Abstract
PH occurs alone or in association with many disorders. Many patients with transthoracic echocardiography (TTE) findings suggesting PH never receive additional evaluation. Patient characteristics and echocardiographic data associated with increased recognition of PH have not been fully evaluated. We evaluated TTE reports at the Cincinnati Veterans Affairs Medical Center from 2005 to 2006 retrospectively for findings highly indicative of PH: Estimated systolic pulmonary artery pressure (sPAP) ≥40 mmHg, increased right atrial or right ventricular (RV) size, or reduced RV function. Only patients with left ventricular ejection fraction (LVEF) ≥50% and no known diagnosis of PH were included. Patient characteristics, TTE findings, provider recognition rates, and subsequent referral for additional evaluation were assessed. A total of 227 of 3,960 (5.7%) TTE reports revealed findings indicating possible PH. Providers acknowledged possible PH in 53 (23.4%) reports. Recognized PH was predicted by increased RV size (odds ratio (OR) = 5.07, P < 0.001), increased right atrial dimension (OR = 6.45, P < 0.001), decreased RV function (OR = 8.86, P < 0.001), and increased PAP (OR = 1.04 corresponding to each unit increase of PAP, P < 0.01). Patients with comorbid obstructive sleep apnea (OSA), interstitial lung disease, and dyspnea were also more likely to be recognized (OR = 3.63, P = 0.021; OR = 10.98, P = 0.004; OR = 2.39, P = 0.007, respectively). The 12-month mortality rate for recognized patients, 11.3% (7/53), was lower than for unrecognized patients, 25.3% (44/174; P = 0.03). Providers recognized less than one in four patients with echocardiographic evidence suggesting PH. Echocardiography reports revealing higher PAP and right heart dilation and dysfunction are associated with increased acknowledgement of possible PH.
Keywords: disease recognition, echocardiography, pulmonary hypertension
Elevation of pulmonary vascular pressures, pulmonary hypertension (PH), is associated with multiple disorders and may cause multiple nonspecific clinical symptoms, radiographic findings, and electrocardiographic changes.[1,2,3] Effective therapies make the early recognition and diagnosis of PH increasingly important, as diagnostic delays are associated with increased mortality.[4,5] Transthoracic Doppler echocardiography (TTE) is a critical tool for the evaluation of suspected PH.[6] Echocardiographic findings of elevated estimated systolic pulmonary artery pressure (sPAP) are frequently the stimulus for right heart catheterization (RHC) to diagnose PH definitively and to determine whether the elevated pressures are due to pulmonary artery hypertension (PAH) or other processes.
Recent analysis of the Registry to Evaluate Early and Long-term Pulmonary Artery Hypertension Disease Management showed that symptoms began more than two years before diagnosis in 21.1% of patients with PAH.[7] The most common symptoms were exertional breathlessness, fatigue, and chest discomfort. Patient characteristics associated with delayed diagnosis included age < 36 years and comorbidities of chronic obstructive pulmonary disease (COPD) or obstructive sleep apnea (OSA). Although echocardiography is frequently an initial study in the evaluation of patients with dyspnea on exertion or chest discomfort and is often the first study to suggest the presence of PH,[8] providers’ acknowledgement of TTE findings suggesting PH is not well studied.
To determine provider recognition rates of possible PH identified by TTE and the clinical and echocardiographic variables associated with PH recognition, we investigated clinicians’ responses to echocardiography reports indicating elevated sPAP.
MATERIALS AND METHODS
This study was conducted at the Cincinnati Veterans Administration Medical Center (VAMC) after approval from the University of Cincinnati Internal Review Board (UC IRB no. 08100301) and the Cincinnati VAMC Research and Development Committee.
