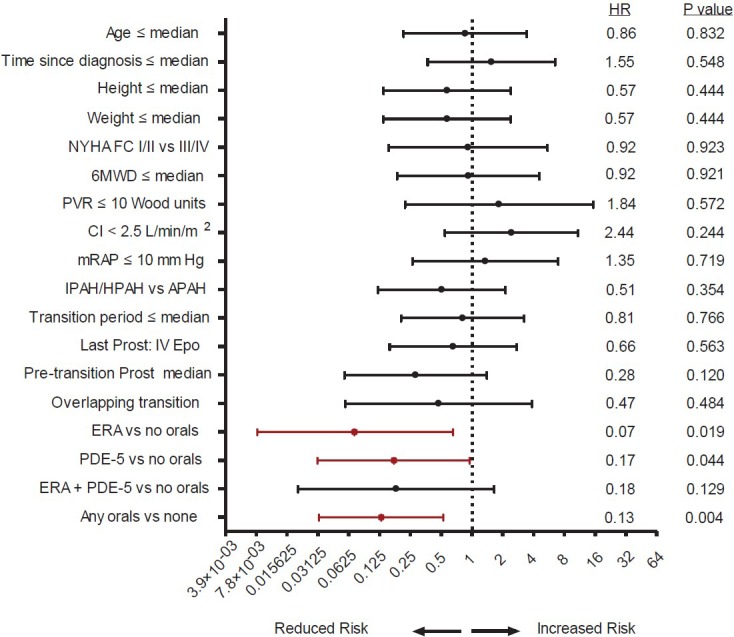
The likelihood of persistence was associated significantly with the use of oral therapy compared with no oral therapy: ERA vs. no orals (P= 0.019); PDE-5 vs. no orals (P= 0.044); any oral vs. no orals (P= 0.004). 6MWD: 6-minute walk distance; APAH: associated with pulmonary arterial hypertension; CI: cardiac index; ERA: endothelin receptor antagonist; Epo: epoprostenol; FC: functional class; HPAH: hereditary pulmonary arterial hypertension; HR: hazard ratio; IPAH: idiopathic pulmonary arterial hypertension; IV: intravenous; mRAP: mean right atrial pressure; NYHA: New York Heart Association; PDE-5: phosphodiesterase type-5; PVR: pulmonary vascular resistance.
DISCUSSION
Disease progression or improvement of the AE profile associated with therapy may prompt a transition from one therapeutic regimen to another. A recent study of 15 patients with PAH reported that transitioning from systemic prostanoids to inhaled trepostinil was well tolerated in patients who were clinically stable, the majority of whom were receiving two or three oral PAH therapies in addition to their prostacyclin therapy.[22] Although it is currently considered unadvisable to transition patients with PAH from parenteral prostacyclin to inhaled therapy, such transitions may be required due to complications associated with parenteral therapy (e.g., pump malfunctions, catheter-related infections, and/or vascular thrombosis)[5] or patient preference (quality of life). The achievement of a successful transition is an important component of effective therapy. This is the largest multicenter study to date to examine predictors of successful transition from parenteral prostacyclin to inhaled iloprost.
The majority of patients (91.9%) in this study underwent an overlapping transition period. Interestingly, among patients with an overlapping transition, a strong correlation was discovered between the use of oral PAH-specific therapies and the achievement of persistence. Persistent patients were more likely to be receiving PDE-5 inhibitors or ERA PAH-specific oral therapies at any time during and at the end of the transition period. Furthermore, among the 29 patients who successfully transitioned off parenteral therapy, four added an additional oral therapy post-transition, suggesting disease progression.
In patients with PAH, there are risks and benefits associated with the transition from parenteral prostacyclin therapy to inhaled iloprost. Associated risks include the potential for a period of inadequate treatment (treatment gap) and rebound PAH. In addition, unknown mechanisms may favor the use of parenteral prostacyclin over inhaled iloprost in certain patient populations. We speculate that such risks may provide an explanation for the unsuccessful transitions encountered in the present study. The benefits of transitioning from parenteral prostacyclin to inhaled iloprost include elimination of AEs associated with IV/SC therapy, minimization of side effects (nausea, vomiting, diarrhea, headache, or other pain), improved tolerability, and improved quality of life.
