Abstract
Hypoxic pulmonary vasoconstriction (HPV) is a compensatory physiological mechanism in the lung that optimizes the matching of ventilation to perfusion and thereby maximizes gas exchange. Historically, HPV has been primarily studied in isolated perfused/ventilated lungs; however, the results of these studies have varied greatly due to different experimental conditions and species. Therefore, in the present study, we utilized the mouse isolated perfused/ventilated lung model for investigation of the role of extracellular Ca2+ and caveolin-1 and endothelial nitric oxide synthase expression on HPV. We also compared HPV using different perfusate solutions: Physiological salt solution (PSS) with albumin, Ficoll, rat blood, fetal bovine serum (FBS), or Dulbecco's Modified Eagle Medium (DMEM). After stabilization of the pulmonary arterial pressure (PAP), hypoxic (1% O2) and normoxic (21% O2) gases were applied via a ventilator in five-minute intervals to measure HPV. The addition of albumin or Ficoll with PSS did not induce persistent and strong HPV with or without a pretone agent. DMEM with the inclusion of FBS in the perfusate induced strong HPV in the first hypoxic challenge, but the HPV was neither persistent nor repetitive. PSS with rat blood only induced a small increase in HPV amplitude. Persistent and repetitive HPV occurred with PSS with 20% FBS as perfusate. HPV was significantly decreased by the removal of extracellular Ca2+ along with addition of 1 mM EGTA to chelate residual Ca2+ and voltage-dependent Ca2+ channel blocker (nifedipine 1 μM). PAP was also reactive to contractile stimulation by high K+ depolarization and U46619 (a stable analogue of thromboxane A2). In summary, optimal conditions for measuring HPV were established in the isolated perfused/ventilated mouse lung. Using this method, we further confirmed that HPV is dependent on Ca2+ influx.
Keywords: hypoxia, pulmonary vasoconstriction, mouse lung, pulmonary artery, endothelial nitric oxide synthase
Hypoxic pulmonary vasoconstriction (HPV), which was first described in 1946 by von Euler and Liljestrand,[1] is an essential physiological response in the lung that optimizes the ventilation/perfusion ratio and maximizes oxygenation of venous blood. Although it has been extensively studied,[2,3] the cellular mechanism involved in HPV is still unclear. One of the hypotheses for the cellular and molecular mechanism of HPV is that the inhibition of voltage-gated K+ channels (Kv) in pulmonary arterial smooth muscle cells (PASMCs) causes membrane depolarization which subsequently leads to Ca2+ influx by the activation of voltage-dependent Ca2+ channel (VDCC).[4,5,6] An increase in the cytosolic Ca2+ concentration [Ca2+]cyt) in PASMCs is a key stimulus for vasoconstriction.[7] In addition, various molecules, ion channels, and signaling pathways have been suggested with regards to the oxygen sensing mechanism of HPV.[3,8,9,10,11,12,13,14,15,16]
To investigate the precise mechanisms of HPV, researchers have employed a variety of methods including intact animals, isolated lungs, isolated pulmonary arteries, isolated PASMCs, and endothelial cells.[4,10,11,16,17,18,19,20,21] The pioneering studies identifying the role of Ca2+ in HPV were conducted by McMurtry et al.[22,23,24,25] in the 1970s using isolated rat lungs. Studies conducted in isolated cells or vessels provide valuable information about the response of individual proteins or genes in specific cell types. However, the results from studies using cells in a culture dish may be vastly different from cells in their physiological setting. The advantage of using the isolated perfused/ventilated lung system is that it excludes the impact of other systemic organs or tissues[26] while maintaining the lung intact and allowing for physiological transport of extracellular substrates via capillary vasculature.[27] Although acute hypoxia causes vasoconstriction in isolated arterial rings and increases [Ca2+]cyt in isolated PASMCs,[2,28,29,30,31] the kinetics of the acute hypoxic response in arterial rings and single cells is rather different from HPV in intact animals and humans. In isolated perfused/ventilated lung preparations, the kinetics and oxygen sensitivity (or the threshold of PO2 that causes vasoconstriction) of HPV are both similar to those observed in intact animals and humans. Therefore, many researchers have used this method to study cellular and molecular mechanisms of HPV and evaluate pulmonary function associated with cardiopulmonary diseases such as pulmonary hypertension, right heart failure, and chronic obstructive pulmonary disease.[32,33,4,35,36,37]
Researchers studying HPV in the isolated lung system have used various animal models such as pigs, sheep, lambs, canines, rabbits, rats, and mice.[21,38,39,40,41,42,43,44] Recently, transgenic and knockout mice have been used to evaluate mechanisms of HPV.[43,45,56,47] Although the isolated perfused/ventilated lung method was widely used to identify the mechanisms of HPV, results have varied due to the use of different species, perfusates, and protocols.[28,48,49,50] Currently, there are few methods describing the isolated perfused/ventilated mouse lung system.[26,51,52] Therefore, in this study, we characterized the isolated perfused mouse lung method using different perfusates and pretone agents in order to define optimal conditions for studying HPV. Using our optimized conditions, we evaluated vascular reactivity in the isolated perfused/ventilated mouse lung and revisited the role of extracellular Ca2+ and endothelial nitric oxide (NO) synthase and caveolin-1 expression in the mechanism of HPV.
MATERIALS AND METHODS
Isolated perfused mouse lung
Experiments using the mice in this study were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Chicago, USA. C57BL/6 mice (22-25 g) were deeply anesthetized with ketamine (100 mg/kg)/xylazine (26 mg/kg) via intra-abdominal injection. After a tracheostomy was performed, mice were ventilated with a gas mixture of 21% O2, 5% CO2 via rodent ventilator (minivent type 845, Harvard Apparatus, USA). Respiratory rate was maintained at 80 breaths/min and tidal volume was 10 mL/kg (~250 ml). Positive end-expiratory pressure was maintained at 2 mmH2O. End-inspiratory plateau pressure (EIP) was measured by a pressure transducer (MPX type 399/2, Hugo Sachs Elektronik-Harvard Apparatus, Germany) which was connected with a tracheal catheter. The mice were secured in the chamber of the isolated perfused lung system (IL-1 Type 839, Harvard Apparatus, USA), the chest was opened by median sternotomy, and then the pericardium, thymus, and fat tissue were carefully removed. In order to prevent blood coagulation, 20 IU heparin was injected into the right ventricle. A stainless steel catheter was inserted into the main pulmonary artery (PA) after performing a right ventriculotomy, and the PA and ascending aorta were tied together using a 6-0 black silk suture. Pulmonary arterial pressure (PAP) was measured using a pressure sensor (P75 Type 379, Hugo Sachs Elektronik-Harvard Apparatus, Germany), which was connected to the PA catheter. The other end of the catheter was connected to a tube for pulmonary perfusion. After a small incision of the left ventricle, the mitral valve was opened using a small hemostat, and the stainless steel cannula was inserted into the left atrium via a left ventriculotomy, which was drained into the reservoir. This cannula was fixed by a black silk suture, which was tied to the left ventricular wall. The pulmonary circulation was maintained in a closed circuit via a peristaltic pump (ISM 834, ISOMATEC, USA). The total volume of perfusate was 50 mL and the flow rate was maintained at 2 mL/min. The PAP, left atrial pressure, and EIP were monitored continuously. Before each cannula was inserted, the pressure was zeroed at the height of the heart. For data acquisition and data storage, Powerlab 8/30, Quad Bridge Amp, and LabChart (AD Instruments, Australia) were used. To maintain the humidity of the opened lung, physiological saline was applied into the thoracic cavity. Temperature was maintained at 36-38°C with a water jacket in the chamber and reservoir. After basal PAP was stabilized for 40-60 minutes, the experiments were performed.
Hypoxic condition for HPV
For observing HPV, mice were ventilated with a normoxic gas mixture (21% O2, 5% CO2, and balanced with N2) and a hypoxic gas mixture (1% O2, 5% CO2, and balanced with N2) in five-minute intervals. The amplitude of HPV was calculated from the change in PAP from basal to peak level.
Solutions and chemicals
The physiological salt solution (PSS) used for the perfusate consisted of the following composition (mM): NaCl 120, KCl 4.3, NaHCO3 19, KH2PO4 1.1, glucose 10, CaCl2 1.8, and MgCl2 1.2 (pH 7.4). In order to protect prostaglandin synthesis, 3.1 mM sodium meclofenamate was added to the perfusate. For isotonic high-K+ solutions (40 mM), NaCl was replaced by an equimolar amount of KCl. A total of 1 mM EGTA was added and CaCl2 was replaced by equimolar MgCl2 for Ca2+-free solutions. To compare the effect of different perfusates on HPV, each chemical was added to the perfusate as follows: (1) 4% albumin (Product No. A7906, Sigma); (2) 4% Ficoll (Product No. F2637, Sigma); (3) DMEM with 4.5 g/L D-glucose, L-glutamine and without sodium pyruvate (Product No. 11965-092, GIBCO); and (4) fetal bovine serum (FBS) (Product No. 16000-044, GIBCO). U46619 was dissolved in DMSO and angiotensin II and nifedipine (Nif) were dissolved in distilled water. All drugs were obtained from Sigma (St. Louis, Mo., USA) and stored at –20°C.