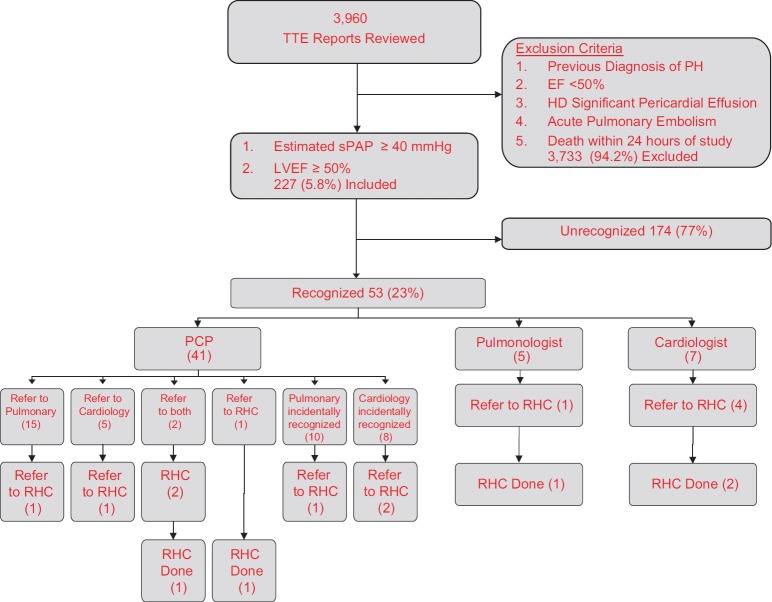
We retrospectively reviewed reports from 3,960 TTEs performed between January 1, 2005 and December 31, 2006. We used a high echocardiographically estimated sPAP threshold, ≥ 40 mmHg, to identify those patients whose PH may warrant further evaluation. Patients with diminished left ventricular systolic function (LVSF), LV ejection fraction (LVEF) < 50%, were excluded to decrease PH that may be caused by left heart disease. When a range for either of these variables was listed, the lower end of the range needed to be ≥ 40 mm Hg or ≥ 50%, respectively. LVEF reported as “normal,” was assumed to be at least 50%. Diastolic dysfunction (DD) was noted but did not exclude patients (Fig. 1).[9] The magnitude of right sided dilation and RV dysfunction was not qualified due to the lack of standardized reporting. TTEs performed on patients with (1) an established diagnosis of PH, (2) acute pulmonary embolism, or (3) immediately after cardiopulmonary resuscitation and TTEs demonstrating hemodynamically significant pericardial effusions were excluded. If a patient had multiple TTEs performed during the study period, only the first study demonstrating evidence of PH was included.
Figure 1.
Flow diagram of study population
At the Cincinnati VAMC, all echocardiograms required completion of a request form recording the study indication including a specific interest in “pulmonary hypertension” and/or “RV function” as well as patient symptoms: Dyspnea, chest pain, edema, and syncope. Age, race, body mass index, smoking status, valvular abnormalities, and relevant comorbidities were also recorded.
We reviewed the electronic medical record of all patients with echocardiograms meeting inclusion criteria for evidence that a provider acknowledged the TTE abnormalities suggesting PH. We evaluated all provider notes written after the echocardiogram and before February 1, 2009 for any of these phrases suggesting the recognition of possible PH: “Pulmonary hypertension,” “right heart failure,” “right heart overload,” “right heart strain,” “elevated pulmonary pressures,” “abnormal pulmonary pressures,” or “cor pulmonale.” If any of these phrases or other reference to abnormal right heart findings appeared in a note, we considered the provider to have recognized possible PH. We determined who acknowledged the TTE results and what further evaluation, if any, was pursued. We did not assess PH management. The duration of follow-up ranged from 25 to 37 months.
Echocardiography
Each TTE was performed using a Philips Sonos 5500 or 7500 Echocardiogram Platform. sPAP was estimated by measuring the right ventricle-right atrium (RV-RA) pressure difference using the modified Bernoulli equation (4v2, v is the highest observed velocity of the tricuspid regurgitant jet) and adding the estimated RA pressure determined by assessing RA size and the size and collapsibility of the inferior vena cava. LVEF was determined by visual estimation. Sonographers measured diastolic parameters using spectral Doppler data assessing mitral valve inflow in conjunction with tissue Doppler measurements obtained at the lateral and septal portions of the mitral annulus. Each cardiologist assessed DD using standard criteria.[10] During the study period, eight cardiologists with level 2 or 3 echocardiographic training interpreted TTEs. All reports used a standardized template.
