Abstract
In the present study, we investigate the antiulcerogenic effect of gallic acid against aspirin plus pyrolus ligation-induced gastric ulcer in rats. Rats were treated with gallic acid (100 and 200 mg/kg) and famotidine (20 mg/kg) for 1 week, followed by induction of gastric ulcer using the aspirin plus pyrolus ligation model. At the end of 4 h after ligation, the rats were sacrificed and ulcer index, gastric juice volume, pH and other biochemical parameter of gastric juice were evaluated. Stomachs of rats were evaluated biochemically to determine oxidant and antioxidant parameters. Pretreatment with gallic acid significantly decreased ulcer index, gastric juice volume, free and total acidity, total protein, DNA content and increased pH and carbohydrates concentration. Gallic acid at a dose of 100 and 200 mg/kg exerted 69.7 and 78.9% ulcer inhibition, respectively. The levels of superoxide dismutase, catalase, reduced glutathione, glutathione reductase, glutathione peroxidise, glucose-6-phosphate dehydrogenase were increased while reduction in myeloperoxidase and lipid peroxidation were observed in the stomach tissues of the drug treated rats. The histopathological studies further confirmed the antiulcer activity of gallic acid. We conclude that the gallic acid possesses antiulcer effect and that these occur by a mechanism that involves attenuation of offensive factors, improvement of mucosal defensive with activation of antioxidant parameters and inhibition of some toxic oxidant parameters.
Keywords: Antioxidant, antisecratoty, antiulcer, aspirin plus pylorus ligation, gallic acid, mucosal defence
Peptic ulcer has become a common health problem affecting a large number of people world-wide causing morbidity and mortality[1]. The pathophysiology of this gastrointestinal disorder is viewed as a net imbalance between mucosal defensive factors such as bicarbonate, prostaglandin, peptides, growth factors and offensive factors such as acid, pepsin[2]. Nonsteroidal drugs, steroids, smoking, alcohol, trauma, shock, Helicobacter pylori and stress have been shown to play a vital role in the pathogenesis of gastric ulcer. Although a wide range of drugs are currently employed in the management of ulcer, but most of them exhibit side effects[2,3]. The use of phytoconstituents to treat major ailments has proved to be clinically effective and less relatively toxic than the existing drugs and also reduces the odious factors serving as a tool in the prevention of peptic ulcer[4].
Gallic acid (3,4,5-trihydroxy benzoic acid) is a naturally abundant polyhydroxy phenolic compound found in gallnuts, grapes, green tea, pineapple, bananas, lemon, strawberries, red wine, etc.[5]. Gallic acid is widely used as dietary herbal supplement[6] ; a recent study demonstrated that about thirty Ayurvedic herbs and their formulation contained a high percentage of gallic acid, and these formulations are widely used for treatment of several diseases in India[7]. Gallic acid possess excellent antioxidant[8], antiobesity[9], hepatoprotective[10], antiasthmatic[11] and anticancer activity[12]. Plant and fruit extracts contains gallic acid as an essential constituent also possess antiulcer activity[13]. A wide number of researches have proved that polyphenolic compounds display a number of pharmacological properties in the gastrointestinal tract, acting as antisecretory, cytoprotective, and antioxidant agents and can be an alternative for the treatment of gastric ulcers[14]. Drugs or formulations that possess powerful antioxidant actions can be more efficient in healing experimentally produced gastric ulcers. However, experimental evidence in favour of antiulcerogenic effect of gallic acid is missing. Therefore, the objective of our study is to assess the antiulcer activity of gallic acid using the aspirin plus pyloric ligation-induced gastric ulcer model in rats, and to evaluate its effects on oxidant and antioxidant parameters in rat stomach tissue.
MATERIALS AND METHODS
Aspirin and famotidine were obtained from Shasan Pharmaceuticals (India). Gallic acid (purity 98%), thiobarbituric acid (TBA), trichloro acetic acid (TCA) and 5,5’-dithiobis (2-nitrobenzoic acid) (DTNB) and ethylenediaminetetraacetic acid (EDTA) were obtained from Sisco Research Laboratories Pvt. Ltd., Mumbai. Bovine serum albumin was purchased from Loba Chemie Pvt. Ltd., Mumbai, and calf thymus DNA was purchased from Sigma Aldrich, USA. All other chemicals used in the study were obtained commercially and were of analytical grade.