Statistical analysis
The data are presented as mean ± standard error of the mean. Paired or unpaired Student's t-tests and one-way analysis of variance with Bonferroni multiple range tests were used for statistical analysis. Significance level was set at P < 0.05.
RESULTS
HPV in PSS with 4% albumin
We compared HPV using different pretone agents in PSS with 4% albumin as a perfusate. Without the pretone agent, exposure to hypoxia negligibly affected PAP. The amplitude of acute hypoxia-induced increase in PAP, or HPV, was only 2.2% ± 0.6% of that induced by 40 mM K+ solution (Fig. 1A). Also, increased PAP induced by hypoxia was not observed after angiotensin II was applied (Fig. 1B). In contrast, in the presence of thromboxane A2 analogue U46619, basal PAP and the amplitude of HPV (or alveolar hypoxia-induced increase in PAP) were dose-dependently increased. When 1 and 2 μM U46619 were applied as a pretone agent, the amplitudes of hypoxia-induced increase in PAP were 3.6 ± 0.1 mmHg (Fig. 1C) and 9.6 ± 1.1 mmHg (Fig. 1D), respectively. These data indicate that (a) acute alveolar hypoxia is unable to increase PAP or cause HPV in the isolated lung perfused with 4% albumin in the absence of a pretone agent, (b) presuperfusion of the pulmonary vasculature with certain pretone agents (e.g., the thromboxane A2 analogue, U46619) confers HPV and dose-dependently enhances the amplitude of HPV, and (c) different pretone agents (or priming factors) affect acute HPV differently under these conditions.
Figure 1.
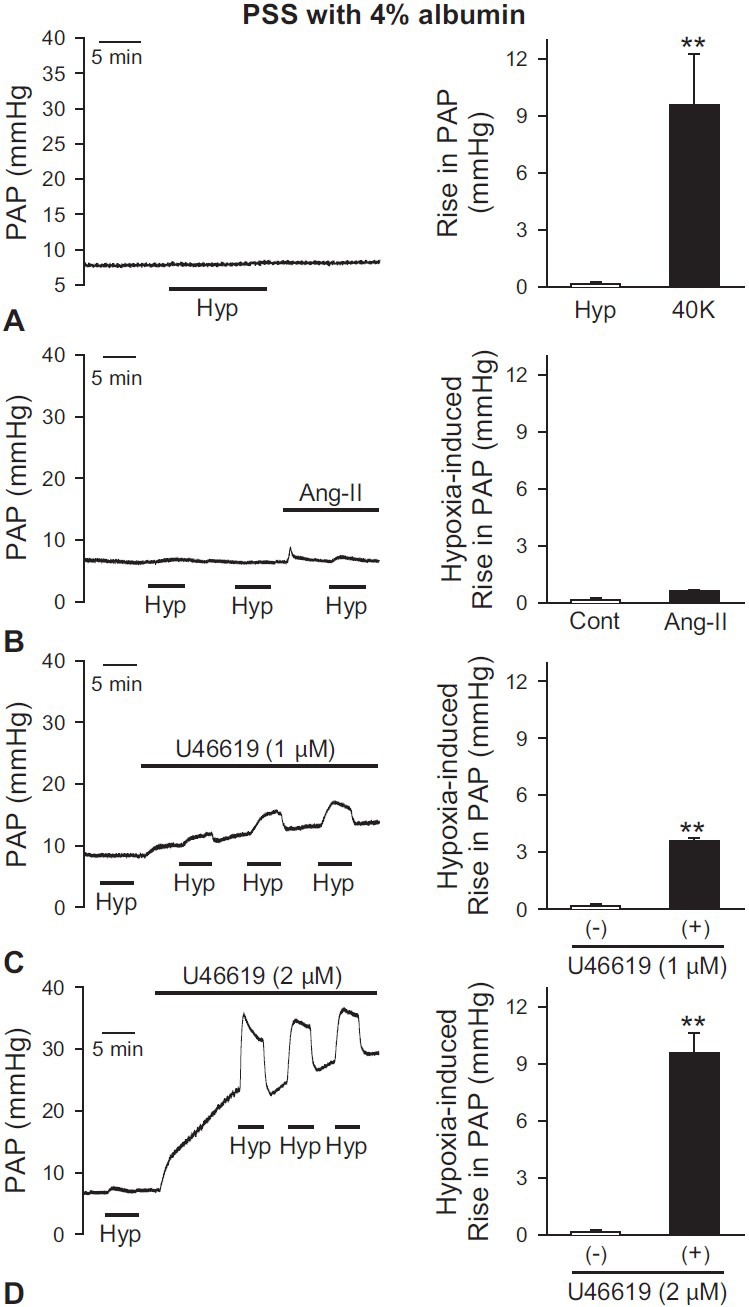
Comparison of HPV using different pretone agents in PSS perfusate with 4% albumin in the isolated perfused/ventilated mouse lung. Flow rate was regularly controlled with 2 mL/min. After stabilization of pulmonary arterial pressure (PAP) for 40-60 min, treatment of hypoxia or drug was applied. Hypoxic (1% O2) and normoxic (21% O2) gas mixture were applied for 5 min intervals via rodent ventilator. (A) Hypoxia (1% O2) alone did not induce vasoconstriction without a pretone agent. (B) Angiotensin II (Ang-II, 0.2 μg/100 μL) did not affect the HPV response. (C and D) In the presence of U46619 (1 μM, C, or 2 μM, (D), baseline PAP was dose-dependently increased and the amplitude of HPV was gradually increased. Mean ± SEM is shown as a bar graph (right panel). *Indicates statistically significant difference from the control value (P< 0.05). Lungs are isolated from two to four mice.
HPV in PSS with 4% Ficoll
In the isolated mouse lung, several studies have used Ficoll as an oncotic agent of perfusate.[32,53] We observed HPV using 4% Ficoll in PSS as a perfusate and pretone agent (angiotensin II). In the absence of angiotensin II, alveolar hypoxia alone did not increase PAP (Fig. 2A). To examine whether the inability of hypoxia to cause HPV was due to low basal PAP, we repeated the experiment when we changed the baseline perfusion rate. The increase of flow rate from 2 to 3 mL/min increased basal PAP but had no effect on HPV (Fig. 2A). However, in the isolated lung perfused with 5% Ficoll in PSS, alveolar hypoxia slightly increased PAP after angiotensin II (0.2 μg) was applied. The amplitude of acute hypoxia-induced increase in PAP was slightly increased (by approximately 2 mmHg) but not statistically significant (P = 0.07, Fig. 2B). These data indicate that, in isolated perfused/ventilated mouse lung, purfusate which includes 4% Ficoll is insufficient for acute hypoxia to cause HPV in the absence or presence of a pretone agent (e.g., angiotensin II).
Figure 2.
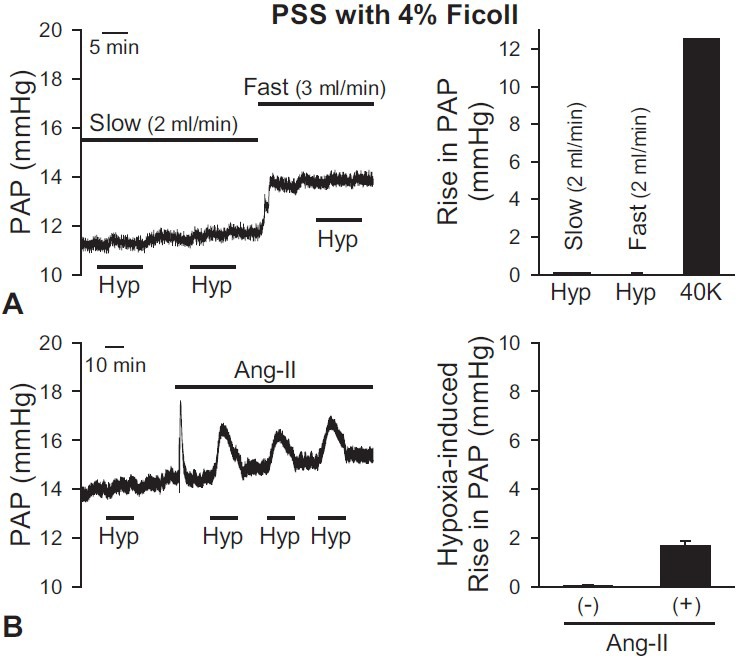
Effects of PSS with 4% Ficoll as a perfusate to induce HPV in the isolated perfused/ventilated mouse lung. After basal PAP was stabilized, the application of hypoxia or pretone agent or change of flow rate was performed. (A) Hypoxia alone (1% O2) did not induce vasoconstriction without a pretone agent. Increasing flow rate of perfusate (from 2 to 3 mL/min) induced the increase of the basal PAP, but not the amplitude of HPV. (B) When Ang-II (0.2 mg/100 ml) as a pretone agent was applied, a strong, transient increase of PAP was induced. After applying Ang-II to return to the basal PAP, hypoxic exposure was applied in the lung. The amplitude of HPV was slightly increased in the presence of angiotensin II but had no significant difference. Mean ± SEM is shown as a bar graph (right panel). Lungs are isolated from two to three mice.
