Abstract
The aim of this work was to formulate Alstonia boonei dried stem bark powder into tablets by wet granulation method using acacia, gelatine and sodium carboxymethyl cellulose as binders at concentrations of 1, 2, 4 and 8% w/w. The phytochemistry of the stem bark of Alstonia boonei was evaluated. The micromeritic properties of the granules prepared were studied. The tablets were evaluated using the necessary official and unofficial tests. The results of the phytochemical analysis showed that alkaloids, tannins, steroids, saponins, glycosides, flavonoids and terpenoids were present while anthroquinones and acidcompounds were absent. Micromeritic studies showed that Alstonia boonei granules had good flowability. The formulated tablets complied with British Pharmacopoeial specification for weight uniformity, hardness (≥5 kgf) and tablet friability (<1%). For disintegration test, tablets formulated with gelatine and acacia at concentrations of 1, 2 and 4% w/w complied with Pharmacopoeial specification. However, tablets formulated with SCMC (1-8% w/w) and 8% w/w of acacia and gelatine failed the disintegration tests (Disintegration time more than 15 min).
Keywords: Alstonia boonei tablets, antimalarial, micromeritic, phyllotaxy, phytochemical analysis
Alstonia boonei De Wild (Apocyanaceae) is a large ever-green and medicinal plant used extensively in West and Central Africa for the treatment of malaria, fever, intestinal helminthes, rheumatism and hypertension[1,2,3]. A.boonei has a history of use in traditional medicine in Nigeria and central Cameroon for malaria treatment, but also for the prevention of disease[4,5]. Alstonia consists of about 50 species widely distributed in the continents of Africa, Asia and America. The stem bark of A. boonei has been listed in the African Pharmacopoeia as an antimalarial drug and it is used in traditional medicine for the treatment of fever, painful micturition, insomnia, chronic diarrhoea, rheumatic pains as antivenom for snake bites and in the treatment of arrow poisoning[4,5,6]. In vitro antiplasmodial activity of A. boonei alkaloids against both drug sensitive and resistant strains of Plasmodium falciparum and in vivo activity against Plasmodium berghei in mice have been reported[4,7,8,9,10]. The antiinflammatory properties of the alcoholic extract of A. boonei have also been reported[10].
Plants with antimalarial and antiinflammatory properties have been of immense ethnomedicinal use to mankind. In view of the popular and wide spread use of herbal products used in such practice, important technical aspects such as standardization and quality control will be of immense benefit in order to enhance their efficacy and improve patient's compliance[11,12,13,14].
Tablet dosage forms are the most popular and preferred drug delivery systems in terms of precision of unit dose, low-cost, patient compliance and good physical and chemical stability and account for 70-80% of all pharmaceutical dosage forms[15]. Tablets have remained the most common dosage form by which medicaments are usually administered to patients because of their advantages over the other dosage forms[16]. Therefore, the aim of the experiment is to study the phytochemical properties of A. boonei stem bark and to formulate A. boonei stem bark powder tablets. Furthermore, the effect of binder type and concentration on the physicochemical properties of A. boonei tablets was studied.
Hydrochloric acid (HCl), lactose (Merck, Germany), maize starch, gelatin, acacia, ethanol 96% (BDH, England), sodium carboxymethyl cellulose (SCMC) and magnesium stearate (May and Baker, England), distilled water (Lion water, Nsukka, Nigeria). A. boonei stem back powder was obtained from a batch processed in our laboratory. All other reagents and solvents were analytical grade and were used as supplied.
The stem bark of A. boonei was collected from a large A. boonei tree located in University of Nigeria, Nsukka in the month of July 2011. The plant material was authenticated by International Centre for Ethnomedicine and Drug Development Nsukka. The organoleptic properties of the plant were as follows: colour: dark green; odour: nutty roasted; taste: bitter; phyllotaxy: whorled arrangement. The voucher specimen of the plant was deposited in the herbarium of the Department of Pharmacognosy and Environmental Medicines, University of Nigeria, Nsukka.
The stem barks of A. boonei were washed thoroughly in water, dried under a shed (below 40° for 3 days and chopped into tiny pieces. The dried stems were milled using a hammer mill (500# grinder/Fuyu Metal, Linyi Fuyu Metal Products Co., Ltd, China) and thereafter, passed through 52 mm sieve (Turgens and Co., Germany).
Phytochemical tests were carried out on the water extract of the powdered stems for the presence of alkaloids, tannins, saponins, flavonoids, steroids, glycosides, anthroquinones, terpenoids and acid compounds. The tests were carried out using the standard procedures of analysis[17,18].