Wistar albino rats of either sex weighing between 150 and 250 g were used. Animals were maintained under standard laboratory conditions (23±2°, 12 h light-dark cycle). All animal procedures have been ratified by institutional animal ethics committee in acquiescence with animal experimentation and care bearing the number 817/04/AC/CPCSEA.
Aspirin plus pylorus ligation-induced ulcer:
Gallic acid, aspirin and famotidine were prepared in 0.5% carboxymethyl cellulose (CMC) as suspension and administered orally once daily at a volume of 10 ml/kg for 7 d using an oral gavage needle. The rats were divided into five groups (n=6). Group I was administered with 0.5% CMC, which served as normal control. Group II received only aspirin (200 mg/kg) and served as ulcer control group, groups III and IV were treated with test drug gallic acid (100 and 200 mg/kg, respectively). Group V received famotidine (20 mg/kg) and served as standard.
From day 5 to day 7, rats in groups III-V received aspirin at a dose of 200 mg/kg, 2 h after the administration of the respective drug treatment. Rats in all the groups were anaesthetised with ether on day 8 after 18 h of fasting. The abdomen was cut opened by a small midline incision below the xiphoid process and pylorus portion of the stomach was lifted out and ligated avoiding traction to the pylorus or damage to its blood supply. At the end of 4 h after ligation, the rats were sacrificed with excess of anaesthetic ether and the stomach was dissected out[3].
Measurement of gastric acid secretion, pH and ulcer index:
The stomach of each animal was carefully excised keeping oesophagus closed; the gastric contents were collected and centrifuged at 1000×g for 10 min. The volume of supernatant was measured and expressed as ml/100 g. The pH of the supernatant was measured using digital pH meter[15]. Stomach was opened along the greater curvature and microscopic observation of the lesions in glandular portion was examined for ulcer area by two investigators. Ulcer index (UI) was calculated by using the arbitrary scoring system as loss of normal morphology -1; discoloration of mucosa −1; mucosal oedema -1; haemorrhages −1; petechial point (until 9) −2; petechial point (>10) −3; ulcer up to 1 mm – n×2; ulcer >1 mm – n×3; perforated ulcer – n×4, where, ‘n’ is the number of ulcer found[16].
Biochemical estimation of gastric juice:
Free and total acidity were determined by titrating 1.0 ml gastric juice with 0.01 N sodium hydroxide (NaOH) using the Topfer's reagent and phenolphthalein respectively as indicator and were expressed as mEq/l/100 g[17].
The protein and individual carbohydrates were estimated by precipitating the mucosubstances by treating the gastric juice with 90% ethanol in 1:9 ratio. The precipitate thus obtained was dissolved in 0.1 N NaOH. This solution was used for the estimation of different carbohydrate such as hexosamine, fucose, hexoses and protein according to the method described by Anoop and Jegadeesan[17].
Pepsin activity was determined using a method, which incorporates the digestion of haemoglobin solution by pepsin ensuring the formation of tyrosine. The formed tyrosine was separated and mixed with alkaline reagent and phenol reagent so as to develop a blue colour, which was estimated using the spectrophotometer at 610 nm[15].
Concentration of DNA in gastric juice was estimated using the following method: Gastric juice was treated with EDTA, albumin and TCA, which were then kept frozen for 48 h. Mixture was then thawed at 37° and centrifuged at 1000×g for 30 min at 4°. The precipitate was taken and washed with TCA several times and added to 5% TCA with constant stirring. The solution was heated at 90° in a boiling water bath. The procedure was repeated again and the supernatant was obtained. This hydrolysate was treated with 60% (w/v) perchloric acid and 2% diphenylamine reagent. The mixture was kept in refrigerator (6-10°) for 48 h and the absorbance was read at 600 nm using the blank. The concentration of DNA was determined from a standard curve prepared earlier with calf thymus DNA and expressed as μg/ml of gastric juice/100 g body weight of animal[18].