HPV in PSS with rat blood
To investigate the effect of blood and the PSS perfusate mixture on HPV in isolated perfused/ventilated mouse lung, we collected blood from rats that were deeply anesthetized with pentobarbital sodium (100 mg/kg). A total of 10 mL whole blood from rat was added to 40 mL PSS perfusate before the experiment. When PSS with 20% rat blood was used as perfusate, the amplitude of alveolar hypoxia-induced increase in PAP was 2.2 ± 0.1 mmHg without angiotensin II (Fig. 3A). After the application of angiotensin II, the amplitude of HPV was slightly increased (Fig. 3B) but was not significantly different from control (Fig. 3B, inset). These data indicate that perfusate comprised of 20% rat blood in PSS is sufficient for alveolar hypoxia to induce a significant increase in PAP; the acute HPV, however, was not enhanced by application of the pretone agent, angiotensin II.
Figure 3.
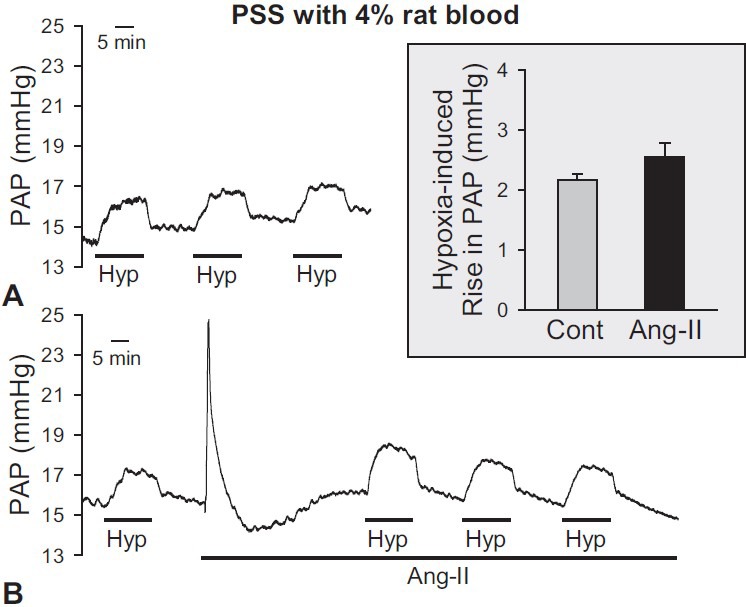
Effects of PSS with rat blood as a perfusate to induce HPV in the isolated perfused/ventilated mouse lung. The blood mixed with perfusate (40 mL PSS and 10 mL whole blood of rat) was used as a perfusate. (A) HPV (1% O2) was repetitively induced in the absence of a pretone agent. (B) Effects of Ang-II (1 mg/100 ml) as a pretone agent on basal PAP and HPV. After the 3rd hypoxic challenge via ventilator in the isolated lung, the injection of Ang- II was applied into the PAP. Application of angiotensin II induced a transient increase of basal PAP; however, the amplitude of HPV did not significantly increase in the presence of Ang-II. (C) Comparison between before (control) and after AII treatment is shown as a bar graph. Bar graph shows mean ± SEM. Lungs are isolated from eight mice.
HPV in DMEM with FBS
In many experiments, it is useful to repeatedly expose the lungs to hypoxic conditions in order to determine the effect of different drugs on HPV. Therefore, we performed hypoxic ventilatation exposure with six trials to evaluate the persistent and repetitive HPV response to alveolar hypoxia using DMEM with 10% FBS as perfusate. During the first trial of exposure to hypoxia, strong vasoconstriction was induced; however, the amplitude of HPV was lower in the second through sixth trials. The amplitude of PAP increase by the sixth hypoxic trial was one third of that of the first hypoxic trial (Fig. 4A). Also, the amplitude of HPV did not increase after the addition of angiotensin II (Fig. 4B). These data indicate that, in isolated perfused/ventilated mouse lung, using cell culture medium (DMEM with 10% FBS) is not good enough for acute hypoxia to induce persistent HPV in the absence or presence of a pretone agent (e.g., angiotensin II).
Figure 4.
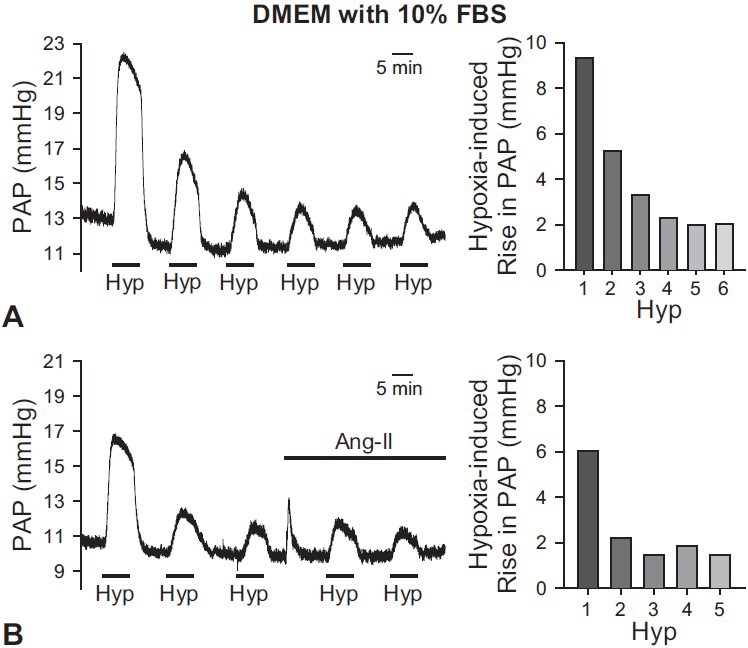
Effects of DMEM with 5%-10% FBS as a perfusate on HPV in the isolated perfused/ventilated mouse lung. After basal PAP was stabilized, hypoxic exposure (1% O2) was repeatedly performed with six challenges. (A) When DMEM with 10% FBS was applied as a perfusate, HPV was strongly induced but the amplitude of HPV or hypoxia-induced increase in PAP was spontaneously decreased after applying the 1st challenge of hypoxia. (B) The amplitudes of HPV using DMEM with 5% FBS were spontaneously decayed. After Ang-II (0.2 mg/100 ml) was applied, HPV was not significantly increased. Bar graphs are shown as mean ± SEM (right panels).
Collectively, the results described above demonstrate that PSS with 4% albumin, 4% Ficoll, or 20% rat blood, and DMEM with 10% FBS, are not optimal perfusates for the isolated perfused/ventilated mouse lung model due to the requirement of a pretone agent and inconsistent responses.
Persistent and repetitive HPV in PSS with 20% FBS
When PSS perfusate with 20% FBS was used in the isolated mouse lung, we applied hypoxic conditions more than six times and observed persistent and repetitive HPV. The mean amplitude of hypoxia-induced increase in PAP was 4.4 ± 0.1 mmHg (Fig. 5A and B). The application of angiotensin II did not significantly affect HPV. The amplitude of HPV with angiotensin II was 5.1 ± 0.3 mmHg (Fig. 5A and B). We also examined the effect of relative long-term exposure to hypoxia in the isolated mouse lung. When the lung was ventilated with a hypoxic gas mixture for three hours, HPV induced a biphasic increase in PAP. PAP rapidly increased by 4.3 ± 1.5 mmHg within the first five minutes of exposure to hypoxia, and then a second increase was observed which was maintained after a slight decrease of PAP (Fig. 5C). The second phase of hypoxia-induced increase in PAP reached a plateau approximately 25-30 minutes after the initial exposure to hypoxia. These results demonstrate that persistent and repetitive HPV can be measured without the need for a pretone agent when using PSS with 20% FBS as the perfusate.
Figure 5.