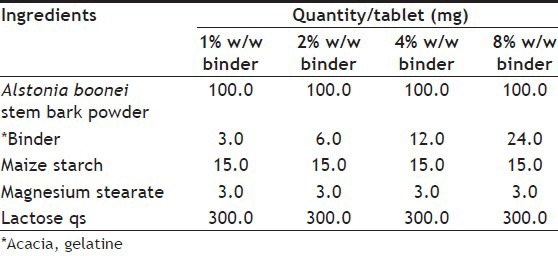
Granules were prepared by wet granulation method using three different binders at concentrations 1, 2, 4 and 8% w/w. Details of granulation are given in Table 1. Lactose was used as filler (8% w/w) and maize starch British Pharmacopoeia (BP) as disintegrant (10% w/w) were dried and mixed for 10 min in a tumbler mixer. The powder mixtures were moistened with the appropriate amount of binder solution. The homogeneous wet mass was then screened through a 1.7 mm sieve and the wet granules dried in a hot air oven at 60° for 1 h. Thereafter, the dried granules were screened through a 1.0 mm sieve.
TABLE 1.
COMPOSITION OF ALSTONIA BOONEI TABLETS
Initially, granules were treated with lubricant, i.e., magnesium stearate. Tablets were prepared by compressing the lubricated granules at 46-48 kgf using a 9.0 mm punch and die set fitted into an automated F3 Manesty single punch tableting machine.
Flow properties of the granules were carried out before compression. Bulk and tapped densities were measured by the following method. About 30 g of the sample was placed in a 100 ml measuring cylinder; the volume occupied by the sample was noted as the bulk volume. The bulk density was obtained by dividing the mass of the sample weighed out by the bulk volume[19,20], as shown in the equation, Bulk density(lB)=Mass of powder (M)/volume of powder (VB).
The cylinder was tapped on a wooden platform by dropping the cylinder from a height of one inch at 2 s interval until there was no change in volume reduction. The volume occupied by the sample was then recorded as the tapped volume. The tapped density was calculated using the formula,Tapped density (lT)=Mass of powder (M)/Tapped volume of powder (VT).
For determination of flow rate and angle of repose, a funnel was properly clamped on to retort stand. The funnel orifice diameter, base diameter and the efflux tubelength were appropriately measured. A 30 g quantity of the granules was weighed out and gradually placed into the funnel with the funnel orifice closed with a shutter. The time taken for the entire sample in the funnel to flow through the orifice was noted. The flow rate was gotten by dividing the mass of the sample by the time of flow in seconds.
The dynamic angle of repose was determined by measuring the height of the heap of powder formed using a cathetometer; the radius was gotten by dividing the diameter by two. Angle of repose (θ) for each granule sample was calculated byequation, θ=tan-1(height of powder heap/radius of powder); and Carr's compressibility indices (%) of the granules were obtained using the formula[19,20], Carr's index (%)=(lT -lB/lT)×100.
Disintegration time test for tablets was conducted using an ErwekaZT 120 basket and rack assembly and 0.1 N HCl maintained at 37.0±1.0° as the disintegration medium. Ten tablets from each batch were used for the test and the procedure being as stipulated in the BP 2009 for normal release tablets[21].
For uniformity of weight, about 20 tablets were randomly selected from each batch. The tablets were weighed individually using an electronic balance (Ohaus Adventurer, China) and the individual weights recorded. The mean weight, standard deviation (SD) and percentage deviation were calculated[21].
Friability test was carried out using 20 tablets randomly selected from each batch of the tablets. The tablets were dedusted and weighed. The tablets were placed into the drum of the friabilator (Erweka GmbH, Germany) and rotated at 25 rpm for 4 min. The tablets were removed from the friabilator, dedusted and reweighed. The friability result was expressed as loss of mass expressed as a percentage of the initial mass[21]. The friability (F) was calculated from the equation, F=(Wo-W/Wo)×100, where Wo and W are the initial and final weights of the tablets, respectively.
Crushing strength test was carried out using a Monsanto-stokes hardness tester. Ten tablets from each batch were randomly selected. Each tablet was placed between the jaws of the hardness tester and force was applied by adjusting the knob of the tester until the tablet integrity failed. The results were recorded in kgf.
Statistical analysis was carried out using the SPSS version 14.0 (SPSS Inc., Chicago, IL. USA). All values are expressed as mean±SD. Data were analysed by one-way analysis of variance. Differences between means were assessed by a two-tailed Student's t-test. P<0.05 was considered statistically significant.
