Abstract
Renal injury is the most common side-effect of aminoglycosides. These antimicrobial drugs are particularly effective against Gram-negative microorganisms. The present study was conducted to investigate the renal protective activity of Cymbopogon citratus in gentamicin-induced nephrotoxicity. Male rabbits were divided into four groups (n=6) including group 1 (0.9% saline treated), group 2 (80 mg/kg/day gentamicin-treated), group 3 (200 mg/kg/day Cymbopogon citratus treated) and group 4 (80 mg/kg/day gentamicin and 200 mg/kg/day Cymbopogon citratus treated). Biochemical kidney functioning parameters, urinary enzymes and histopathological examination were performed. The results of the present study showed that simultaneous administration of Cymbopogon citrates and gentamicin significantly protected alteration in body weight, blood urea nitrogen, serum creatinine, creatinine clearance, serum uric acid, serum electrolytes, urinary volume, urinary protein, urinary lactate dehydrogenase and urinary alkaline phosphatase induced by gentamicin. Histological examination of the kidney also suggested the same. It is concluded from the current study that co-administration of Cymbopogon citratus with gentamicin for 3 weeks successfully prevented renal damage associated with aminoglycosides.
Keywords: Aminoglycoside, antioxidant, Cymbopogon citratus, lemon grass, renal injury
Aminoglycosides are bactericidal drugs, which are obtained from different species of Streptomyces. These drugs have a number of chemical, pharmacological, toxicological and antimicrobial properties[1]. In spite of broad spectrum antimicrobial properties, they are mostly used for the treatment of Gram-negative bacterial infections. Gentamicin is one of the most commonly used aminoglycosides, which has specific concentration dependent bactericidal activity with low chances of bacterial resistance[2]. Despite all these qualities, they have been drug of fear owing to their nephrotoxic properties. If the administration of aminoglycosides in higher doses proved safe then there would be no need to develop new indications against aerobic gram negative bacilli[3]. Cymbopogon citratus (Gramineae) commonly known as lemongrass. It is widely used as tea in the form of hot decoction. The decoction is medicinally used as analgesic, antipyretic, antiseptic, sedative, carminative and stomachic[4]. The plant has been reported to have strong antimicrobial activity against a large number of microorganisms except Pseudomonas aeruginosa[5]. Laboratory studies showed that it has strong antioxidant properties[6]. Further, it has been presented with antifungal activity against Aspergillus fumigatus and Trichophyton rubrum[7] and is used in the treatment of oral thrush in human immunodeficiency virus/acquired immunodeficiency syndrome patients[8].
If the fear of nephrotoxicity associated with aminoglycosides is allayed, Gram-negative infections will better be treated by using these drugs. Therefore, C. citratus was investigated for the renal-protective effects owing to its strong flavonoid contents and antioxidant properties, which may help in the inhibition of oxidative injury and may serve as protectant against gentamicin-induced toxicity.
Injection Refobacin® contained 80 mg gentamicin (Merck, Pakistan) were purchased from a local pharmacy in Abbottabad, Pakistan. Dried leaves of C. citratus (3 kg) were purchased from local market, identified and authenticated by Government College, Abbottabad, Pakistan. A voucher specimen (1020) was submitted to the Botany department of the same institute.
The plant material was ground with the help of grinding machine (ZK-115, Japan) and extracted with 70% ethanol. The ethanol extract was evaporated under reduced pressure with the help of rotary evaporator (R-210, Germany)[9]. Preliminary phytochemical study was performed for the presence of alkaloids, glycosides, terpenes, saponins, tannins, flavonoids and carbohydrates[10].
Total 24 male rabbits of mixed breed (1-1.5 kg) were kept for acclimatization in the animal house of Frontier Medical College, Abbottabad. The study was approved from the Scientific Society University of Malakand Pakistan. All animals were maintained on the same diet with 12 h light and dark cycles. Animals were randomly divided into four groups with six rabbits in each group. Control group animals (group C) were treated with isotonic saline (2 ml, i.m), whereas gentamicin-treated animals (group G) were administered gentamicin (80 mg/kg/day, i.m) for a period of 21 days. Group GC-ci animals were treated with co-therapy of gentamicin (80 mg/kg/day, i.m) and C. citratus (200 mg/kg/day, p.o) while group C-ci animals were administered C. citratus (200 mg/kg/day, p.o).