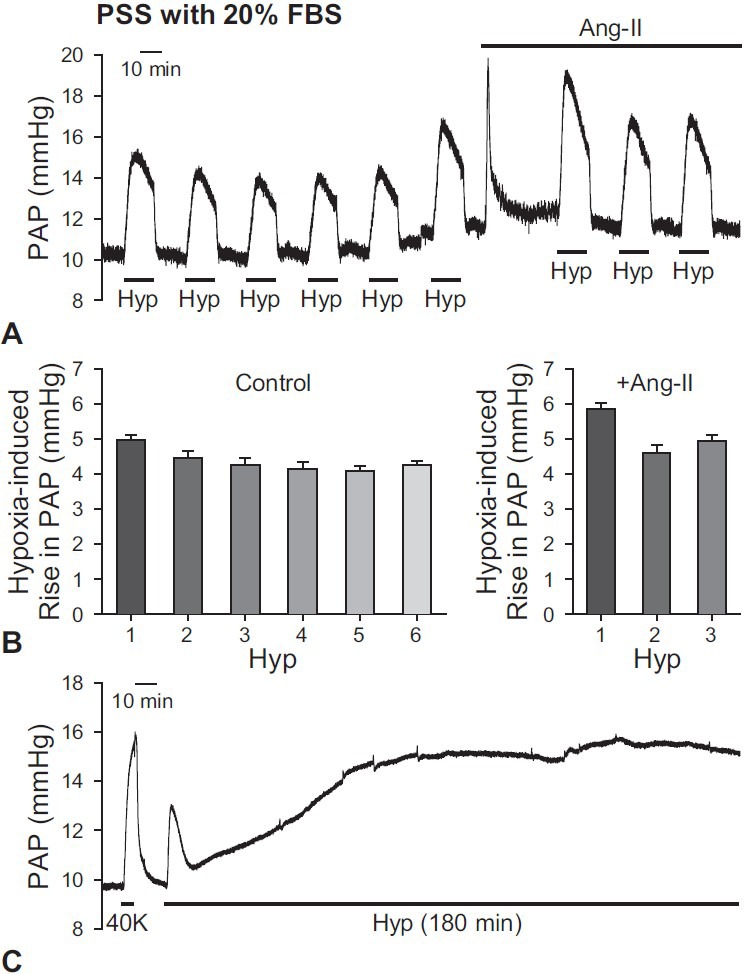
Effects of PSS with 20% FBS as a perfusate on HPV in isolated perfused/ventilated mouse lung. (A) Representative trace of HPV is shown. Consistent and repetitive HPV was observed with PSS perfusate with 20% FBS. The amplitudes of HPV were increased by the application of Ang-II (0.2 mg/100 ml). (B) Bar graphs are shown as mean ± SEM. The amplitude of HPV from 1st to 6th trials of hypoxia without a pretone agent (left panel) and the amplitude of HPV with Ang-II (right panel) are shown as a bar graph. (C) Representative trace of HPV for 180 min. When hypoxia (1% O2) was applied via ventilator, the amplitude of HPV was transiently increased for the first 5 min, and then secondary increase was slowly shown and maintained. Lungs are isolated from two to six mice.
Effects of extracellular Ca2+ on HPV
Since HPV was first described in 1946,[1] many researchers have identified the importance of the effect of extracellular Ca2+ on HPV.[3,30,54,55] In the isolated perfused mouse lung, however, there is little evidence about the role of extracellular Ca2+.[43] Therefore, we evaluated the role of extracellular Ca2+ on HPV using a Ca2+-free solution and a Ca2+ channel blocker. When a Ca2+- free solution was applied, the amplitude of HPV was significantly decreased and then recovered after the removal of the Ca2+-free solution (Fig. 6A). During hypoxic conditions, HPV was completely abolished by the change of perfusate from the PSS solution with 1.8 mM CaCl2 to the Ca2+-free solution (Fig. 6B). In addition, application of nifidipine, a selective blocker of VDCCs, after observing HPV three times, significantly decreased the amplitude of HPV. The amplitude of the acute hypoxia-induced increase in PAP in the presence of 1 μM Nif was 22.5% ± 0.6% of control (P < 0.01, Fig. 6C). These data provide compelling evidence that acute hypoxia-mediated pulmonary vasoconstriction is significantly dependent on a rise in cytosolic [Ca2+] due to Ca2+ influx through VDCCs.
Figure 6.
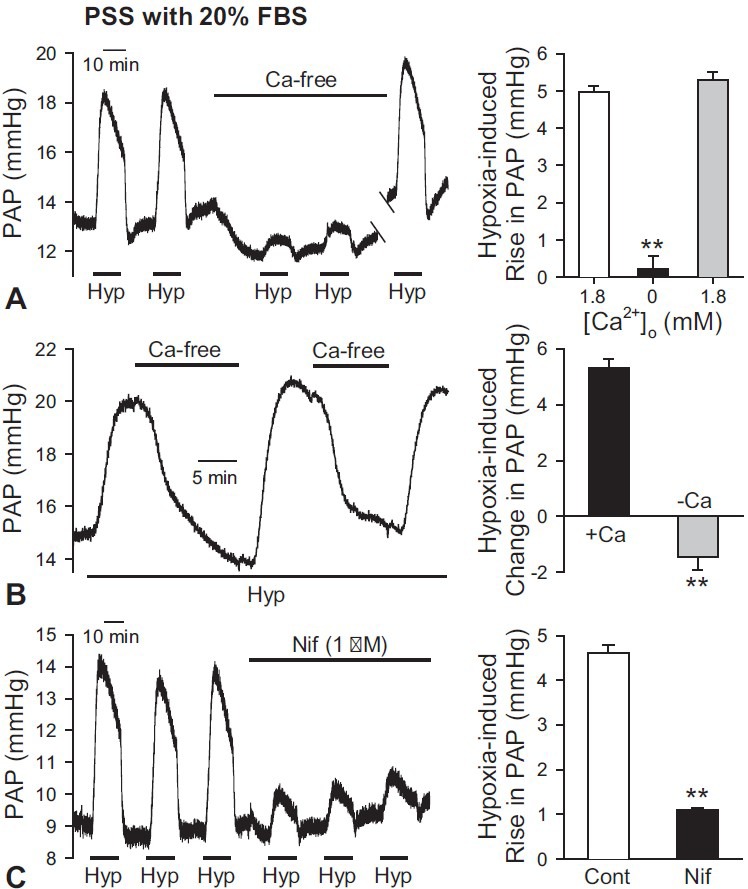
Involvement of extracellular Ca2+ influx through VDCC in HPV using PSS perfusate with 20% FBS in the isolated perfused/ventilated mouse lung. (A) Removal of extracellular Ca2+ (Ca-free) significantly decreased the basal PAP and inhibited HPV. After restoration of extracellular Ca2+ in the perfusate to 1.8 mM Ca2+ again, HPV was recovered as before (left panel). Bar graphs are shown as mean ± SEM (right panel). **P < 0.01 vs. open and grey bars. (B) Application of Ca2+-free (-Ca) solution during hypoxic conditions (1% O2) decreased the amplitude of the hypoxia-induced increase in PAP (left panel). 1 mM EGTA was included in the Ca2+-free solution. Bar graphs are shown as mean ± SEM (right panels). +Ca, solution including 1.8 mM Ca2+. **Indicates statistically significant difference from the control value (P < 0.01). Lungs are isolated from three to six mice. (C) Effect of Nif on basal PAP and HPV was shown as a representative trace (left panel). After the 3rd challenge of hypoxic exposure, Nif (1 mM) was applied in the perfusate. The 4th to 6th challenges of hypoxia were performed in the presence of Nif. Application of Nif decreased amplitudes of HPV. Bar graphs are shown as mean ± SEM (right panel). **Indicates statistically significant difference from the control value (P < 0.01). Lungs are isolated from six mice.
Measuring HPV using isolated perfused/ventilated mouse lung: Comparisons of contractile responses in different knockout mice
The purpose of this study was to optimize the isolated perfused/ventilated lung system to further investigate mechanisms of HPV, and therefore, we compared HPV and contractile responses in lungs from knockout mice. Some researchers have suggested that endothelial nitric oxide synthase (eNOS) activity is directly suppressed by caveolin-1 (Cav1),[56,57] and Cav1 knockout (Cav1-/-) mice eventually develop pulmonary hypertension due to pathologic eNOS hyperactivity.[58,59,60,61] Also, eNOS is one of the primary signaling proteins thought to play a role in HPV.[62,63,64,65] Therefore, we compared 40K+-induced contraction, basal PAP, HPV, and hypoxic treatment for 90 minutes in the isolated perfused/ventilated mouse lung from mice genetically deficient in Cav1, eNOS, or eNOS/Cav1. The amplitudes of 40K+-induced contraction and basal PAP in NOS3-/- (eNOS deletion) and NOS3-/-/Cav1-/- mice were significantly higher than those of the control mice (Fig. 7A and C). The amplitude of the 40K+-induced contraction and basal PAP in NOS3-/-/Cav1-/- mice was 301.2% ± 45.5% and 147.8% ± 7.8% of control, respectively. However, HPV was not significantly enhanced by eNOS or eNOS/Cav1 deletion, although the basal PAP was significantly increased in NOS3-/- and NOS3-/-/Cav1-/- mice in comparison to wild-type (WT) controls (Fig. 7B and C). Cav1 deletion had no effect on HPV, basal PAP, or 40K+-induced contraction.
Figure 7.
