Abstract
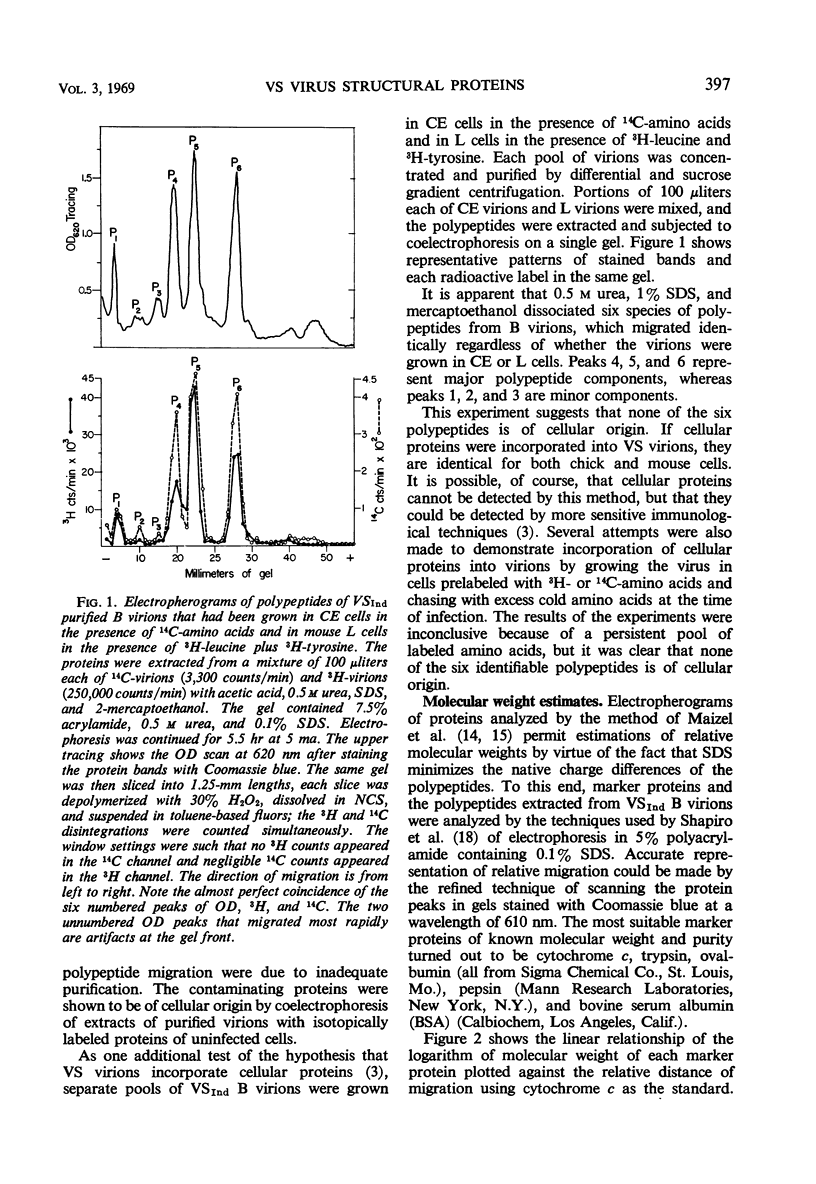
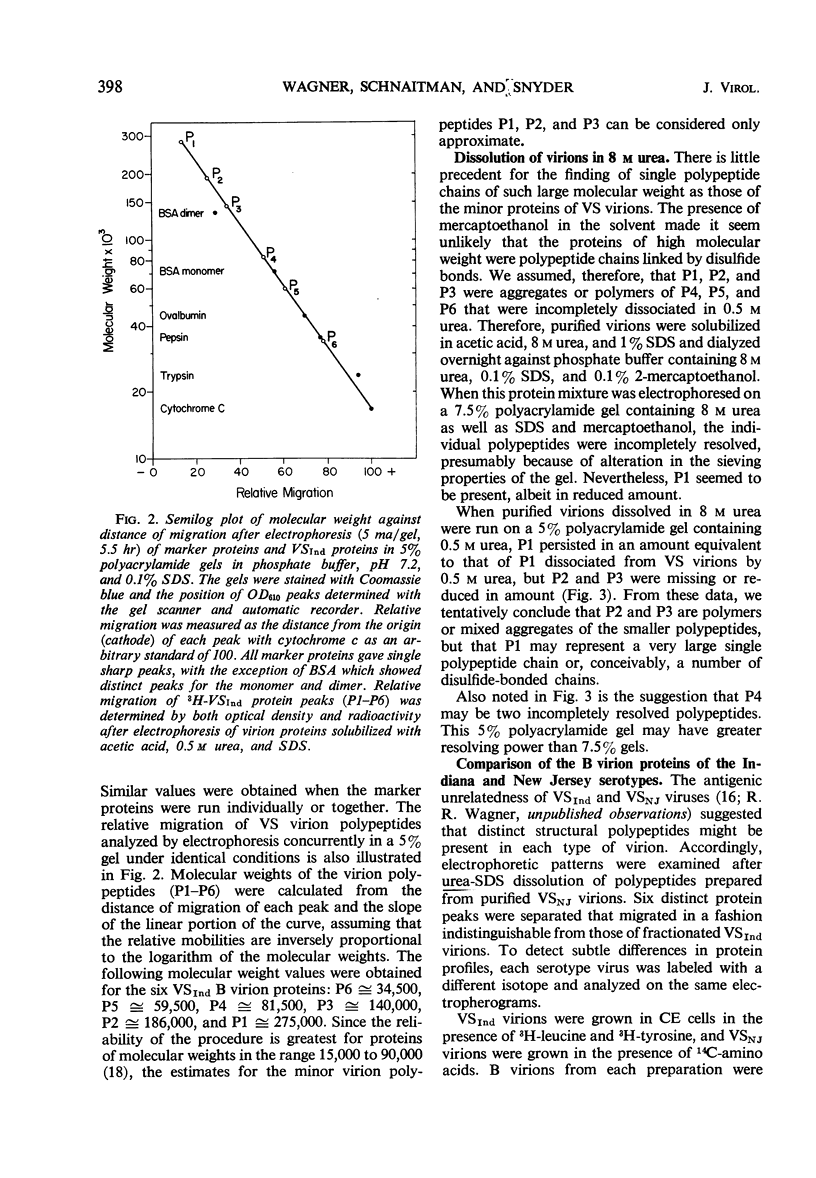
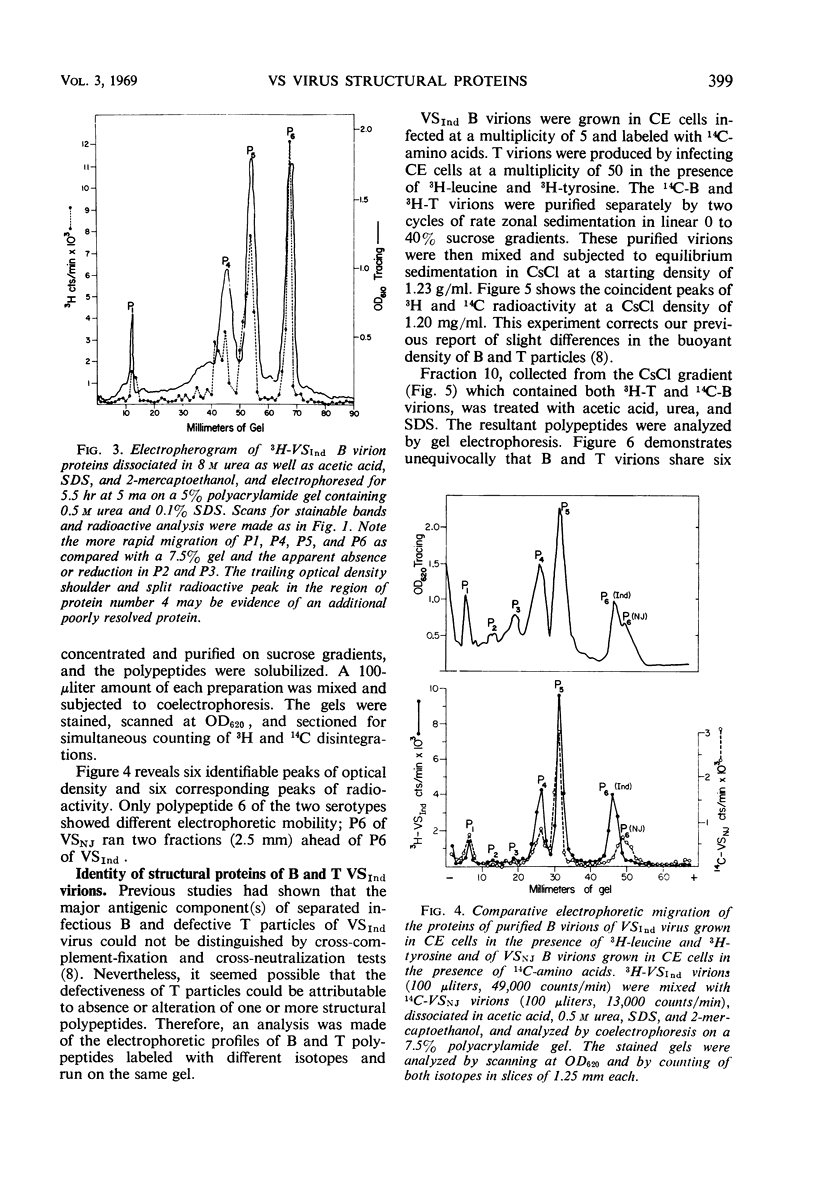
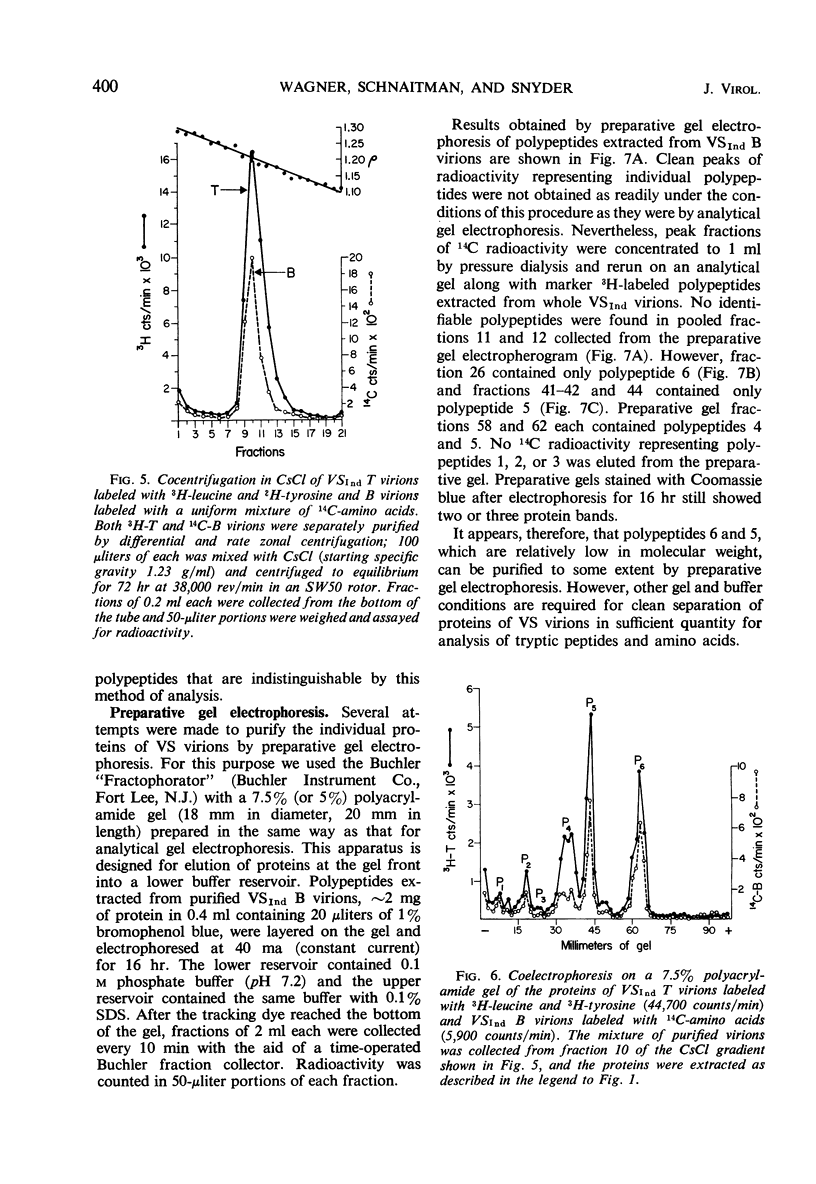
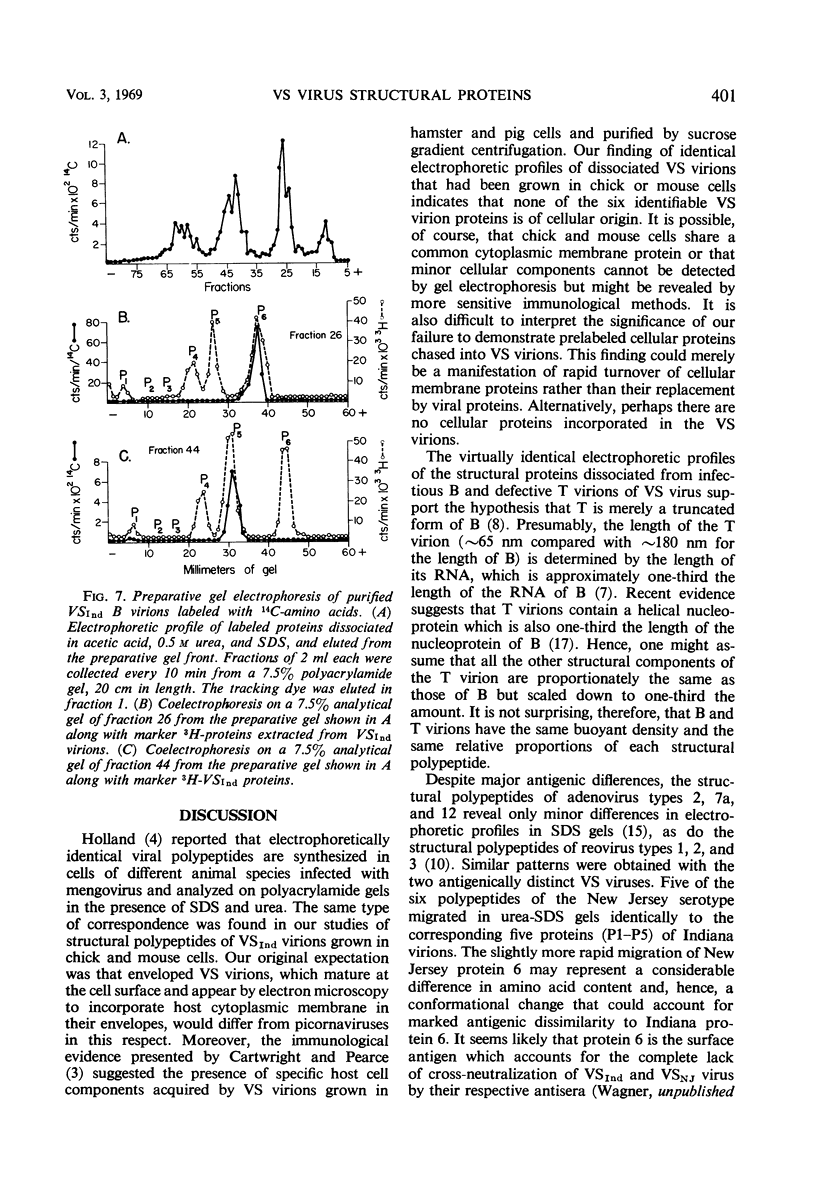
Three major and three minor structural proteins were identified by polyacrylamide gel electrophoresis of purified infectious virions of the Indiana serotype of vesicular stomatitis (VS) virus disrupted with acetic acid, 0.5 m urea, sodium dodecyl sulfate (SDS), and 2-mercaptoethanol. Molecular weights of the six virion proteins were estimated by comparative electrophoretic migration of known marker proteins in the presence of SDS. The following values were obtained: major proteins P6 ≅ 34,500, P5 ≅ 59,500, and P4 ≅ 81,500; minor proteins P3 ≅ 140,000, P2 ≅ 186,000, and P1 ≅ 275,000. P1 did not disaggregate in 8 m urea, but P2 and P3 did. The possibility that P1 is an uncleaved large polypeptide chain could not be ruled out. Six identical protein components were