One limitation of the present study was that only patients who could be successfully transitioned for ≥ 24 hours were eligible for study inclusion. The number of patients who initiated transition but failed to achieve a transition duration of 24 hours remains unknown. In addition, the patients in this study appeared to have stable PAH, as evidenced by the frequent presence of a well-preserved mean right atrial pressure, cardiac index, and 6MWD before transition to inhaled iloprost. Furthermore, patients were on relatively low doses of parenteral prostacyclins before transition, suggesting that they may have been relatively intolerant of parenteral prostacyclins and therefore may not have been receiving optimal doses of these agents before transition. This scenario would facilitate selection of patients who are more likely to achieve a successful transition. Another limitation was that the duration of patient follow-up was limited and clinical parameters were not collected during the post-transition period. Consequently, it remains unknown whether such patients were able to continue long-term without parenteral prostacyclin treatment or whether their long-term outcomes would be improved, worsened, or equivalent to those of similar patients who remained on parenteral prostacyclin. Finally, the uncontrolled, retrospective study design precludes the establishment of firm conclusions based on the present findings. Consequently, these results are intended to facilitate the development of additional testable hypotheses and do not in any way recommend a change in patient therapy or promote transitions from parenteral therapy to inhaled iloprost monotherapy. Clinicians should continue to follow routine protocols for IV transition. Patients who are transitioned must be followed closely for evidence of deterioration that would prompt resumption of parenteral prostacyclins or the addition of other therapies.
In selected patients, transitioning from a parenteral prostacyclin to inhaled iloprost appears safe and feasible, and is associated with a high rate of long-term success. Concomitant use of oral drugs during and post-transition appeared to facilitate persistence. Additional studies are needed to define selection criteria and potentially develop a transition algorithm.
ACKNOWLEDGMENTS
Medical writing support was provided by Scarlett Geunes-Boyer, PhD, of inScience Communications, Springer Healthcare.
Footnotes
Source of Support: This study was sponsored by Actelion Pharmaceuticals US, Inc.
Conflict of Interest: Richard N. Channick has received consulting fees from Actelion and research grants from Gilead Sciences. Robert P. Frantz has received reimbursement for the cost of travel to investigator meetings from Actelion and has served as a consultant for Pfizer. Steven M. Kawut has received consulting fees, advisory board fees, speaking fees, unrestricted educational grants, and/or research funding from Pfizer, Actelion, Bayer, Novartis, Merck, Gilead, United Therapeutics, and Lung Rx. Harold Palevsky has received either consulting fees, speaking fees, and/or research funding from Actelion, Bayer, GeNO, Gilead, Lung LLC, Pfizer, and United Therapeutics. Ramagopal Tumuluri has served as a speaker for Actelion. Roxana Sulica has received research funding from Actelion, Gilead, and United Therapeutics. Paula O. Lauto has no conflicts of interest to report. Wade W. Benton is an employee of Actelion. Bennett de Boisblanc has received research support from Actelion, GlaxoSmithKline, and Ikaria.
REFERENCES
- 1.Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997;336:111–7. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 2.Sitbon O, McLaughlin VV, Badesch DB, Barst RJ, Black C, Galiè N, et al. Survival in patients with class III idiopathic pulmonary arterial hypertension treated with first line oral bosentan compared with an historical cohort of patients started on intravenous epoprostenol. Thorax. 2005;60:1025–30. doi: 10.1136/thx.2005.040618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLaughlin V, Humbert M, Coghlan G, Nash P, Steen V. Pulmonary arterial hypertension: the most devastating vascular complication of systemic sclerosis. Rheumatology (Oxford) 2009;48(Suppl 3):iii25–31. doi: 10.1093/rheumatology/kep107. [DOI] [PubMed] [Google Scholar]
- 4.Rubin LJ, Mendoza J, Hood M, McGoon M, Barst R, Williams WB, et al. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Ann Intern Med. 1990;112:485–91. doi: 10.7326/0003-4819-112-7-485. [DOI] [PubMed] [Google Scholar]