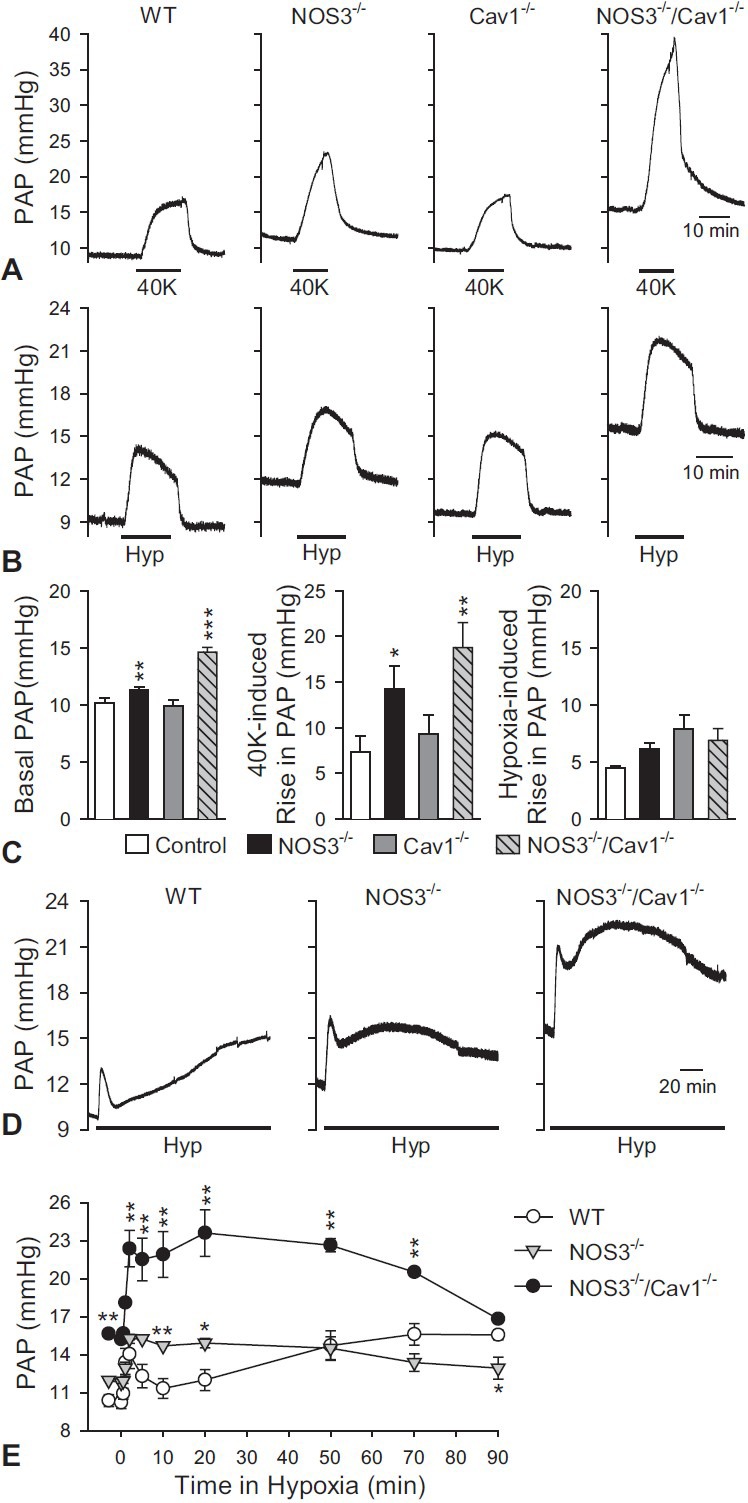
Comparison of 40K+-induced contraction, HPV, and vasoconstrictive response to 90 minutes-hypoxic ventilation among mice deleted selectively for endothelial nitric oxide synthase (NOS3-/-), caveolin-1 (Cav1-/-), and NOS3-/-/Cav1-/- in the isolated perfused/ventilated mouse lung. (A) 40K+-induced contraction (or increase in PAP) was significantly enhanced by eNOS deletion (NOS3-/-) or eNOS/Cav1 deletion (NOS3-/-/Cav1-/-) in comparison to WT control mice. (B) Basal PAP in the NOS3-/- or NOS3-/-/Cav1-/- mice was significantly higher than balsa PAP in control or Cav1-/- mice. HPV was also enhanced by eNOS/ Cav1 deletion; however, neither Cav1 nor eNOS deletion alone had any effect on HPV. (C) Summarized data (mean ± SEM) showing the basal PAP (left panel), the amplitude of 40K-mediated increase in PAP (middle panel) and hypoxia-induced increase in PAP (right panel) in isolated perfused/ventilated lungs of WT, NOS3-/-, Cav1-/- and NOS3-/-/Cav1-/- mice. *P < 0.05, **P < 0.01 vs. WT control. (D) Effect of hypoxic treatment for 90 min was shown as a representative data in WT control, NOS3-/-, and NOS3-/-/Cav1-/- mice. Horizontal broken lines indicate the level of the basal PAP in WT control mice. (E) Summarized data (mean ± SEM) showing the time courses of prolonged (80 min) hypoxia-induced increase in PAP in WT control (open circles), NOS3-/-, and NOS3-/-/Cav1-/- mice. *P < 0.05, **P < 0.01 vs. WT control mice. Lungs are isolated from three to six mice.
Last, biphasic vasoconstrictions were induced by hypoxic treatment for 90 minutes (also see Fig. 5C) in all types of mice (Fig. 7D). In NOS3-/- and NOS3-/-/Cav1-/- mice, basal PAP and the transient increase in PAP induced by acute hypoxia were not significantly changed in comparison to WT control mice, whereas the second phase of HPV was actually enhanced and accelerated (Fig. 7E). These data indicate that deletion of eNOS enhances and accelerates the second phase of HPV in isolated perfused/ventilated lungs, while deletion of eNOS and Cav1 further enhanced the acute hypoxia-induced increase in PAP.
DISCUSSION
In the present study, we determined optimal conditions for isolated perfused/ventilated mouse lung perfusion in order to study HPV. The application of PSS with 20% FBS as perfusate was shown to be ideal for generating persistent and repetitive HPV, whereas hypoxic ventilation exposure did not increase PAP using other perfusate solutions such as PSS containing 4% albumin, 4% Ficoll, or rat blood, and DMEM containing 5-10% FBS.
With the PSS solution with 4% albumin or 4% Ficoll as a perfusate, the amplitude of HPV was under 1 mmHg. This amplitude of HPV was similar with results from other researchers who compared transgenic and WT mice using 4% Ficoll as an oncotic agent in PSS.[32] In these conditions using PSS with 4% albumin or Ficoll, pretone agents such as angiotensin II and U46619 were required to increase the hypoxia-induced increase in PAP (Figures 1 and 2). Specifically, U46619 dose-dependently induced repetitive HPV when applied to PSS perfusate containing 4% albumin (Fig. 1C and D). U46619 has widely been used as a pretone agent in isolated tension measurements for PA.[15,18] However, because the basal PAP and EIP were also dramatically increased by U46619, it was difficult to measure HPV for a long time before causing pulmonary edema.
In isolated perfused/ventilated rat lung experiments, some researchers have used a mixture of rat blood and PSS solution as a perfusate.[21,44,66,67] When whole blood was applied in the perfusate, the amplitude of HPV increased more than two-fold, potentially because of the increase in viscosity or presence of vasoreactive molecules in blood,[21,23,42,68,69] although the absolute amplitude of HPV or alveolar hypoxia-induced increase in PAP was still small (Fig. 3).
In contrast to HPV in response to PSS with 4% albumin or Ficoll or rat blood, in the first challenge of hypoxic exposure, the absolute amplitude of the HPV in DMEM with 10% FBS increased by 9.3 mmHg. This amplitude of HPV is similar to results from previous studies in rats.[21,44,68] However, after the first trial, HPV decreased and the amplitude of the sixth trial was 2.0 mmHg (Fig. 4A). The spontaneously declining HPV did not recover upon application of angiotensin II Figure 4B.
To evaluate the effect of drugs or treatments on HPV, one must be able to measure consistent and repetitive HPV responses. Persistent HPV was observed when PSS with 20% FBS was perfused in the isolated mouse lung (Fig. 5A and B). From the first to the sixth challenge, the absolute amplitudes of HPV were about 4.5 mmHg in absence of pretone agent. To our knowledge, this is the first demonstration of persistent HPV responses in the isolated mouse lung perfused with PSS containing 20% FBS.
PSS or RPMI media was also used in many other studies as a perfusate in the isolated perfused lung, while albumin, Ficoll, or rat blood was added to the perfusate as an oncotic agent.[26,35,44,52,53] In the present study, the sustained HPV was only demonstrated with PSS with 20% FBS as a perfusate. Use of PSS containing 20% FBS as perfusate was suitable to measure acute hypoxic pressor responses of the lung vasculature to alveolar hypoxia for a few minutes as well as for chronic hypoxic exposure for more than two to three hours. When the exposure to hypoxia for three hours was applied, HPV was shown as a biphasic increase in PAP, a transient increase in PAP (which lasted approximately 10 minutes), followed by a gradual increase that reached a plateau about 30 minutes after initial hypoxic exposure (Fig. 5C and 7D), left panel). This was similar to previous results using different perfusates in the isolated perfused/ventilated lung and PA from different animals.[18,26,30,54,70] Several researchers mentioned that the first peak in five minutes reflected an acute HPV. The second phase, which was prolonged vasoconstriction, might be related to the loss of endothelium-dependent vasorelaxation or an increase of vasoconstrictor production and release, which are involved in the development of pulmonary hypertension.[54,55,70] In the present study, however, our data demonstrated that the second phase of HPV, or hypoxia-induced increase in PAP, was actually enhanced and accelerated in isolated perfused/ventilated lungs of NOS3-/- and NOS3-/-/Cav1-/- mice (Fig. 7D and E). Furthermore, the proposed perfusate condition used in this study to measure HPV for long periods might be useful for the further investigation, in which it is required to identify PAP responses to prolonged hypoxia and their mechanisms.