Blood urea nitrogen (BUN) was measured by modified Bertholot's indophenol assay while serum creatinine was measured by Jaffe reaction[11]. Commercially available reagents (ProDia International, UAE) were used with the help of power lab 300 (Merck, Germany). While serum uric acid was measured by using commercially available reagents (Egyptian Company for Biotechnology, Egypt) with the help of power lab 300 (Merck, Germany). Further, creatinine clearance was calculated by using the formula; Creatinine clearance=(Urinary creatinine concentration/serum creatinine concentration)×urinary volume.
Serum calcium was measured by cresolphthalein complexone method[12], using commercially available reagents (Randox Laboratories Limited, UK). However, serum sodium and serum potassium was measured by using flame photometer (PFP-7, England)[12]. Urinary protein, alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) was measured by using commercially available reagents (DiaSys Diagnostic GmbH, Germany) according to the German Society of Clinical Chemistry[13,14].
Both kidneys were isolated on the last day of the experimental period and fixed in formalin solution (10%). The tissues were dehydrated with ascending grades of ethanol and cleared with the help of xylene solution. The cleared tissues were imbedded in paraffin wax and cut down into a number of sections (<3 μ). Slides were stained with haematoxylin and eosin dyes and examined under light microscope (Leitz, Germany).
Results were expressed as mean±standard error and compared by using one-way analysis of variance followed by Dunnett-test with the help of Graph-Pad prism (version-v). The difference was considered significant when the P value was less than 0.05.
Although human's susceptibility to gentamicin may be less than that of animals but the extent of cell injury is similar in both cases even in therapeutic doses[15]. However, renal functional and morphological alterations are observed only when the drug is given in at least 5-10 folds higher doses[16]. Keeping this hypothesis in mind, daily dose of 80 mg/kg of gentamicin was used in the current study to induce significant nephrotoxicity.
Preliminary phytochemical study of C. citratus revealed the presence of a large amount of tannins and terpenes, moderate amount of carbohydrates and flavonoids and a mild amount of alkaloids, saponins and glycosides. The flavonoid contents have been presented with antioxidant properties[6] and the antioxidant properties of the plant might responsible for their nephroprotective activities.
Gentamicin has been reported with significant weight loss in a dose dependent manner[17]. Similarly, in the current study, gentamicin-treated animals significantly lost their body weight (10.795±1.09% vs. control group 0.155±0.91%) provides a state of negative nitrogen balance, which thus gave other statement of substantial potassium loss[18]. Further group GC-ci and C-ci lost their body weight (2.03±0.27% and −0.91±0.81%, respectively) significantly different from gentamicin-treated animals. Estimation of BUN has been thought to be the most important biomarker for the assessment of renal injury[19]. BUN increased significantly in gentamicin-treated animals (54.18±2.60 mg/dl vs. control group 14.1±1.12 mg/dl) on the last day of the study period. However, group GC-ci and C-ci animals were significantly different from gentamicin-treated animals (19.23±1.0 mg/dl and 13.0±0.77 mg/dl respectively). Serum creatinine was also increased in gentamicin-treated animals significantly from the control group animals, group GC-ci and C-ci animals as given in Table 1. The relation between serum creatinine and tubular necrosis has also been presented with the suggestion that necrotic debris in the lumen of tubules might responsible for the elevated serum creatinine[20].
TABLE 1.