- 5.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 6.Badesch DB, Tapson VF, McGoon MD, Brundage BH, Rubin LJ, Wigley FM, et al. Continuous IV epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann Intern Med. 2000;132:425–34. doi: 10.7326/0003-4819-132-6-200003210-00002. [DOI] [PubMed] [Google Scholar]
- 7.Simonneau G, Barst RJ, Galiè N, Naeije R, Rich S, Bourge RC, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800–4. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 8.Tapson VF, Gomberg-Maitland M, McLaughlin VV, Benza RL, Widlitz AC, Krichman A, et al. Safety and efficacy of IV treprostinil for pulmonary arterial hypertension: A prospective, multicenter, open-label, 12-week trial. Chest. 2006;129:683–8. doi: 10.1378/chest.129.3.683. [DOI] [PubMed] [Google Scholar]
- 9.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 10.Barst RJ, Galiè N, Naeije R, Simonneau G, Jeffs R, Arneson C, et al. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur Respir J. 2006;28:1195–203. doi: 10.1183/09031936.06.00044406. [DOI] [PubMed] [Google Scholar]
- 11.Barst RJ, Gibbs JS, Ghofrani HA, Hoeper MM, McLaughlin VV, Rubin LJ, et al. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S78–84. doi: 10.1016/j.jacc.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olschewski H, Simonneau G, Galiè N, Higenbottam T, Naeije R, Rubin LJ, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–9. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 13.Barst RJ, Rubin LJ, McGoon MD, Caldwell EJ, Long WA, Levy PS. Survival in primary pulmonary hypertension with long-term continuous intravenous prostacyclin. Ann Intern Med. 1994;121:409–15. doi: 10.7326/0003-4819-121-6-199409150-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kallen AJ, Lederman E, Balaji A, Trevino I, Petersen EE, Shoulson R, et al. Bloodstream infections in patients given treatment with intravenous prostanoids. Infect Control Hosp Epidemiol. 2008;29:342–9. doi: 10.1086/529552. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC) Bloodstream infections among patients treated with intravenous epoprostenol or intravenous treprostinil for pulmonary arterial hypertension-seven sites, United States, 2003-2006. MMWR Morb Mortal Wkly Rep. 2007;56:170–2. [PubMed] [Google Scholar]
- 16.Rich JD, Glassner C, Wade M, Coslet S, Arneson C, Doran A, et al. The effect of diluent pH on bloodstream infection rates in patients receiving IV treprostinil for pulmonary arterial hypertension. Chest. 2012;141:36–42. doi: 10.1378/chest.11-0245. [DOI] [PubMed] [Google Scholar]
- 17.Kitterman N, Poms A, Miller DP, Lombardi S, Farber HW, Barst RJ. Bloodstream infections in patients with pulmonary arterial hypertension treated with intravenous prostanoids: insights from the REVEAL REGISTRY®. Mayo Clin Proc. 2012;87:825–34. doi: 10.1016/j.mayocp.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barst RJ. Diagnosis and treatment of pulmonary artery hypertension. Curr Opin Pediatr. 1996;8:512–9. doi: 10.1097/00008480-199610000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Ivy DD, Doran AK, Smith KJ, Mallory GB, Jr, Beghetti M, Barst RJ, et al. Short- and long-term effects of inhaled iloprost therapy in children with pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51:161–9. doi: 10.1016/j.jacc.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schenk P, Petkov V, Madl C, Kramer L, Kneussl M, Ziesche R, et al. Aerosolized iloprost therapy could not replace long-term IV epoprostenol (prostacyclin) administration in severe pulmonary hypertension. Chest. 2001;119:296–300. doi: 10.1378/chest.119.1.296. [DOI] [PubMed] [Google Scholar]
- 21.Reddy MT, Patel H, Ventura HO. Successful transition from intravenous to inhaled prostacyclin in a patient with pulmonary hypertension and right ventricular failure. Congest Heart Fail. 2008;14:285–6. doi: 10.1111/j.1751-7133.2008.00006.x. [DOI] [PubMed] [Google Scholar]
- 22.de Jesus Perez VA, Rosenzweig E, Rubin LJ, Poch D, Bajwa A, Park M, et al. Safety and efficacy of transition from systemic prostanoids to inhaled treprostinil in pulmonary arterial hypertension. Am J Cardiol. 2012;110:1546–50. doi: 10.1016/j.amjcard.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Cramer JA, Bradley-Kennedy C, Scalera A. Treatment persistence and compliance with medications for chronic obstructive pulmonary disease. Can Respir J. 2007;14:25–9. doi: 10.1155/2007/161652. [DOI] [PMC free article] [PubMed] [Google Scholar]