Statistical methods
The binary outcome of recognized PH was predicted by individual clinical factors using both unadjusted and adjusted methods to compute logistical regression models. The unadjusted method used one factor or predictor at a time to predict the outcome; in the adjusted method, patient's demographics were added as controlling covariates to adjust for prediction of the factor in the logistical model. One-year survival rates were estimated using Kaplan-Meier curves and compared using a logrank test. Numerical variables were summarized using medians (ranges), and compared between groups using Wilcoxon rank sum tests. Categorical variables were summarized using frequencies (%) and compared between groups using Fisher's exact tests. All statistical computations were performed using SAS 9.2 (SAS, Cary, NC). P < 0.05 were considered statistically significant.
RESULTS
Clinical characteristics and TTE findings
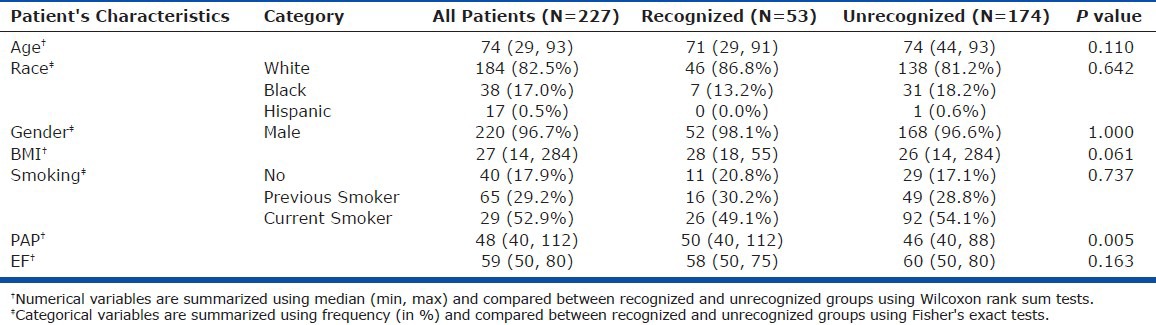
Of the 3,960 TTE reports, 227 (5.7%) contained findings suggesting PH, preserved LVEF, and no excluding conditions (Fig. 1). These 227 patients formed the study group. Their demographics and clinical characteristics are presented in Table 1. Providers acknowledged elevated pulmonary pressures in 53 of the 227 patients (23.3%).
Table 1.
Summary of patient characteristics
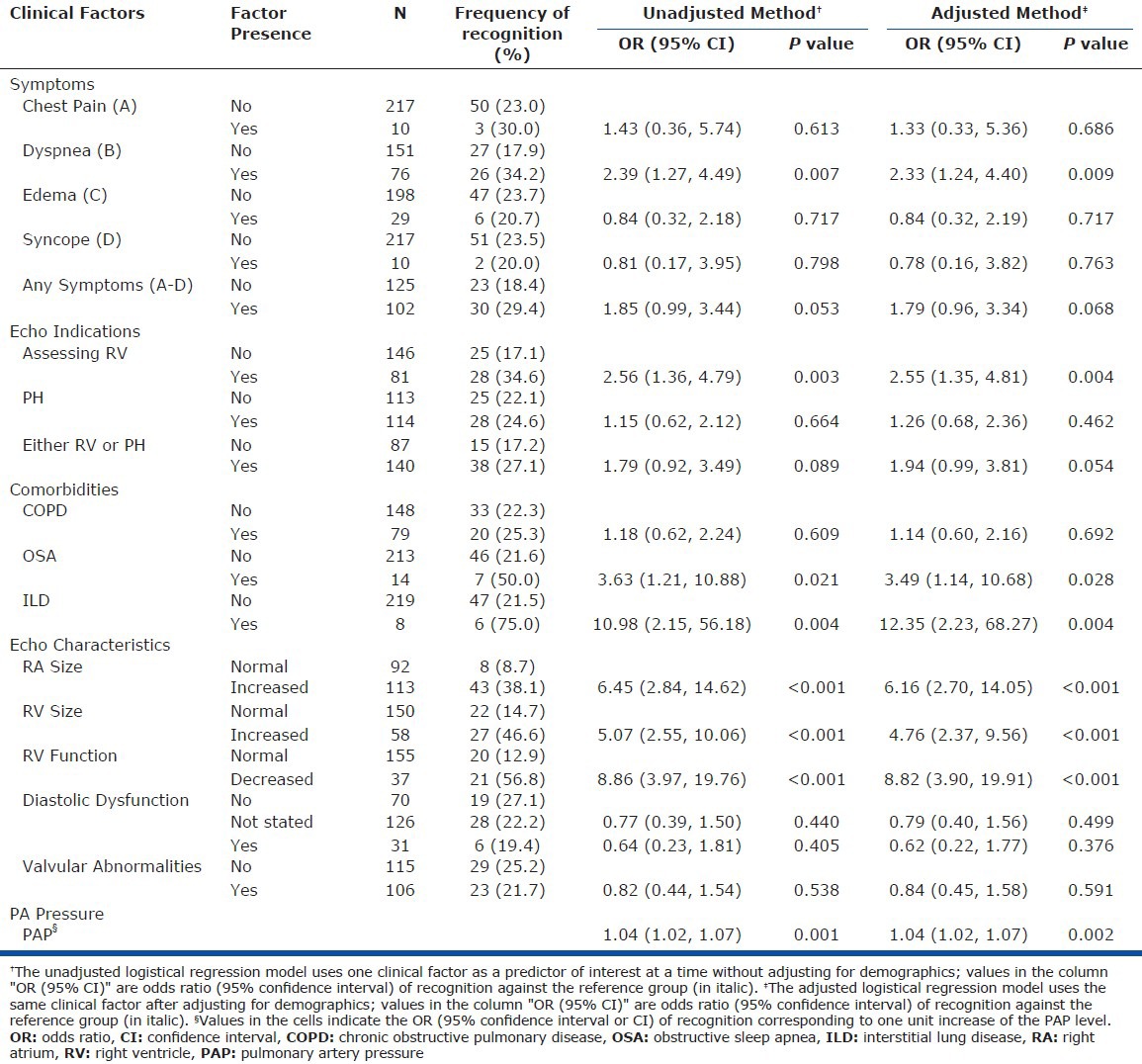
Among the 81 patients who underwent TTE for an indication of RV assessment, findings of PH were acknowledged in 28 (34.6%) patients. The OR of PH recognition was 2.56 (P = 0.003) for the TTE indication of RV assessment. TTEs performed to assess for PH were not associated with an increased acknowledgement of the presence of findings suggesting PH (P = 0.664).