BODY WEIGHT, SERUM BUN, SERUM CREATININE, CREATININE CLEARANCE AND SERUM URIC ACID
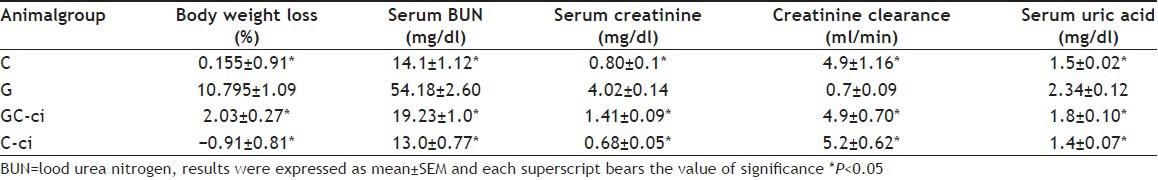
Serum uric acid increased significantly in gentamicin-treated animals on the last day of the study period (2.34±0.12 mg/dl vs. control group 1.5±0.02 mg/dl). However, group GC-ci and C-ciwas significant from gentamicin-treated animals (1.8±0.10 mg/dl and 1.4±0.07 mg/dl, respectively). Serum uric acid is the final metabolite of purine; therefore, any change in the glomerular filtration rate may lead to increase serum uric acid[21]. Creatinine clearance decreased in gentamicin-treated animals significantly different from the control group animals. Further, creatinine clearance of group GC-ci and C-ci were significantly different from gentamicin-treated group as given in Table 1. As gentamicin-induced renal damage is associated with a significant rise in serum BUN, serum creatinine and fall in glomerular filtration rate[16].
Serum potassium decreased significantly in gentamicin-treated animals (3.43±0.17 mEq/l vs. control group 5.10±0.24 mEq/l) on the last day of the study period, which correlate with the previous reports[18,22,23]. Group GC-ci and C-ci was significantly different from gentamicin-treated animals (4.11±0.18 mEq/l and 5.16±0.32 mEq/l, respectively). The hypokalemia induced by gentamicin has also been reported previously[16] and is thought to be owing to the depression of Na+-K+-ATPase activity, which is induced by gentamicin[24].
Serum calcium was also decreased significantly in gentamicin-treated animals (7.68±0.21 mg/dl vs. control group 9.72±0.25 mg/dl) as reported previously[23]. Further, group GC-ci and C-ciwas significantly different from gentamicin-treated animals as given in Table 2. However, in opposition, gentamicin has been reported with no effect on serum calcium[18]. Resistance to the action of parathyroid hormones occurs in the renal toxicity may have a significant role in the induction of hypocalcaemia[23]. Serum sodium remained statistically unchanged in all experimental groups, ranged between 137.67±1.09 mEq/l and 140.17±1.01 mEq/l as given in Table 2. However, an abnormal urinary excretion of sodium, potassium, calcium and magnesium in gentamicin-treated animals was reported previously[16].
TABLE 2.
SERUM ELECTROLYTES AND URINARY PROTEIN EXCRETION
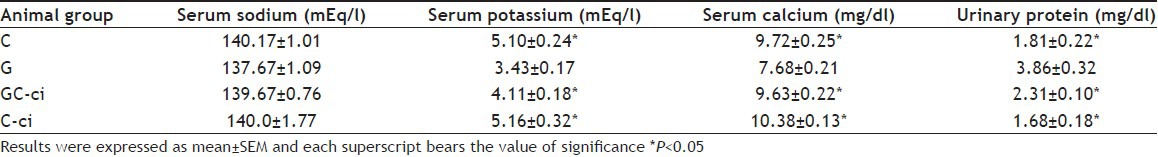
Increase in urinary protein excretion was observed in gentamicin-treated animals (3.86±0.32 mg/dl vs. control group 1.81±0.22 mg/dl) on the last day of the study period. Further group GC-ci and C-ci was significantly different from gentamicin-treated animals (2.31±0.10 mg/dl and 1.68±0.18 mg/dl, respectively). Excessive protein excretion may because of proximal tubular damage associated with gentamicin administration, may lead to cellular damage[25].