Several previous studies have used whole blood or a mixture of blood and PSS as the perfusate for the isolated perfused/ventilated rat lung model;[21,23,67] however, few studies use rat blood in the perfusate for mouse studies. The blunted HPV we see when using PSS plus 20% rat blood could be due to differences in the composition of rat blood versus mouse blood or possibly due to an immune response. The use of PSS plus 20% FBS in our studies gave the optimal response over a long period of time. It is advantageous that the perfusate composition be as simple as possible in order for the investigation of basic mechanisms of HPV by avoiding the addition of complex molecules in the perfusate.[26] The addition of 20% FBS to the perfusate may provide the necessary nutrients and growth factors required to maintain the integrity of vascular function during the extended experimental time. Additionally, the FBS may contain basal pretone or priming factors similar to the blood that are necessary for the vessels to maintain their function in an experimental setting.
The goal of these studies was to optimize the isolated perfused/ventilated mouse lung model for the study of HPV. In the literature, several studies have reported very different levels of response to hypoxia, with a hypoxic-induced change in PAP ranging from 1 to 8 mmHg.[26,71] A portion of the differences in hypoxic-induced change in PAP could be due to the use of different strains of mice.[72] It has been reported that there is a greater than two-fold difference in the hypoxic-induced change in PAP between BALB/c and C57BL/6 mice under the same conditions.[26] The differences in the response to hypoxia are also seen between species. Using the rat isolated perfused/ventilated lung model, a range in hypoxic-induced change in PAP from 4 to 14 mmHg has been reported.[21,23,73,74] Variable degrees of pulmonary vascular reactivity are seen in other species as well. Cattle are hyper-reactors to hypoxic stress, while sheep are hyporeactors.[75] These examples emphasize the importance of using an optimized perfusion solution in order to obtain the most accurate and comparable results.
One of the major mechanisms responsible for inducing HPV is an increase in cytosolic [Ca2+] in PASMCs. Regulating resting membrane potential (Em) in PASMCs is mediated by the activity of K+ channels[76] and Na+/K+ ATPase. Although Kv channels have been mainly studied as a determinant of resting Em[28,77], two-pore domain channels (TASK)[78,79,80] and KCNQ channels[81] were also suggested to contribute to the regulation of resting Em. When the PA was exposed to acute hypoxia, the inhibition of these K+ channels induced membrane depolarization and the opening of VDCC, which increased Ca2+ influx and induced vasoconstriction.[3,54] In several cases, the role of Ca2+ has been proven using isolated pulmonary arteries, cells, or lungs from other species[81,82,83] but not in the isolated perfused/ventilated mouse lungs. Our results demonstrate that HPV was nearly abolished in the absence of extracellular Ca2+ (using a Ca2+-free solution as a perfusate), and that Nif-sensitive VDCCs play a major role in the acute hypoxia-induced increase in PAP (Fig. 6).
To confirm the optimal conditions in the isolated perfused/ventilated mouse lung, we compared vasoconstrictive responses using mice deleted selectively for eNOS, Cav1, and eNOS/Cav1. Increased basal PAP in eNOS null mice was similar to previous studies using a NOS inhibitor,[37,44,65] Interestingly, 40K+-induced contraction and basal PAP were enhanced in the NOS3-/-/Cav1-/- mice, but the HPV was not changed. Since Cav1 expression is known to regulate eNOS activity and ROS generation,[57,84] further investigation is required to elucidate the combined effect of eNOS and Cav1 on PAP and HPV.
Taken together, we established and optimized conditions for measuring HPV in the isolated perfused/ventilated mouse lung. Using PSS with 20% FBS as a perfusate, a repetitive and persistent HPV, with an amplitude of about 5 mmHg, is obtained without any pretone agents. HPV in the isolated perfused/ventilated mouse lung is dependent on cytosolic Ca2+, which is similar to other data from isolated arteries, and lungs from other species. We suggest that this design is useful for defining mechanisms of HPV and related pathogenic diseases via comparison with transgenic and WT mice.
ACKNOWLEDGMENTS
This work was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL115014, HL066012, and HL098053).
Footnotes
Source of Support: None
Conflict of Interest: None declared.
REFERENCES
- 1.von Euler US, Liljestrand G. Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol Scand. 1946;12:301–20. [Google Scholar]
- 2.Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev. 2012;92:367–520. doi: 10.1152/physrev.00041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan JX, editor. Boston: Kluwer Academic Publishers; 2004. Hypoxic Pulmonary Vasoconstriction: Cellular and Molecular Mechanisms. [Google Scholar]
- 4.Archer SL, Wu XC, Thébaud B, Nsair A, Bonnet S, Tyrrell B, et al. Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: Ionic diversity in smooth muscle cells. Circ Res. 2004;95:308–18. doi: 10.1161/01.RES.0000137173.42723.fb. [DOI] [PubMed] [Google Scholar]
- 5.Osipenko ON, Tate RJ, Gurney AM. Potential role for Kv3.1b channels as oxygen sensors. Circ Res. 2000;86:534–40. doi: 10.1161/01.res.86.5.534. [DOI] [PubMed] [Google Scholar]
- 6.Sham JS, Crenshaw BR, Jr, Deng LH, Shimoda LA, Sylvester JT. Effects of hypoxia in porcine pulmonary arterial myocytes: Roles of K V channel and endothelin-1. Am J Physiol Lung Cell Mol Physiol. 2000;279:L262–72. doi: 10.1152/ajplung.2000.279.2.L262. [DOI] [PubMed] [Google Scholar]
- 7.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–5. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 8.Hirenallur SD, Detweiler ND, Haworth ST, Leming JT, Gordon JB, Rusch NJ. Furegrelate, a thromboxane synthase inhibitor, blunts the development of pulmonary arterial hypertension in neonatal piglets. Pulm Circ. 2012;2:193–200. doi: 10.4103/2045-8932.97605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard LS, Crosby A, Vaughan P, Sobolewski A, Southwood M, Foster ML, et al. Distinct responses to hypoxia in subpopulations of distal pulmonary artery cells contribute to pulmonary vascular remodeling in emphysema. Pulm Circ. 2012;2:241–9. doi: 10.4103/2045-8932.97616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michelakis ED, Thebaud B, Weir EK, Archer SL. Hypoxic pulmonary vasoconstriction: Redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells. J Mol Cell Cardiol. 2004;37:1119–36. doi: 10.1016/j.yjmcc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol. 2005;98:390–403. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- 12.Papamatheakis DG, Patel JJ, Blood Q, Merritt TT, Longo LD, Wilson SM. Depolarization-dependent contraction increase after birth and preservation following long-term hypoxia in sheep pulmonary arteries. Pulm Circ. 2012;2:41–53. doi: 10.4103/2045-8932.94832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pham I, Wuerzner G, Richalet JP, Peyrard S, Azizi M. Bosentan effects in hypoxic pulmonary vasoconstriction: Preliminary study in subjects with or without high altitude pulmonary edema-history. Pulm Circ. 2012;2:28–33. doi: 10.4103/2045-8932.94824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pozeg ZI, Michelakis ED, McMurtry MS, Thébaud B, Wu XC, Dyck JR, et al. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003;107:2037–44. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- 15.Rodman DM, Yamaguchi T, O’Brien RF, McMurtry IF. Hypoxic contraction of isolated rat pulmonary artery. J Pharmacol Exp Ther. 1989;248:952–9. [PubMed] [Google Scholar]
- 16.Wang J, Weigand L, Foxson J, Shimoda LA, Sylvester JT. Ca2+ signaling in hypoxic pulmonary vasoconstriction: Effects of myosin light chain and Rho kinase antagonists. Am J Physiol Lung Cell Mol Physiol. 2007;293:L674–85. doi: 10.1152/ajplung.00141.2007. [DOI] [PubMed] [Google Scholar]