TTE findings suggesting PH were acknowledged in 26 of 76 patients (34.2%) with dyspnea but in only 27 of 151 (17.9%) individuals who were not breathless (Table 2). The OR of PH recognition was 2.39 (P = 0.007) for the presence of dyspnea. Chest pain, edema, and syncope were not associated with the acknowledgement of possible PH. Among comorbidities, both OSA and interstitial lung disease (ILD) positively predicted PH recognition, with ORs of 3.63 (P = 0.021) and 10.98 (P = 0.004), respectively.
Table 2.
Odds ratio of recognition predicted by clinical factors
The median estimated sPAP was significantly higher in the recognized compared with the unrecognized cohort (50 vs 46 mmHg, P = 0.005). Among TTEs with sPAPs ≥50 mmHg (n = 100), 29 were recognized whereas 71 were unrecognized (Table 1). sPAP was a significant predictor of PH recognition, the OR (95% confidence interval [CI] of recognition was 1.04 (1.02, 1.07) for each unit increase in the sPAP (P = 0.001).
Providers were more likely to recognize PH when RA or RV size was increased (OR = 5.07 and 6.45, respectively) or RV function was impaired (OR = 8.86, P < 0.001) for all three findings. A total of 46 of the 53 (86.8%) patients with acknowledged PH had at least one of these characteristics. Among patients with RV dysfunction (n = 20), the median estimated sPAP was 61 mmHg. Some degree of DD occurred in more than half of the patients in each group but did not correlate with patterns of recognition.