Antioxidant activity of gallic acid in stomach tissue:
After microscopic examination, the mucosa of the stomach was scraped from the glandular part and suspended in 5.0 ml of cooled 0.15 mol KCl - 10 mmol potassium phosphate buffer (pH 7.4) containing 0.1% Triton X-100 and centrifuged at 1000×g for 10 min. The supernatant of both the tissues were collected and used for estimation of several antioxidant activities.
Superoxide dismutase (SOD) activity was determined by the inhibition of autocatalysed adrenochrome formation in the presence of tissue homogenate at 480 nm. The activity was expressed as μmol/min/mg protein[19].
Decomposition of hydrogen peroxide (H2O2) in the presence of catalase (CAT) was determined at 254 nm. CAT activity was defined as the amount of enzyme required to decompose 1 μmol of H2O2/min, at 25° and pH 7. Results were expressed as μmol H2O2/min/mg protein[20].
Total reduced glutathione (GSH) was determined based on the reaction of GSH with DTNB to give a compound that absorbs at 412 nm. Briefly, 0.5 ml of supernatant was mixed with 2.0 ml of 0.3 mol/l disodium hydrogen phosphate solution. Aliquot of 0.2 ml solution of DTNB (0.4 mg/ml, 1% sodium citrate) was added and the absorbance was measured immediately after mixing at 412 nm. Results were expressed in μmol GSH/min/mg protein[21].
Glutathione reductase (GSSH) activity was estimated spectrophotometrically by measuring the rate of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidation at 340 nm. GSSH activity was expressed as μmol NADPH oxidised/min/mg protein at 30°[22].
Glutathione peroxidase (GPx) activity was determined spectrophotometrically using the following method. Briefly, 100 μl tissue homogenate solutions was mixed with 800 μl of 100 mmol/l potassium phosphate buffer (pH 7.4), containing 1 mmol/l EDTA, 1 mmol/l sodium azide, 0.2 mmol/l NADPH, 1 U/ml GSSH and 1 mmol/l GSH. After 5 min incubation, the reaction was started by the addition of 100 μl 2.6 mmol H2O2 and the absorbance change at 340 nm in 3 min was recorded at 37°. Various controls were carried out and suitably subtracted. GPx activity was expressed as μmol NADPH oxidised/min/mg protein at 37°[9].
Lipid peroxidation was indices by the formation of malondialdehyde (MDA) and hydroperoxide (HP). The estimation of MDA was carried out using the TBA reactive substance test while estimation of HP was carried out after tissue homogenate was treated with Fox reagent. The results were expressed as nmol/min/mg tissue protein[23].
Myeloperoxidase (MPx) activity was determined by the following method. Briefly, 100.0 μl of the homogenate was added to 1.9 ml of 10 mmol/l phosphate buffers (pH 6.0) and 1.0 ml of 1.5 mmol/l O-dianisidine hydrochloride containing 0.0005% (w/v) hydrogen peroxide. The changes in absorbance at 450 nm of each sample were recorded on an UV/Vis spectrophotometer. MPx activities in tissues were expressed as μmol/min/mg protein[24].
Glucose-6-phosphate dehydrogenase (G6PDH) activity was assayed by measuring the increase in absorbance, which occurs at 340 nm due to NADPH being reduced to NADPH. This reaction takes place when two electrons were transferred from glucose-6-phosphate to NADPH catalysed by the enzyme G6PDH in the reaction. The activity was expressed in terms of μmol/min/mg protein[25].
Histopathology:
The damaged or ulcerated parts of stomachs were kept in 10% formalin for 24 h. The tissue was embedded in paraffin and cut into 5 μm thick sections. The sections were stained with haematoxylin-eosin for histopathological examination.
Statistical analysis:
The data are expressed as mean±standard error mean and statistical comparisons were analysed using the statistical package for social science (SPSS Inc., Chicago, IL, USA) version 10.0 employing one-way analysis of variance followed by Dunnett's test. The results were considered statistically significant if the P values were less than 0.05.