- 17.Cogolludo A, Moreno L, Bosca L, Tamargo J, Perez-Vizcaino F. Thromboxane A2-induced inhibition of voltage-gated K+ channels and pulmonary vasoconstriction: Role of protein kinase Czeta. Circ Res. 2003;93:656–63. doi: 10.1161/01.RES.0000095245.97945.FE. [DOI] [PubMed] [Google Scholar]
- 18.Leach RM, Robertson TP, Twort CH, Ward JP. Hypoxic vasoconstriction in rat pulmonary and mesenteric arteries. Am J Physiol. 1994;266:L223–31. doi: 10.1152/ajplung.1994.266.3.L223. [DOI] [PubMed] [Google Scholar]
- 19.Murtha YM, Allen BM, Orr JA. The role of protein kinase C in thromboxane A2-induced pulmonary artery vasoconstriction. J Biomed Sci. 1999;6:293–5. doi: 10.1007/BF02253571. [DOI] [PubMed] [Google Scholar]
- 20.Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, et al. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci U S A. 2006;103:19093–8. doi: 10.1073/pnas.0606728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SJ, Yoo HY, Earm YE, Kim SJ, Kim JK, Kim SD. Role of arachidonic acid-derived metabolites in the control of pulmonary arterial pressure and hypoxic pulmonary vasoconstriction in rats. Br J Anaesth. 2011;106:31–7. doi: 10.1093/bja/aeq268. [DOI] [PubMed] [Google Scholar]
- 22.McMurtry IF, Davidson AB, Reeves JT, Grover RF. Inhibition of hypoxic pulmonary vasoconstriction by calcium antagonists in isolated rat lungs. Circ Res. 1976;38:99–104. doi: 10.1161/01.res.38.2.99. [DOI] [PubMed] [Google Scholar]
- 23.McMurtry IF, Hookway BW, Roos S. Red blood cells play a crucial role in maintaining vascular reactivity to hypoxia in isolated rat lungs. Chest. 1977;71:253–6. doi: 10.1378/chest.71.2_supplement.253. [DOI] [PubMed] [Google Scholar]
- 24.McMurtry IF, Petrun MD, Reeves JT. Lungs from chronically hypoxic rats have decreased pressor response to acute hypoxia. Am J Physiol. 1978;235:H104–9. doi: 10.1152/ajpheart.1978.235.1.H104. [DOI] [PubMed] [Google Scholar]
- 25.McMurtry IF, Rounds S, Stanbrook HS. Studies of the mechanism of hypoxic pulmonary vasoconstriction. Adv Shock Res. 1982;8:21–33. [PubMed] [Google Scholar]
- 26.Weissmann N, Akkayagil E, Quanz K, Schermuly RT, Ghofrani HA, Fink L, et al. Basic features of hypoxic pulmonary vasoconstriction in mice. Respir Physiol Neurobiol. 2004;139:191–202. doi: 10.1016/j.resp.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Rhoades RA. Isolated perfused lung preparation for studying altered gaseous environments. Environ Health Perspect. 1984;56:43–50. doi: 10.1289/ehp.845643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, et al. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest. 1998;101:2319–30. doi: 10.1172/JCI333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harder DR, Madden JA, Dawson C. Hypoxic induction of Ca2+-dependent action potentials in small pulmonary arteries of the cat. J Appl Physiol. 1985;59:1389–93. doi: 10.1152/jappl.1985.59.5.1389. [DOI] [PubMed] [Google Scholar]
- 30.Weir EK, Archer SL. The mechanism of acute hypoxic pulmonary vasoconstriction: The tale of two channels. FASEB J. 1995;9:183–9. doi: 10.1096/fasebj.9.2.7781921. [DOI] [PubMed] [Google Scholar]
- 31.Yuan XJ, Goldman WF, Tod ML, Rubin LJ, Blaustein MP. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am J Physiol. 1993;264:L116–23. doi: 10.1152/ajplung.1993.264.2.L116. [DOI] [PubMed] [Google Scholar]
- 32.Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, et al. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L656–64. doi: 10.1152/ajplung.00090.2003. [DOI] [PubMed] [Google Scholar]
- 33.Zhao L, Mason NA, Morrell NW, Kojonazarov B, Sadykov A, Maripov A, et al. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation. 2001;104:424–8. doi: 10.1161/hc2901.093117. [DOI] [PubMed] [Google Scholar]
- 34.Fagan KA, Fouty BW, Tyler RC, Morris KG, Jr, Hepler LK, Sato K, et al. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest. 1999;103:291–9. doi: 10.1172/JCI3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanderpool RR, Naeije R, Chesler NC. Impedance in isolated mouse lungs for the determination of site of action of vasoactive agents and disease. Ann Biomed Eng. 2010;38:1854–61. doi: 10.1007/s10439-010-9960-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuchscherer HA, Vanderpool RR, Chesler NC. Pulmonary vascular remodeling in isolated mouse lungs: effects on pulsatile pressure-flow relationships. J Biomech. 2007;40:993–1001. doi: 10.1016/j.jbiomech.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 37.Spohr F, Busch CJ, Teschendorf P, Weimann J. Selective inhibition of guanylate cyclase prevents impairment of hypoxic pulmonary vasoconstriction in endotoxemic mice. J Physiol Pharmacol. 2009;60:107–12. [PubMed] [Google Scholar]
- 38.Nelin LD, Dawson CA. The effect of N omega-nitro-L-arginine methylester on hypoxic vasoconstriction in the neonatal pig lung. Pediatr Res. 1993;34:349–53. doi: 10.1203/00006450-199309000-00022. [DOI] [PubMed] [Google Scholar]
- 39.Schnader J, Undem B, Peters SP, Sylvester JT. Effects of nordihydroguaiuretic acid on sulfidopeptide leukotriene activity and hypoxic pulmonary vasoconstriction in the isolated sheep lung. Prostaglandins. 1993;46:5–19. doi: 10.1016/0090-6980(93)90058-f. [DOI] [PubMed] [Google Scholar]
- 40.Gordon JB, Hortop J, Hakim TS. Developmental effects of hypoxia and indomethacin on distribution of vascular resistances in lamb lungs. Pediatr Res. 1989;26:325–9. doi: 10.1203/00006450-198910000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Drop LJ, Toal KW, Geffin GA, DD OK, Hoaglin DC, Daggett WM. Pulmonary vascular responses to hypercalcemia and hypocalcemia in the dog. Anesthesiology. 1989;70:825–36. doi: 10.1097/00000542-198905000-00020. [DOI] [PubMed] [Google Scholar]
- 42.Baek EB, Yoo HY, Park SJ, Kim HS, Kim SD, Earm YE, et al. Luminal ATP-induced contraction of rabbit pulmonary arteries and role of purinoceptors in the regulation of pulmonary arterial pressure. Pflugers Arch. 2008;457:281–91. doi: 10.1007/s00424-008-0536-z. [DOI] [PubMed] [Google Scholar]
- 43.Fuchs B, Rupp M, Ghofrani HA, Schermuly RT, Seeger W, Grimminger F, et al. Diacylglycerol regulates acute hypoxic pulmonary vasoconstriction via TRPC6. Respir Res. 2011;12:20. doi: 10.1186/1465-9921-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo HY, Park SJ, Bahk JH, Kim SJ. Inhibition of hypoxic pulmonary vasoconstriction of rats by carbon monoxide. J Korean Med Sci. 2010;25:1411–7. doi: 10.3346/jkms.2010.25.10.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keserü B, Barbosa-Sicard E, Popp R, Fisslthaler B, Dietrich A, Gudermann T, et al. Epoxyeicosatrienoic acids and the soluble epoxide hydrolase are determinants of pulmonary artery pressure and the acute hypoxic pulmonary vasoconstrictor response. FASEB J. 2008;22:4306–15. doi: 10.1096/fj.08-112821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Francis BN, Wilkins MR, Zhao L. Tetrahydrobiopterin and the regulation of hypoxic pulmonary vasoconstriction. Eur Respir J. 2010;36:323–30. doi: 10.1183/09031936.00188809. [DOI] [PubMed] [Google Scholar]
- 47.Roth M, Rupp M, Hofmann S, Mittal M, Fuchs B, Sommer N, et al. Heme oxygenase-2 and large-conductance Ca2+-activated K+ channels: Lung vascular effects of hypoxia. Am J Respir Crit Care Med. 2009;180:353–64. doi: 10.1164/rccm.200806-848OC. [DOI] [PubMed] [Google Scholar]
- 48.Spöhr F, Busch CJ, Reich C, Motsch J, Gebhard MM, Kuebler WM, et al. 4-Aminopyridine restores impaired hypoxic pulmonary vasoconstriction in endotoxemic mice. Anesthesiology. 2007;107:597–604. doi: 10.1097/01.anes.0000281897.13703.fd. [DOI] [PubMed] [Google Scholar]
- 49.Bernal PJ, Leelavanichkul K, Bauer E, Cao R, Wilson A, Wasserloos KJ, et al. Nitric-oxide-mediated zinc release contributes to hypoxic regulation of pulmonary vascular tone. Circ Res. 2008;102:1575–83. doi: 10.1161/CIRCRESAHA.108.171264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keserü B, Barbosa-Sicard E, Schermuly RT, Tanaka H, Hammock BD, Weissmann N, et al. Hypoxia-induced pulmonary hypertension: Comparison of soluble epoxide hydrolase deletion vs. inhibition. Cardiovasc Res. 2010;85:232–40. doi: 10.1093/cvr/cvp281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parker JC, Gillespie MN, Taylor AE, Martin SL. Capillary filtration coefficient, vascular resistance, and compliance in isolated mouse lungs. J Appl Physiol. 1999;87:1421–7. doi: 10.1152/jappl.1999.87.4.1421. [DOI] [PubMed] [Google Scholar]