Post-TTE evaluation
The type of ordering provider and what diagnostic action was taken are shown in Figure 1. Primary care providers (PCP) who ordered the TTE and recognized PH (41/53) made a subspecialty referral to either pulmonology (15/41), cardiology (5/41), or to both (2/41). One patient was referred directly by the PCP to RHC (1/41). No PCP decided on watchful waiting or follow up TTE. Eighteen patients (34%) were followed by a pulmonologist or cardiologist for a non-PH related disease and the subspecialist incidentally recognized the abnormal TTE whereas the PCP who ordered the test did not. A pulmonologist or a cardiologist ordered the TTE and recognized findings of PH in twelve patients. The median time between TTE performance and provider recognition was 17 days (range 0-933, mean 151 days). For studies not recognized within thirty days (n = 25), the median time to recognition was 219 days (range 43-933).
Twenty of the recognized patients’ TTE reports were evaluated by cardiologists, and seven (35%) were referred for RHC but only 43% (3/7) of the RHCs were performed. Pulmonologists evaluated thirty TTE reports and referred three (10%) to RHC. Of the remaining three patients, one was referred directly by the PCP and the other two were comanaged by a pulmonologist and cardiologist. The 13 patients who were referred for RHC had a median sPAP of 59 mmHg.
Among the five patients who had an RHC, one had an elevated mean PAP of 50 mmHg with a pulmonary capillary wedge pressure (PCWP) of 25 mmHg attributed to left heart failure and one had PAH, mean PAP 51 mmHg, attributed to pulmonary vascular disease associated with his known HIV. The other three patients had a normal mean PAP.
The all-cause, 12-month mortality rate was 11.3% (7/53) for recognized patients and 25.3% (44/174) for unrecognized patients (P = 0.03) (Fig. 2). Mortality rates at the end of 24 months were 22.6% (12/53) and 33.3% (58/174) for recognized and unrecognized patients, respectively, (P = 0.17).
Figure 2.
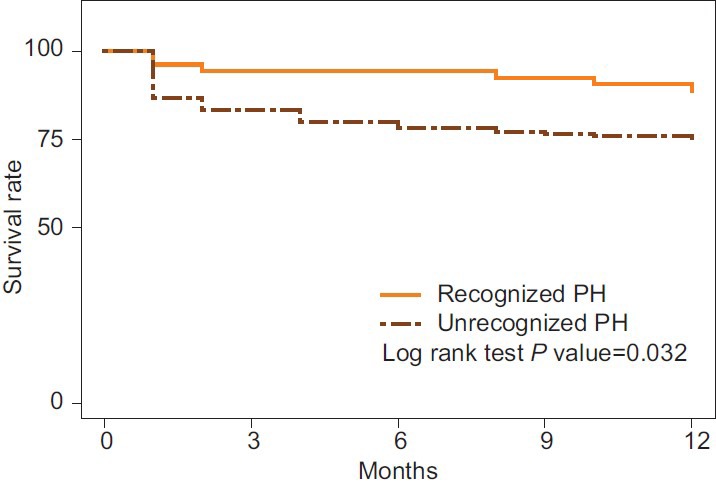
Kaplan-Meier curve of all cause 12 month mortality in the recognized and unrecognized groups.
DISCUSSION
Recent insights into the pathophysiology of PAH have led to the development of effective therapies that improve survival and quality of life (QoL).[3] Before treatment can be initiated, PAH must be recognized and diagnosed. The most common clinical symptoms of PAH—breathlessness, fatigue, syncope, and chest discomfort—are nonspecific and are often attributed to other disorders. PAH is frequently associated with other pulmonary and cardiac diseases that may further confound its identification. RHC is necessary to confirm the presence of PAH, but TTE is frequently used as an initial, noninvasive screening study. In our review of 3,960 TTE reports, 5.7% demonstrated findings suggesting PH. However, PH was recognized in only 23.4% of these cases and far fewer patients underwent RHC for definitive diagnosis. The mean time between TTE performance and PH recognition was 151 days. In a disease that can be rapidly progressive, earlier recognition and initiation of effective therapy may improve survival and QoL.
Under-recognition of disorders occurs frequently and is a common cause of medical errors. Investigations of myocardial infarction, pulmonary embolism, depression, and COPD suggest atypical manifestations, disease processes with overlapping symptoms, confounding comorbidities, and potentially inaccurate screening modalities contribute to disease under-recognition.[11,12,13,14,15,16,17,18] As with these other under-recognized disorders, there is no defining constellation of symptoms that clearly indicates the presence of PH.