The measurement of the flow properties of granules is essential before tableting and capsule filling because variation in particle flow will automatically cause variation in tablet/capsule weight and active ingredient variation. The flow property of bulk material results from the cohesive forces acting on individual particles such as van der Waals, electrostatic, surface tension, interlocking and friction[19]. The flow of powder during manufacturing dictates the quality of the product in terms of weight, hardness and content uniformity of the tablets[19].
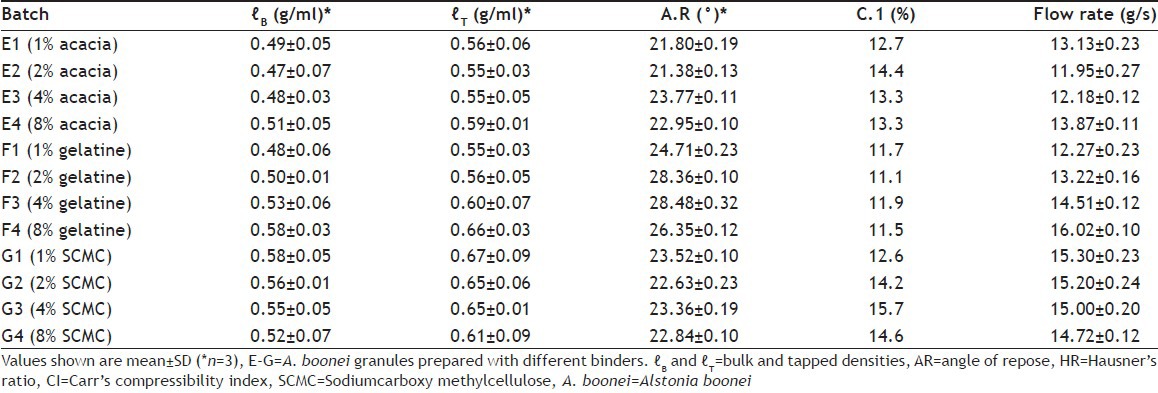
The results of micromeritic properties of A. boonei stem bark powder granules presented in Table 2 showed that the values fell within the acceptable range for good powder flow. Values of angle of repose were significantly (P<0.05) below 30°, which showed that the granules had low interparticulate friction and hence good flowability. The results of Carr's compressibility index showed that the granules were within the specified limits for good powder flow and ranged from 11.1 to 15.7%.
TABLE 2.
MICROMERITIC PROPERTIES OF ALSTONIA BOONEI STEM BARK GRANULES
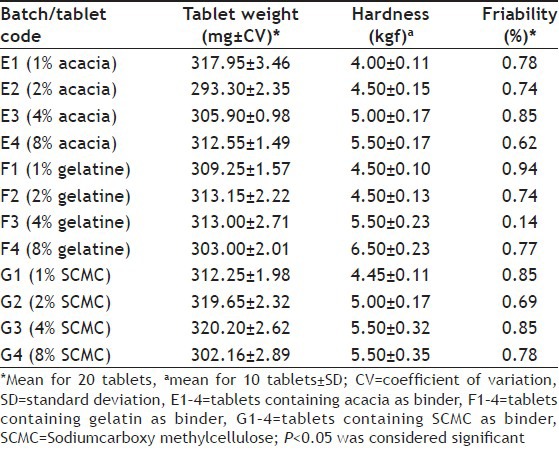
The properties of tablets containing A. boonei stem bark powder presented in Table 3 showed that the tablets exhibited good hardness-friability profile. Tablets hardness ranged from 4.00±0.11 to 5.50±0.17 kgf for tablets formulated with 1 and 8% w/w acacia, 4.50±0.10-6.50±0.23 kgf for tablets formulated with 1 and 8% w/w gelatin and 4.45±0.11-5.50±0.35 kgf for tablets formulated with SCMC. All the A. boonei stem bark powder tablets complied with BP specification for tablets friability of <1%. Results of tablets weight uniformity presented in Table 3 showed that all the batches of tablets passed the uniformity of weight test and deviations obtained complied with BP standards of not more than 5% for tablets weighing 250 mg or more[21].
TABLE 3.