- 52.von Bethmann AN, Brasch F, Nüsing R, Vogt K, Volk HD, Müller KM, et al. Hyperventilation induces release of cytokines from perfused mouse lung. Am J Respir Crit Care Med. 1998;157:263–72. doi: 10.1164/ajrccm.157.1.9608052. [DOI] [PubMed] [Google Scholar]
- 53.Littler CM, Morris KG, Jr, Fagan KA, McMurtry IF, Messing RO, Dempsey EC. Protein kinase C-epsilon-null mice have decreased hypoxic pulmonary vasoconstriction. Am J Physiol Heart Circ Physiol. 2003;284:H1321–31. doi: 10.1152/ajpheart.00795.2002. [DOI] [PubMed] [Google Scholar]
- 54.Mauban JR, Remillard CV, Yuan JX. Hypoxic pulmonary vasoconstriction: Role of ion channels. J Appl Physiol. 2005;98:415–20. doi: 10.1152/japplphysiol.00732.2004. [DOI] [PubMed] [Google Scholar]
- 55.Ward JP, Aaronson PI. Mechanisms of hypoxic pulmonary vasoconstriction: Can anyone be right? Respir Physiol. 1999;115:261–71. doi: 10.1016/s0034-5687(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 56.Bauer PM, Yu J, Chen Y, Hickey R, Bernatchez PN, Looft-Wilson R, et al. Endothelial-specific expression of caveolin-1 impairs microvascular permeability and angiogenesis. Proc Natl Acad Sci U S A. 2005;102:204–9. doi: 10.1073/pnas.0406092102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272:18522–5. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 58.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–52. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 59.Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, et al. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci U S A. 2002;99:11375–80. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wunderlich C, Schmeisser A, Heerwagen C, Ebner B, Schober K, Braun-Dullaeus RC, et al. Chronic NOS inhibition prevents adverse lung remodeling and pulmonary arterial hypertension in caveolin-1 knockout mice. Pulm Pharmacol Ther. 2008;21:507–15. doi: 10.1016/j.pupt.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, et al. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest. 2009;119:2009–18. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki K, Naoki K, Kudo H, Nishio K, Sato N, Aoki T, et al. Impaired hypoxic vasoconstriction in intraacinar microvasculature in hyperoxia-exposed rat lungs. Am J Respir Crit Care Med. 1998;158:602–9. doi: 10.1164/ajrccm.158.2.9709073. [DOI] [PubMed] [Google Scholar]
- 63.Fagan KA, Tyler RC, Sato K, Fouty BW, Morris KG, Jr, Huang PL, et al. Relative contributions of endothelial, inducible, and neuronal NOS to tone in the murine pulmonary circulation. Am J Physiol. 1999;277:L472–8. doi: 10.1152/ajplung.1999.277.3.L472. [DOI] [PubMed] [Google Scholar]
- 64.Nelson RS, Eichinger MR. Role of nitric oxide (NO) in pulmonary dysfunction associated with experimental cirrhosis. Respir Physiol. 2001;126:65–74. doi: 10.1016/s0034-5687(00)00227-9. [DOI] [PubMed] [Google Scholar]
- 65.Vaughan DJ, Brogan TV, Kerr ME, Deem S, Luchtel DL, Swenson ER. Contributions of nitric oxide synthase isozymes to exhaled nitric oxide and hypoxic pulmonary vasoconstriction in rabbit lungs. Am J Physiol Lung Cell Mol Physiol. 2003;284:L834–43. doi: 10.1152/ajplung.00341.2002. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura T, Vollmar B, Menger MD, Inui K, Wada H, Schafers HJ. Heme oxygenase does not contribute to control of basal vascular tone in isolated blood-perfused rat lung. J Heart Lung Transplant. 2004;23:599–605. doi: 10.1016/S1053-2498(03)00232-8. [DOI] [PubMed] [Google Scholar]
- 67.Yoo HY, Park SJ, Seo EY, Park KS, Han JA, Kim KS, et al. Role of thromboxane A2-activated nonselective cation channels in hypoxic pulmonary vasoconstriction of rat. Am J Physiol Cell Physiol. 2012;302:C307–17. doi: 10.1152/ajpcell.00153.2011. [DOI] [PubMed] [Google Scholar]
- 68.Deem S, Min JH, Moulding JD, Eveland R, Swenson ER. Red blood cells prevent inhibition of hypoxic pulmonary vasoconstriction by nitrite in isolated, perfused rat lungs. Am J Physiol Heart Circ Physiol. 2007;292:H963–70. doi: 10.1152/ajpheart.00812.2006. [DOI] [PubMed] [Google Scholar]
- 69.Deem S, Swenson ER, Alberts MK, Hedges RG, Bishop MJ. Red-blood-cell augmentation of hypoxic pulmonary vasoconstriction: Hematocrit dependence and the importance of nitric oxide. Am J Respir Crit Care Med. 1998;157:1181–6. doi: 10.1164/ajrccm.157.4.9707165. [DOI] [PubMed] [Google Scholar]
- 70.Weissmann N, Winterhalder S, Nollen M, Voswinckel R, Quanz K, Ghofrani HA, et al. NO and reactive oxygen species are involved in biphasic hypoxic vasoconstriction of isolated rabbit lungs. Am J Physiol Lung Cell Mol Physiol. 2001;280:L638–45. doi: 10.1152/ajplung.2001.280.4.L638. [DOI] [PubMed] [Google Scholar]
- 71.Busch CJ, Spohr FA, Motsch J, Gebhard MM, Martin EO, Weimann J. Effects of ketamine on hypoxic pulmonary vasoconstriction in the isolated perfused lungs of endotoxaemic mice. Eur J Anaesthesiol. 2010;27:61–6. doi: 10.1097/EJA.0b013e328329affb. [DOI] [PubMed] [Google Scholar]
- 72.Gomez-Arroyo J, Saleem SJ, Mizuno S, Syed AA, Bogaard HJ, Abbate A, et al. A brief overview of mouse models of pulmonary arterial hypertension: Problems and prospects. Am J Physiol Lung Cell Mol Physiol. 2012;302:L977–91. doi: 10.1152/ajplung.00362.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dumas JP, Goirand F, Bardou M, Dumas M, Rochette L, Advenier C, et al. Role of potassium channels and nitric oxide in the relaxant effects elicited by beta-adrenoceptor agonists on hypoxic vasoconstriction in the isolated perfused lung of the rat. Br J Pharmacol. 1999;127:421–8. doi: 10.1038/sj.bjp.0702575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robertson TP, Ward JP, Aaronson PI. Hypoxia induces the release of a pulmonary-selective, Ca2+-sensitising, vasoconstrictor from the perfused rat lung. Cardiovasc Res. 2001;50:145–50. doi: 10.1016/s0008-6363(01)00192-4. [DOI] [PubMed] [Google Scholar]
- 75.Grover RF, Vogel JH, Averill KH, Blount SG., Jr Pulmonary hypertension. individual and species variability relative to vascular reactivity. Am Heart J. 1963;66:1–3. doi: 10.1016/0002-8703(63)90062-0. [DOI] [PubMed] [Google Scholar]
- 76.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 77.Yuan XJ. Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Circ Res. 1995;77:370–8. doi: 10.1161/01.res.77.2.370. [DOI] [PubMed] [Google Scholar]
- 78.Olschewski A, Li Y, Tang B, Hanze J, Eul B, Bohle RM, et al. Impact of TASK 1 in human pulmonary artery smooth muscle cells. Circ Res. 2006;98:1072–80. doi: 10.1161/01.RES.0000219677.12988.e9. [DOI] [PubMed] [Google Scholar]
- 79.Gurney AM, Osipenko ON, MacMillan D, McFarlane KM, Tate RJ, Kempsill FE. Two-pore domain K channel, TASK-1, in pulmonary artery smooth muscle cells. Circ Res. 2003;93:957–64. doi: 10.1161/01.RES.0000099883.68414.61. [DOI] [PubMed] [Google Scholar]
- 80.Gurney AM, Joshi S. The role of twin pore domain and other K+ channels in hypoxic pulmonary vasoconstriction. Novartis Found Symp. 2006;272:218–28. [PubMed] [Google Scholar]
- 81.Joshi S, Balan P, Gurney AM. Pulmonary vasoconstrictor action of KCNQ potassium channel blockers. Respir Res. 2006;7:31. doi: 10.1186/1465-9921-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259:C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- 83.Ng LC, Gurney AM. Store-operated channels mediate Ca2+ influx and contraction in rat pulmonary artery. Circ Res. 2001;89:923–9. doi: 10.1161/hh2201.100315. [DOI] [PubMed] [Google Scholar]
- 84.Lobysheva I, Rath G, Sekkali B, Bouzin C, Feron O, Gallez B, et al. Moderate caveolin-1 downregulation prevents NADPH oxidase-dependent endothelial nitric oxide synthase uncoupling by angiotensin II in endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31:2098–105. doi: 10.1161/ATVBAHA.111.230623. [DOI] [PubMed] [Google Scholar]