In our cohort, dyspnea correlated with increased recognition of PH. Breathlessness is the most common presenting symptom in PAH.[19] Chest pain, edema, and syncope are manifestations of more advanced disease[6] but were not associated with increased recognition. Even though breathlessness is correlated with the recognition of PH, this symptom is not specific and may be caused by a myriad of other disorders. Additionally, providers recognized PH more frequently when either OSA or ILD was present, whereas comorbid COPD, DD, or valvular heart disease did not affect PH recognition. These correlations may reflect the provider's knowledge of symptoms and conditions associated with PH.
Unawareness of guidelines may also lead to disease under-recognition, as demonstrated by the chronic kidney disease (CKD) population.[20,21,22,23] Glomerular filtration rate (GFR) rather than creatinine level is recommended for the accurate diagnosis of CKD.[24] Providers face a bigger challenge when interpreting TTE reports because there are no widely accepted, evidence-based guidelines for the echocardiographic recognition of PH. Recently, the European Society of Cardiology and European Respiratory Society (ESC/ERS) released “arbitrary criteria” for echocardiographic evidence of PH.[25] Assuming RA pressures of 5 mmHg, an estimated sPAP ≤36 mmHg was classified as “PH unlikely;” an sPAP of 37-50 mmHg was classified as “PH possible;” and an sPAP of >50 mmHg was classified as “PH likely.” This classification, in addition to previously published values for PA pressures in echocardiographically normal patients,[26] supports our use of 40 mmHg as the threshold value that should alert the provider of abnormally elevated pulmonary pressures.
Our findings suggest that clinicians are more likely to recognize PH when the estimated sPAP exceeds 50 mmHg, correlating with the ESC/ERS “PH likely” category. Although the median estimated sPAP was significantly higher in the recognized compared with the unrecognized cohort (50 vs 46 mmHg, P = 0.005), this 4 mmHg difference may not be clinically significant and is likely within the range of error of the echocardiographic estimation of sPAP. Interestingly, the sPAP threshold of 40-50 mmHg is very similar to the conclusions of studies of patients with systemic sclerosis, which suggest that a TTE-estimated sPAP of 45-50 mmHg should trigger RHC.[27,28] In addition, we did find that the odds of PH recognition increased by 1.04 for every 1 mmHg rise in the estimated sPAP. Although our findings are concordant with the ECS/ERS classification, further clinical investigations are needed to establish evidence-based guidelines for TTE findings that should provoke further diagnostic evaluation. A decision analysis algorithm will need to account for patient age, gender, weight, and level of activity, all of which can influence expected PA pressures.
The National Health and Nutrition Examination Survey showed that reporting format is another important component of disease recognition.[22] Providers often use serum creatinine rather than GFR to assess renal function causing the underdiagnosis of CKD in older patients.[29] In our study, echocardiographic data were reported in a standardized computerized template, but the content of the report summary was at the discretion of the interpreting cardiologist and consequently contained variable details about right heart function and size. As clinicians review TTE reports, they may be reading the summary and missing important information that is embedded amidst the technical details of the TTE. Further, we observed no better recognition of PH by providers who specifically requested right heart data or estimation of PAP than by those who requested TTEs for other reasons. Thus, TTE findings of PH may be ignored or missed even by clinicians who suspect PH. The use of a standardized TTE summary that includes an evaluation of sPAP and right heart function might improve the acknowledgement of findings suggesting PH and increase the number of patients who undergo prompt further evaluation.
Once a provider recognized abnormal TTE findings suggesting PH, only one quarter of these patients were referred for further evaluation. Several reasons may explain this low referral rate. First, sPAP measured by TTE is an estimate of pressures measured by RHC.[6] Primary care providers may be reluctant to refer all patients with abnormal TTE findings to a specialist due to a perceived unacceptably high rate of discordance between TTE findings and RHC proven PH. Similarly, this imperfect correlation may dissuade specialists from ordering an RHC given the risk of procedural complications. Some providers may have attributed the elevated sPAP to concurrent disorders that might cause PH and decided not to pursue further evaluation. Finally, providers who are unsure of the next appropriate diagnostic step or who do not have relationships with PH specialists may forgo further evaluation.