RESULTS AND DISCUSSION
Nonsteroidal analgesic and antiinflammatory drugs like aspirin can effects on oxidant and antioxidant mechanisms and interfere prostaglandin synthesis through cyclooxygenase pathways, produce neutrophil and oxygen radical dependent microvascular injury leading to mucosal damage. Aspirin acts directly by increasing the H+ ion transport while on the mucosal epithelial cells, it decreases mucin, surface-active phospholipids, bicarbonate secretion and microvasculature damage by generation of free radicals[26]. In pylorus-ligated rats the gastric acid secretion is an important factor for generation of ulceration. When aspirin was administered to pylorus-ligated rats it further aggravated the acidity and the resistance of the gastric mucosa was decreased thereby imposing extensive damage to the glandular region of the stomach[27]. Aspirin plus pylorus ligation-induced animals models was used to investigate the antiulcerogenic activity of gallic acid, and ulcer control group show significant differences in ulcer parameters when compared to healthy control group.
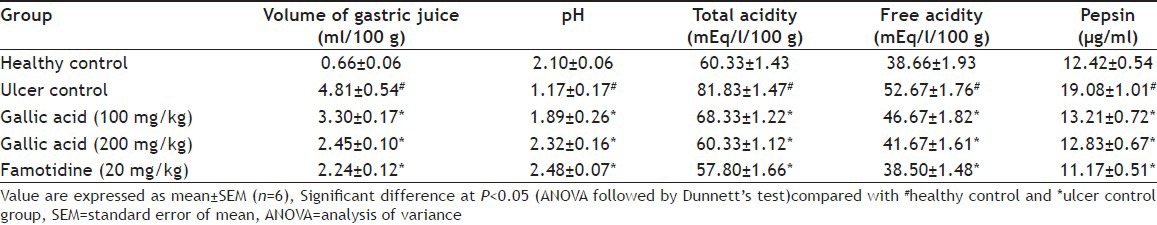
Aspirin plus pylorus ligation-induced animals showed extensive gastric lesions that were confined to the glandular portion of the stomach, which is evidenced by an increase in ulcer index (UI) when compared to untreated control. Pre-treatment with gallic acid (100 and 200 mg/kg) produced 69.66 and 78.93% inhibition of UI, respectively (fig. 1). Treatment with gallic acid caused a decrease in gastric juice volume, total acidity, free acidity and pepsin concentration. Both the dose produced a significant effect, but the higher dose found more effective and produced almost similar effect to that of standard drug. Total and free acidity also decreases significantly (P<0.05) compare to ulcer control group. Pepsin concentration also significantly decreased after treatment with gallic acid. Gastric pH was significantly improved in the ulcer control group after treatment with gallic acid and famotidine (Table 1). These effects may contribute to the cytoprotective effect of gallic acid.
Fig. 1.
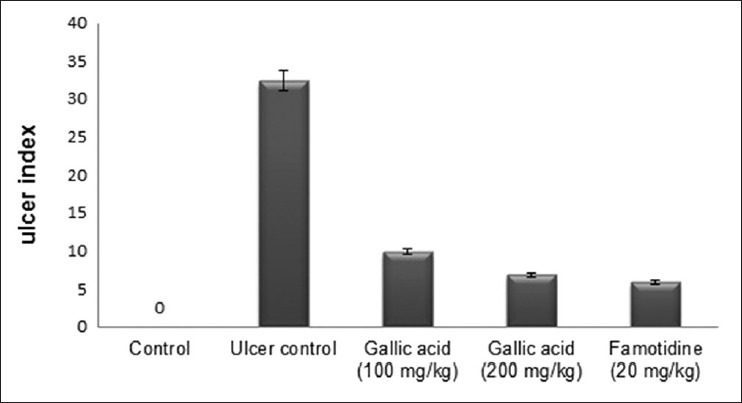
Effect of gallic acid on ulcer index.
Effect of gallic acid on ulcer index in the stomach of aspirin plus pylorus-ligated rats. Gallic acid 100 and 200 mg/kg and famotidine 20 mg/kg showed 69.7, 78.9 and 82.0 % inhibition of ulcers, respectively.
TABLE 1.