PROPERTIES OF ALSTONIA BOONEI STEM BARK POWDER TABLETS
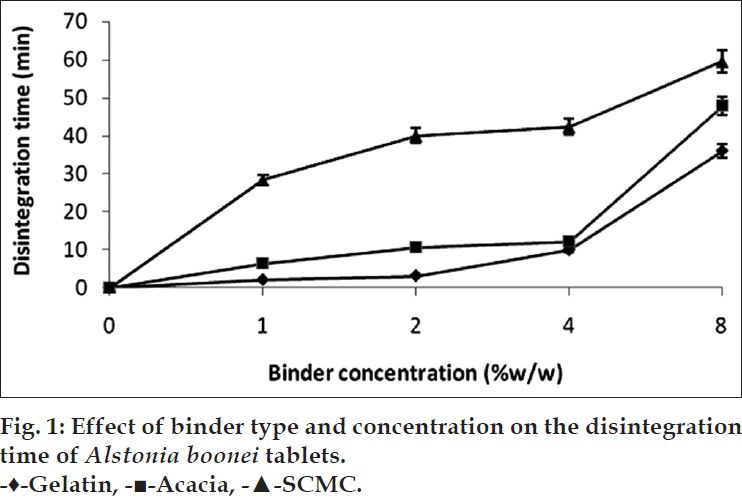
The results of tablets disintegration time test of A. boonei tablets formulated with different binder types and varying concentration of binders are shown in fig. 1. From the results, tablets formulated with gelatine and acacia at concentrations of 1, 2 and 4% w/w complied with BP 2009 specification for normal release tablets. However, tablets formulated with SCMC (1-8% w/w) and 8% w/w of acacia and gelatine failed the disintegration time test for normal release tablets as there disintegration times were significantly above 15 min (P<0.05).
Fig. 1.
The results of the phytochemical screening of the plant stem bark are shown in Table 4. The results indicated the presence of very important phytochemicals in the stem bark. Phytochemical analysis of A. boonei stem bark revealed the presence of alkaloids, saponins, tannins, saponins, steroids, flavonoids and cardiac glycosides in substantial quantities. Anthroquinones and acid compounds were however, not found in the plant stem bark. The presence of alkaloids in high concentration in the plant stem bark explains the traditional use of the plant for the treatment of malaria. The medicinal plants that are moderately rich in alkaloids and tannins have potential health promoting effects[22,23]. Similarly, saponins have anticarcinogenic properties and other health benefits[24]. Alshawsh et al., reported that tannins may have antiplasmodial activity[2]. The stem bark of the plant also contains glycosides. Cardiac glycosides are used to treat heart problems that may result from severe malaria attack. Phytochemicals are non-nutritive plant chemicals that have protective or disease preventive properties. Plants produce these chemicals substances to protect themselves and they are also believed to protect humans against certain diseases[25,26].
TABLE 4.
RESULTS OF THE PHYTOCHEMICAL SCREENING OF ALSTONIA BOONEI STEMBARK

ACKNOWLEDGMENTS
The authors are thankful to Mr. A. O. Ozioko, a consultant taxonomist with the International Centre for Ethnomedicine and Drug Development (InterCEDD) Nsukka, Nigeria, for plant material authentication.
Footnotes
Chime, et al.: Alstonia Boonei Stem Bark Powder Tablets
REFERENCES
- 1.Terashima H. Names, use and attributes of plants and animals among the Ituri forest foragers: A comparative ethnobotanical and ethnozoological study. Afr Study Monogr. 2003;28:7–24. [Google Scholar]
- 2.Alshawsh MA, Mothana RA, AlShamahy HA, Alsllami SF, Lindequist U. Assessment of antimalarial activity against Plasmodium falciparum and phytochemical screening of some Yemeni medicinal plants. Evid Based Complement Alternat Med. 2009;6:453–6. doi: 10.1093/ecam/nem148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betti JL. An ethnobotanical study of medicinal plants among the Baka Pygmiesinthedjabiospherereserve, Cameroon. Afr Study Monogr. 2004;25:1–27. [Google Scholar]
- 4.Majekodunmi SO, Adegoke OA, Odeku OA. Formulation of the extract of the stem bark of Alstonia boonei as tablet dosage form. Trop J Pharm Res. 2008;7:987–94. [Google Scholar]
- 5.Tepongning RN, Lucantoni L, Nasuti CC, Dori GU, Yerbanga SR, Lupidi G, et al. Potential of a Khaya ivorensis-Alstonia boonei extract combination as antimalarial prophylactic remedy. J Ethnopharmacol. 2011;137:743–51. doi: 10.1016/j.jep.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 6.Ojewole AO. Studies on the pharmacology of echitamine, an alkaloid-from the stem bark of Alstonia boonei L.(Apocynaceae) Int J Crude Drug Res. 1984;22:121–43. [Google Scholar]