Limitations
We conducted a single institution retrospective chart review. Our study population limits the application of our findings to the general population. The prevalence of PAH among our patients, though unknown, is likely less than the general population, given that most VAMC patients are male and PAH predominantly affects females. Additionally, these patients have a high prevalence of left heart disease, increasing the risk of associated post-capillary pulmonary hypertension. Although TTEs were performed and read in a standardized fashion, five cardiac sonographers and eight cardiologists were involved during the study period. We were unable to determine inter- and intra-observer variability as several sonographers and cardiologists no longer work at our institution. A standardized report template was used, but each report summary and the emphasis of abnormal results were variable. Although most veterans receive the majority of their medical care within the VHA, we were unable to account for recognition of elevated sPAP by non-VA providers and, therefore, may have underestimated the actual acknowledgement rate. The majority of the patients were older men with significant comorbidities including OSA and COPD that may have influenced the results. Finally, the VHA utilizes a comprehensive EMR that incorporates all medical tests including echocardiography reports; this level of access to medical information may increase the access to echocardiography reports and influence the recognition of findings suggesting PH.
Future directions
Guidelines to assist providers in the interpretation of echocardiographic evidence of PH including a predictive tool that incorporates comorbidities, symptoms, and clinical suspicion should be validated in a prospective trial. In addition, adherence to the guidelines for the echocardiographic reporting of abnormal right heart indices may improve provider recognition of PH.[30]
Footnotes
Source of Support: None
Conflict of Interest: None declared.
REFERENCES
- 1.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 2.Runo JR, Loyd JE. Primary pulmonary hypertension. Lancet. 2003;361:1533–44. doi: 10.1016/S0140-6736(03)13167-4. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: Developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–94. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: The impact of epoprostenol therapy. Circulation. 2002;106:1477–82. doi: 10.1161/01.cir.0000029100.82385.58. [DOI] [PubMed] [Google Scholar]
- 5.Provencher S, Jais X, Yaici A, Sitbon O, Humbert M, Simonneau G. Clinical challenges in pulmonary hypertension: Roger S. Mitchell lecture. Chest. 2005;128:622–8S. doi: 10.1378/chest.128.6_suppl.622S-a. [DOI] [PubMed] [Google Scholar]
- 6.Rubin LJ. American College of Chest Physicians. Diagnosis and management of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:7–10S. doi: 10.1378/chest.126.1_suppl.7S. [DOI] [PubMed] [Google Scholar]
- 7.Brown LM, Chen H, Halpern S, Taichman D, McGoon MD, Farber HW. Delay in recognition of pulmonary arterial hypertension: Factors identified from the REVEAL Registry. Chest. 2011;140:19–26. doi: 10.1378/chest.10-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houtchens J, Martin D, Klinger JR. Diagnosis and management of pulmonary arterial hypertension. Pulm Med. 2011;2011:845864. doi: 10.1155/2011/845864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez VA, Haddad F, Zamanian RT. Diagnosis and management of pulmonary hypertension associated with left ventricular diastolic dysfunction. Pulm Circ. 2012;2:163–9. doi: 10.4103/2045-8932.97598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khouri SJ, Maly GT, Suh DD, Walsh TE. A practical approach to the echocardiographic evaluation of diastolic function. J Am Soc Echocardiogr. 2004;17:290–7. doi: 10.1016/j.echo.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Tiemens BG, Ormel J, Simon GE. Occurrence, recognition, and outcome of psychological disorders in primary care. Am J Psychiatry. 1996;153:636–44. doi: 10.1176/ajp.153.5.636. [DOI] [PubMed] [Google Scholar]