EFFECT OF GALLIC ACID ON GASTRIC PARAMETERS IN ASPIRIN PLUS PYLORUS LIGATION-INDUCED ULCER RATS
The levels protein, and DNA concentration in the gastric juice of animals, which received varying dose of gallic acid and famotidine was found to decrease significantly (P<0.05) proving the efficacy of the treatment (Table 2). Increase in protein content of gastric juice resulted in a decrease in total carbohydrate and protein ratio in aspirin plus pylorus ligated rat leading to the damage of gastric mucosa[19]. Treatment with gallic acid decreased the plasma protein leakage from gastric mucosa by strengthening the mucosal barrier. The strengthening of mucosal defence is further exemplified by a decrease in cell exfoliation[21] as seen from the reduction in DNA content of the gastric juice. Results suggest the increase in mucosal defence capacity of the gallic acid.
TABLE 2.
EFFECT OF GALLIC ACID ON TOTAL PROTEIN AND MUCOSAL CARBOHYDRATE CONTENT, PEPSIN AND DNA CONCENTRATION IN GASTRIC JUICE OF ASPIRIN PLUS PYLORUS-LIGATED RATS
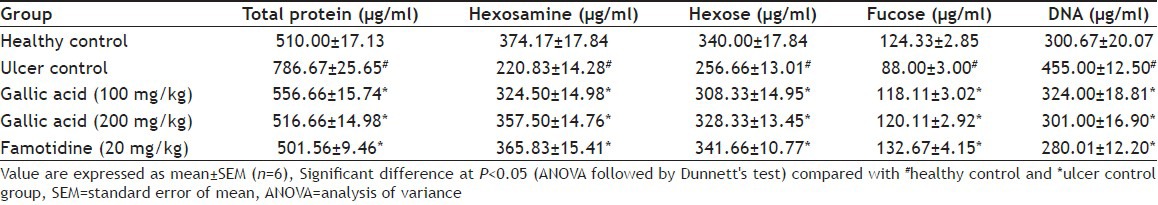
Total hexoses, hexosamine and fucose concentration was also increased significantly in gallic acid (100 and 200 mg/kg) and standard drug treated group (Table 2). Mucus acts as defensive factor by preventing physical damage to mucosa leading to a lesser degree of ulceration. The individual carbohydrates such as hexoses, fucose and hexosamine concentrations are the index of mucin content in gastric juice[19]. Administration of gallic acid resulted in a significant increase in glycoprotein content in gastric mucosa and a net increase in the synthesis of mucus contributing to its ulcer protective role.
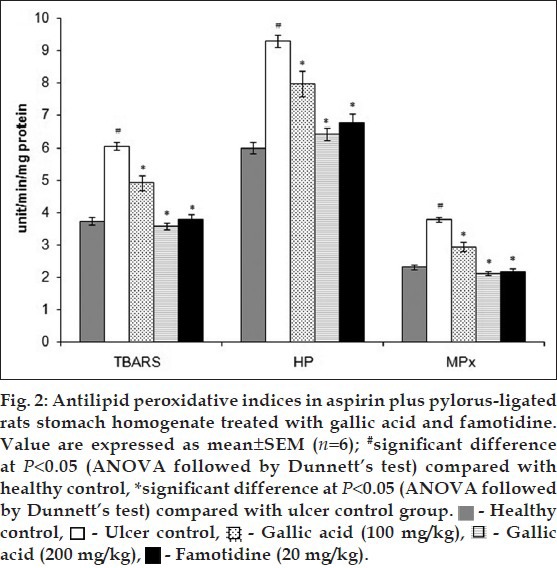
Free radicals are detrimental to the integrity of biological tissue and mediate their injury. Oxidative damage of the gastric mucosal cell membrane by reactive oxygen species (ROS) is the major contributing factor for gastric ulceration. Increased production of ROS causes a decrease in membrane permeability, activities of enzyme and receptors and activation of cells[26,27,28,29]. A significant decrease (P<0.05) in the levels of enzymatic and nonenzymatic antioxidant enzymes such as SOD, CAT, GSH, GPx, GSSH and G6PDH were observed in ulcer control animals when compared to normal rats (Table 3). G6PDH is a cytoplasmic enzyme and acts as a supporter of the primary antioxidant enzymes. It provides coenzymes and substrate to the primary antioxidant enzymes thus playing a protective role against ROS induced oxidative stress[30]. Administration of gallic acid and famotidine escalated the levels of all enzymatic and non-enzymatic antioxidant enzymes to normal justifying the protection offered by them in conditions of gastric damage. Infiltration of neutrophils into gastric mucosal tissue is a critical process in the pathogenesis of gastric ulcer and can be checked by MPx activity. Increased lipid peroxidation is one of the major indications of ROS generation, which can be indices by the increased level of TBARS and HP[23,24,31]. The levels lipid peroxidation parameters such as MDA and HP were significantly elevated in the ulcerative stomach homogenate (fig. 2). A similar increment in level of MPx was also observed in ulcer control. These high levels of MDA, HP and MPx were profoundly reduced in case of rats, which received gallic acid and famotidine in a dose dependent manner. Polyhydroxy phenolic substances like gallic acid having potent antioxidant properties are anticipated to exert protective effects on ulceration.