- 7.Wright CW, Allen D, Phillipson JD, Kirby GC, Warhurst DC, Massiot G, et al. Alstonia species: Are they effective in malaria treatment? J Ethnopharmacol. 1993;40:41–5. doi: 10.1016/0378-8741(93)90087-l. [DOI] [PubMed] [Google Scholar]
- 8.Vasanth S, Gopal RH, Rao RH, Rao RB. Plant antimalarial agents. Ind J Sci Res. 1990;49:68–77. [Google Scholar]
- 9.Awe SO, Opeke OO. Effects of Alstonia congensis on Plasmodium berghei in mice. Fitoterapia. 1990;61:225–9. [Google Scholar]
- 10.Osadebe PO. Antiinflmmatory properties of the root bark of A. boonei. Niger J Nat Prod Med. 2002;6:39–41. [Google Scholar]
- 11.Bonati A. How and why should westandardize phytopharmaceutical drugs for clinical validation? J Ethnopharmacol. 1991;32:195–7. doi: 10.1016/0378-8741(91)90117-v. [DOI] [PubMed] [Google Scholar]
- 12.Elisabetsky E, Amador TA, Albuquerque RR, Nunes DS, CarvalhoAdo C. Analgesic activity of Psychotria colorata (Willd exR. and S.) Muell. Arg. alkaloids. J Ethnopharmacol. 1995;48:77–83. doi: 10.1016/0378-8741(95)01287-n. [DOI] [PubMed] [Google Scholar]
- 13.Patwardhan B. Ethnopharmacology and drug discovery. J Ethnopharmacol. 2005;100:50–2. doi: 10.1016/j.jep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Bulus A, Abdul KH. Studies on the use of Zizyphus spina-christi against pain in rats and mice. Afr J Biotechnol. 2007;6:1317–24. [Google Scholar]
- 15.Nachaegari SK, Bansal AK. Coprocessed excipients for solid dosage forms. Pharm Technol. 2004:52–64. [Google Scholar]
- 16.Okoye EI, Onyekweli AO, Olobayo OK, Arhewoh MI. Brittle fracture index (BFI) as a tool in the classification, grouping and ranking of some binders used in tablet formulation: Lactose tablets. Sci Res Ess. 2010;5:500–6. [Google Scholar]
- 17.Harborne JB. London: Academic Press; 1993. Phytochemistry; pp. 89–131. [Google Scholar]
- 18.Sofowora H. Medicinal Plants and Traditional Medicine in Africa. 2nd ed. Ibadan Nigeria: Spectrum Books Ltd., Sunshine House; 1993. Screening plants for bioactive agents; pp. 134–56. [Google Scholar]
- 19.Trease GE, Evans WC. 15th ed. London: Saunders Publishers; 2002. Pharmacology; pp. 42–4. (221-306). 331-93. [Google Scholar]
- 20.Yüksel N, Türkmen B, Kurdoğlu AH, Başaran B, Erkin J, Baykara T. Lubricant efficiency of magnesium stearate indirect compressible powder mixtures comprising cellactose® 80 and pyridoxine hydrochloride. FABAD J Pharm Sci. 2007;32:173–83. [Google Scholar]
- 21.Aulton ME. 3rd ed. Edinburg: Churchill Livingstone; 2007. Pharmaceutics; the Science of Dosage Form Design; pp. 197–210. [Google Scholar]
- 22.Vol. 111. London: The Commision Office; 2009. British Pharmacopoaeia; pp. 6578–85. [Google Scholar]
- 23.Ikewuchi CC, Ikewuchi JC. Chemical profile of Pleurotus tuberregium (Fr) Sings Sclerotia. Pac J Sci Tech. 2008;10:28–30. [Google Scholar]
- 24.Jigam AA, Helmina O, Dauda BE, Okogun JO. Polygalloyl tannin isolated from the root of Acacianil oticadel. (Leguminoseae) is effective against Plasmodium berghei in mice. J Med Plants Res. 2010;4:1169–75. [Google Scholar]
- 25.Olajide OA, Awe SO, Makinde JM, Ekhelar AI, Olusola A, Morebise O, et al. Studies on the antiinflammatory, antipyretic and analgesic properties of Alstonia boonei stem bark. J Ethnopharmacol. 2000;71:179–86. doi: 10.1016/s0378-8741(99)00200-7. [DOI] [PubMed] [Google Scholar]
- 26.Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4:685–8. [Google Scholar]