dissociated from Indiana VS virions grown in chick and mouse cells; no cellular proteins could be detected in purified virions. Of six proteins identified in virions of the New Jersey serotype, only the smallest protein (P6) could be distinguished from any of the six proteins of the Indiana serotype on the basis of migration in SDS gels. The defective T particles of Indiana VS virus contained the same six proteins in essentially the same proportions as those of the infectious B virions. Only P6 and P5 could be cleanly separated by preparative gel electrophoresis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown F., Cartwright B., Almeida J. D. The antigens of vesicular stomatitis virus. I. Separation and immunogenicity of three complement-fixing components. J Immunol. 1966 Mar;96(3):537–545. [PubMed] [Google Scholar]
- Brown F., Cartwright B., Crick J., Smale C. J. Infective virus substructure from vesicular stomatitis virus. J Virol. 1967 Apr;1(2):368–373. doi: 10.1128/jvi.1.2.368-373.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Pearce C. A. Evidence for a host cell component in vesicular stomatitis virus. J Gen Virol. 1968 Mar;2(2):207–214. doi: 10.1099/0022-1317-2-2-207. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J. Virus-directed protein synthesis in different animal and human cells. Science. 1968 Jun 21;160(3834):1346–1348. doi: 10.1126/science.160.3834.1346. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Greenawalt J. W., Wagner R. R. Defective T particles of vesicular stomatitis virus. I. Preparation, morphology, and some biologic properties. Virology. 1966 Oct;30(2):161–172. doi: 10.1016/0042-6822(66)90092-4. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Comparative sedimentation coefficients of RNA extracted from plaque-forming and defective particles of vesicular stomatitis virus. J Mol Biol. 1966 Dec 28;22(2):381–384. doi: 10.1016/0022-2836(66)90143-4. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loh P. C., Shatkin A. J. Structural proteins of reoviruses. J Virol. 1968 Nov;2(11):1353–1359. doi: 10.1128/jvi.2.11.1353-1359.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS W. L., HANSON R. P. Immunodiffusion studies on the antigenic relationships within and between serotypes of vesicular stomatitis virus. Am J Vet Res. 1962 Jul;23:896–899. [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Summers D. F. Evidence for differences in size and composition of the poliovirus-specific polypeptides in infected HeLa cells. Virology. 1968 Sep;36(1):48–54. doi: 10.1016/0042-6822(68)90115-3. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Nakai T., Howatson A. F. The fine structure of vesicular stomatitis virus. Virology. 1968 Jun;35(2):268–281. doi: 10.1016/0042-6822(68)90267-5. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Simpson R. W., Hauser R. E. Structural components of vesicular stomatitis virus. Virology. 1966 Aug;29(4):654–667. doi: 10.1016/0042-6822(66)90289-3. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Pfefferkorn E. R., Darnell J. E., Jr Identification of the membrane protein and "core" protein of Sindbis virus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):533–537. doi: 10.1073/pnas.59.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishler P. V., Epstein C. J. A convenient method of preparing polyacrylamide gels for liquid scintillation spectrometry. Anal Biochem. 1968 Jan;22(1):89–98. doi: 10.1016/0003-2697(68)90262-5. [DOI] [PubMed] [Google Scholar]
- Viñuela E., Algranati I. D., Ochoa S. Synthesis of virus-specific proteins in Escherichia coli infected with the RNA bacteriophage MS2. Eur J Biochem. 1967 Mar;1(1):3–11. doi: 10.1007/978-3-662-25813-2_2. [DOI] [PubMed] [Google Scholar]
- WAGNER R. R., LEVEY A. H., SNYDER R. M., RATCLIFF G. A., Jr, HYATT D. F. BIOLOGIC PROPERTIES OF TWO PLAQUE VARIANTS OF VESICULAR STOMATITIS VIRUS (INDIANA SEROTYPE). J Immunol. 1963 Jul;91:112–122. [PubMed] [Google Scholar]