TABLE 3.
ANTIOXIDANT ACTIVITY OF GALLIC ACID IN RAT STOMACH TISSUE
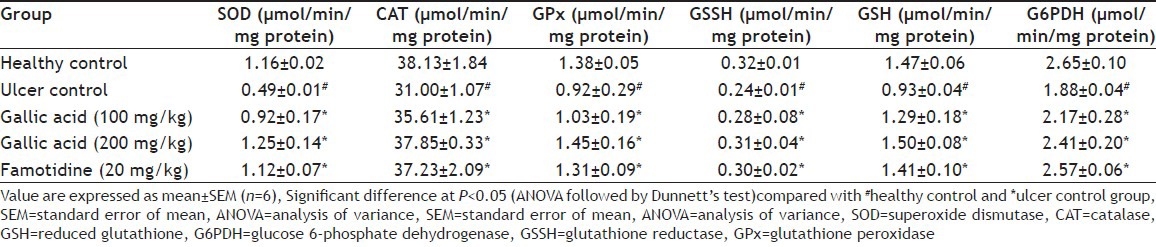
Fig. 2.
Histopathological observation also confirmed the ulcer protective effect of gallic acid. Gallic acid (200 mg/kg) and famotidine treated group does not showed any ulceration though less inflammation in submucosa can be observed (fig. 3). These observations further support the antiulcer effect of gallic acid.
Fig. 3.
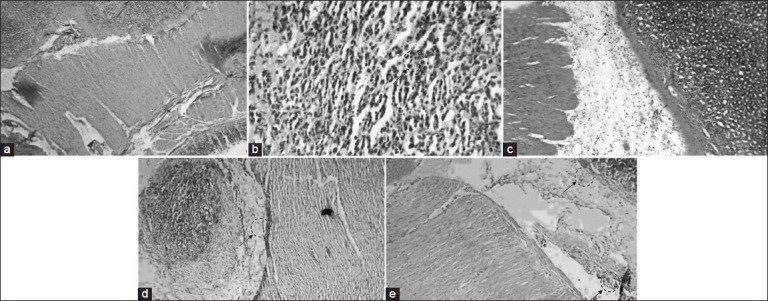
Histopathological examination of gastric mucosal tissue section of normal animals, ulcerated rat after treatment with and without individual drugs or combination treatment.
The stomachs were stained with heamatoxylin and eosin (Magnification 100×). (a) Normal control rat. (b) Ulcerated rat stomach, shows erosion with distorted gastric glands and damaged mucosal epithelium with inflammation and congestion. (c) Ulcerated rat pre-treated with gallic acid (100 mg/kg) presence of mild ulceration and inflammation and congestion of submucosa. (d) Ulcerated rat pre-treated with gallic acid (200 mg/kg), shows gastric mucosa with mild inflammation and congestion of submucosa but no ulceration (e) Ulcerated rat pre-treated with of famotidine (20 mg/kg), shows mild inflammation of submucosa with no evidence of ulceration.