- 12.de Torbal A, Boersma E, Kors JA, van Herpen G, Deckers JW, van der Kuip DA, et al. Incidence of recognized and unrecognized myocardial infarction in men and women aged 55 and older: The Rotterdam Study. Eur Heart J. 2006;27:729–36. doi: 10.1093/eurheartj/ehi707. [DOI] [PubMed] [Google Scholar]
- 13.Ryu JH, Olson EJ, Pellikka PA. Clinical recognition of pulmonary embolism: Problem of unrecognized and asymptomatic cases. Mayo Clin Proc. 1998;73:873–9. doi: 10.4065/73.9.873. [DOI] [PubMed] [Google Scholar]
- 14.Yamasaki A, Hashimoto K, Hasegawa Y, Okazaki R, Yamamura M, Harada T, et al. COPD is frequent in conditions of comorbidity in patients treated with various diseases in a university hospital. Int J Chron Obstruct Pulmon Dis. 2010;5:351–5. doi: 10.2147/COPD.S12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tapson VF, Humbert M. Incidence and prevalence of chronic thromboembolic pulmonary hypertension: From acute to chronic pulmonary embolism. Proc Am Thorac Soc. 2006;3:564–7. doi: 10.1513/pats.200605-112LR. [DOI] [PubMed] [Google Scholar]
- 16.Sartorius N, Ustün TB, Costa e Silva JA, Goldberg D, Lecrubier Y, Ormel J, et al. An international study of psychological problems in primary care. Preliminary report from the World Health Organization Collaborative Project on ‘Psychological Problems in General Health Care’. Arch Gen Psychiatry. 1993;50:819–24. doi: 10.1001/archpsyc.1993.01820220075008. [DOI] [PubMed] [Google Scholar]
- 17.Sigurdsson E, Thorgeirsson G, Sigvaldason H, Sigfusson N. Unrecognized myocardial infarction: Epidemiology, clinical characteristics, and the prognostic role of angina pectoris. The Reykjavik Study. Ann Intern Med. 1995;122:96–102. doi: 10.7326/0003-4819-122-2-199501150-00003. [DOI] [PubMed] [Google Scholar]
- 18.Tylee A, Walters P. Underrecognition of anxiety and mood disorders in primary care: Why does the problem exist and what can be done? J Clin Psychiatry. 2007;68(Suppl 2):27–30. [PubMed] [Google Scholar]
- 19.Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107:216–23. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 20.Ryan TP, Sloand JA, Winters PC, Corsetti JP, Fisher SG. Chronic kidney disease prevalence and rate of diagnosis. Am J Med. 2007;120:981–6. doi: 10.1016/j.amjmed.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 21.McClellan WM, Knight DF, Karp H, Brown WW. Early detection and treatment of renal disease in hospitalized diabetic and hypertensive patients: Important differences between practice and published guidelines. Am J Kidney Dis. 1997;29:368–75. doi: 10.1016/s0272-6386(97)90197-9. [DOI] [PubMed] [Google Scholar]
- 22.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 23.Ouseph R, Hendricks P, Hollon JA, Bhimani BD, Lederer ED. Under-recognition of chronic kidney disease in elderly outpatients. Clin Nephrol. 2007;68:373–8. doi: 10.5414/cnp68373. [DOI] [PubMed] [Google Scholar]
- 24.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 25.Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC), European Respiratory Society (ERS), International Society of Heart and Lung Transplantation (ISHLT) Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219–63. doi: 10.1183/09031936.00139009. [DOI] [PubMed] [Google Scholar]
- 26.McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104:2797–802. doi: 10.1161/hc4801.100076. [DOI] [PubMed] [Google Scholar]
- 27.Launay D, Mouthon L, Hachulla E, Pagnoux C, de Groote P, Remy-Jardin M, et al. Prevalence and characteristics of moderate to severe pulmonary hypertension in systemic sclerosis with and without interstitial lung disease. J Rheumatol. 2007;34:1005–11. [PubMed] [Google Scholar]
- 28.Proudman SM, Stevens WM, Sahhar J, Celermajer D. Pulmonary arterial hypertension in systemic sclerosis: The need for early detection and treatment. Intern Med J. 2007;37:485–94. doi: 10.1111/j.1445-5994.2007.01370.x. [DOI] [PubMed] [Google Scholar]
- 29.Swedko PJ, Clark HD, Paramsothy K, Akbari A. Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med. 2003;163:356–60. doi: 10.1001/archinte.163.3.356. [DOI] [PubMed] [Google Scholar]
- 30.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]