The present results suggest that gallic acid has in vivo antioxidant and antiulcerogenic effect on gastric lesion induced by aspirin plus pyrolus ligation. Gallic acid appears to exert its antiulcer effects by increased mucosal defensive and decreased offensive factors. Gallic acid also appears to activate antioxidant mechanisms and inhibit toxic oxidant mechanisms in stomach tissues, which is also responsible for its potent antiulcer effect.
Footnotes
Sen, et al.: Antiulcerogenic Effect of Gallic Acid
REFERENCES
- 1.Agrawal AK, Rao CV, Sairam K, Joshi VK, Goel RK. Effect of Piper longum Linn., Zingiber officianalis Linn and Ferula species on gastric ulceration and secretion in rats. Indian J Exp Biol. 2000;38:994–8. [PubMed] [Google Scholar]
- 2.Hoogerwerf WA, Pasricha PJ. Pharmacotherapy of gastric acidity, peptic ulcers, and gastroesophageal reflux disease. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw-Hill Medical Publishing Division; 2006. pp. 967–81. [Google Scholar]
- 3.Umamaheswari M, Asokkumar K, Rathidevi R, Sivashanmugam AT, Subhadradevi V, Ravi TK. Antiulcer and in vitro antioxidant activities of Jasminum grandiflorum L. J Ethnopharmacol. 2007;110:464–70. doi: 10.1016/j.jep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Jainu M, Devi CS. Antiulcerogenic and ulcer healing effects of Solanum nigrum (L.) on experimental ulcer models: Possible mechanism for the inhibition of acid formation. J Ethnopharmacol. 2006;104:156–63. doi: 10.1016/j.jep.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 5.Madlener S, Illmer C, Horvath Z, Saiko P, Losert A, Herbacek I, et al. Gallic acid inhibits ribonucleotide reductase and cyclooxygenases in human HL-60 promyelocytic leukemia cells. Cancer Lett. 2007;245:156–62. doi: 10.1016/j.canlet.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Roberts AT, Martin CK, Liu Z, Amen RJ, Woltering EA, Rood JC, et al. The safety and efficacy of a dietary herbal supplement and gallic acid for weight loss. J Med Food. 2007;10:184–8. doi: 10.1089/jmf.2006.272. [DOI] [PubMed] [Google Scholar]
- 7.Borde VU, Pangrikar PP, Tekale SU. Gallic acid in Ayurvedic herbs and formulations. Recent Res Sci Technol. 2011;3:51–4. [Google Scholar]
- 8.Yen G, Duh P, Tsai H. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002;79:307–13. [Google Scholar]
- 9.Hsu CL, Yen GC. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br J Nutr. 2007;98:727–35. doi: 10.1017/S000711450774686X. [DOI] [PubMed] [Google Scholar]
- 10.Jadon A, Bhadauria M, Shukla S. Protective effect of Terminalia belerica Roxb. and gallic acid against carbon tetrachloride-induced damage in albino rats. J Ethnopharmacol. 2007;109:214–8. doi: 10.1016/j.jep.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 11.Dorsch W, Bittinger M, Kaas A, Müller A, Kreher B, Wagner H. Antiasthmatic effects of Galphimia glauca, gallic acid, and related compounds prevent allergen- and platelet-activating factor-induced bronchial obstruction as well as bronchial hyperreactivity in guinea pigs. Int Arch Allergy Immunol. 1992;97:1–7. doi: 10.1159/000236088. [DOI] [PubMed] [Google Scholar]
- 12.Faried A, Kurnia D, Faried LS, Usman N, Miyazaki T, Kato H, et al. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Int J Oncol. 2007;30:605–13. doi: 10.3892/ijo.30.3.605. [DOI] [PubMed] [Google Scholar]
- 13.Govindarajan R, Vijayakumar M, Singh M, Rao ChV, Shirwaikar A, Rawat AK, et al. Antiulcer and antimicrobial activity of Anogeissus latifolia. J Ethnopharmacol. 2006;106:57–61. doi: 10.1016/j.jep.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Sumbul S, Ahmad MA, Mohd A, Mohd A. Role of phenolic compounds in peptic ulcer: An overview. J Pharm Bioallied Sci. 2011;3:361–7. doi: 10.4103/0975-7406.84437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debnath PK, Gode KD, Das DG, Sanyal AK. Effects of propranolol on gastric secretion in albino rats. Br J Pharmacol. 1974;51:213–6. doi: 10.1111/j.1476-5381.1974.tb09649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dias PC, Foglio MA, Possenti A, de Carvalho JE. Antiulcerogenic activity of crude hydroalcoholic extract of Rosmarinus officinalis L. J Ethnopharmacol. 2000;69:57–62. doi: 10.1016/s0378-8741(99)00133-6. [DOI] [PubMed] [Google Scholar]
- 17.Anoop A, Jegadeesan M. Biochemical studies on the antiulcerogenic potential of Hemidesmus indicus R.Br.var. indicus. J Ethnopharmacol. 2003;84:149–56. doi: 10.1016/s0378-8741(02)00291-x. [DOI] [PubMed] [Google Scholar]
- 18.Mukhopadhyaya K, Bhattacharya D, Chakraborty A, Goel RK, Sanyal AK. Effect of banana powder (Musa sapientum var. paradisiaca) on gastric mucosal shedding. J Ethnopharmacol. 1987;21:11–9. doi: 10.1016/0378-8741(87)90089-4. [DOI] [PubMed] [Google Scholar]
- 19.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 20.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 21.Ellman GK. The sulphydryl groups. Ach Biochem Biophys. 1959;32:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 22.Racker E. Glutathione reductase from bakers’ yeast and beef liver. J Biol Chem. 1955;217:855–65. [PubMed] [Google Scholar]
- 23.Rukkumani R, Aruna K, Varma PS, Rajasekaran KN, Menon VP. Comparative effects of curcumin and an analog of curcumin on alcohol and PUFA induced oxidative stress. J Pharm Pharm Sci. 2004;7:274–83. [PubMed] [Google Scholar]
- 24.Odabasoglu F, Cakir A, Suleyman H, Aslan A, Bayir Y, Halici M, et al. Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J Ethnopharmacol. 2006;103:59–65. doi: 10.1016/j.jep.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 25.Cartier P, Leroux JP, Marchand JC. Methods of determination of tissue glycolytic enzymes. Ann Biol Clin (Paris) 1967;25:109–36. [PubMed] [Google Scholar]
- 26.Scheiman JM. NSAIDs, gastrointestinal injury, and cytoprotection. In: Dubois RN, Giardiello FM, editors. NSAIDs, Eicosonoids, and the Gastroenteric Tract. Vol. 25. Philadelphia: Saunders; 1996. pp. 279–98. [DOI] [PubMed] [Google Scholar]
- 27.Sanmugapriya E, Venkataraman S. Antiulcerogenic potential of Strychnos potatorum Linn seeds on aspirin plus pyloric ligation-induced ulcers in experimental rats. Phytomedicine. 2007;14:360–5. doi: 10.1016/j.phymed.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 28.Demir S, Yilmaz M, Köseoğlu M, Akalin N, Aslan D, Aydin A. Role of free radicals in peptic ulcer and gastritis. Turk J Gastroenterol. 2003;14:39–43. [PubMed] [Google Scholar]
- 29.Sairam K, Priyambada S, Aryya NC, Goel RK. Gastroduodenal ulcer protective activity of Asparagus racemosus: An experimental, biochemical and histological study. J Ethnopharmacol. 2003;86:1–10. doi: 10.1016/s0378-8741(02)00342-2. [DOI] [PubMed] [Google Scholar]
- 30.Swarnakar S, Ganguli K, Kundu P, Benergee A, Maity P, Sharma AV. Curcumin regulates expression and activity of matrix metalloproteinases 9 and 2 during prevention and healing of indomethacin induced gastric ulcer. J Biol Chem. 2005;280:9409–15. doi: 10.1074/jbc.M413398200. [DOI] [PubMed] [Google Scholar]
- 31.Ramesh B, Pugalendi KV. Antihyperglycemic effect of umbelliferone in streptozotocin-diabetic rats. J Med Food. 2006;9:562–6. doi: 10.1089/jmf.2006.9.562. [DOI] [PubMed] [Google Scholar]