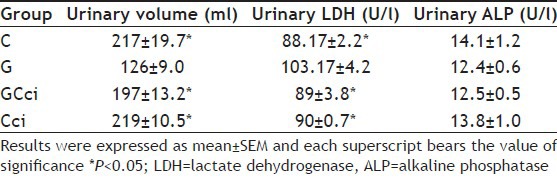
Urinary volume decreased in gentamicin-treated animals (168±11.9 ml/24 h vs. control group 200±9.1 ml/24 h) on the last day of the study period. Further group GC-ci and G-ci was significantly different from gentamicin-treated animals as given in Table 3. Some of the researchers studied tubular brush border enzymes to investigate the renal damage[26]. Therefore, urinary enzymes including LDH and ALP were studied. LDH excretion was increased in gentamicin-treated animals significantly different from the control group animals may because of leaking of cytosolic LDH due to ruptured cell membrane[27]. Group GC-ci and C-ci were significantly different from gentamicin-treated animals as given in Table 3. Gentamicin has been reported with significant nephrotoxicity associated with a significant increase in urinary ALP and LDH[28]. However, ALP was remained same in the current study in all experimental groups including gentamicin-treated and control group animals as given in Table 3.
TABLE 3.
URINARY VOLUME AND ENZYMES EXCRETION
Histopathological examination of gentamicin-treated animals revealed the presence of regenerating cells and necrosis observed side-by-side as reported previously[16,23]. However, control group animals showed normal glomerular and tubular structures with no detectable amount of necrosis fig. 1a and b. Gentamicin-treated animals were observed with increased cellularity and atropic glomeruli. Necrosis of most of the proximal tubules was seen in which tubular and cellular pattern was lost. However, medulla presented a number of ruptured tubules with granular casts (fig. 1c and d). Animals treated with simultaneous administration of gentamicin and C. citratus presented normal glomeruli and tubules with hazy appearance and regenerative activities (fig. 1e and f). Further, animals treated with C. citratus exhibited intact parenchyma with no evidence of tubular necrosis or any significant abnormality in glomeruli (fig. 1g and h). In the current study, the presence of hyaline and granular casts in the proximal tubules of gentamicin-treated animals causes’ tubular degeneration may lead to leaking of proteins. The tubular back-leak of filtrate due to obstruction may cause depression of glomerular filtration rate lead to renal failure[26]. Further, animals treated with co-therapy of C. citratus and gentamicin showed vacuoles in their proximal tubular cells; may because of pinocytotic activity. This pinocytotic activity may due to amino acids, proteins and salts. The exact mechanism is not clear but not related with substantial cellular injury[22].
Fig. 1.
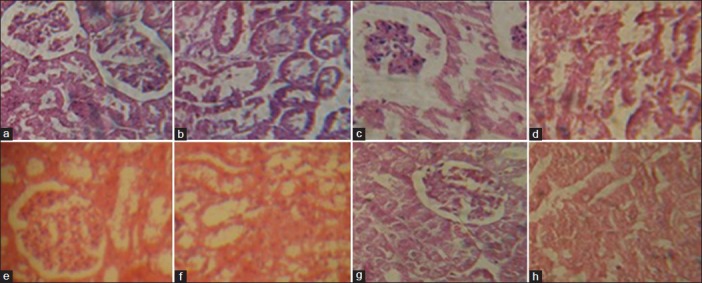
Histopathogical examination of control, toxic and extract-treated animals.
Photomicrographs of the control group animals: (a) renal cortex presenting normal glomeruli with no evidence of necrosis and (b) renal medulla shows normal tubules with no major abnormality. Photomicrographs of gentamicin-treated animals: (c) renal cortex presenting atropic glomeruli with hydropic changes and necrotic proximal tubules with the loss of cellular pattern and (d) Renal medulla shows ruptured tubules with a number of cast cells. Photomicrographs of animals treated with co-therapy of Cymbopogon citratus and gentamicin: (e) renal cortex presenting normal glomeruli and (f) renal medulla shows normal tubules with hazy appearance and regenerative activity. Photomicrographs of animals treated with C. citratus: (g) renal cortex presenting normal structures and (h) renal medulla shows normal tubules with no significant necrosis.
Co-administration of daily dose of 200 mg/kg of C. citratus for 3 weeks successfully prevented renal damage associated with aminoglycosides assessed by renal functioning parameters and histopathological examination.
ACKNOWLEDGMENTS
Higher Education Commission, Government of Pakistan is highly acknowledged for their financial support through indigenous PhD scholarship (batch-iv). Also authors thanks Prof. Umar Farooq, Government College, Abbottabad, Pakistan for identification and authentication of plant material.
Footnotes
Ullah, et al.: Cymbopogon citratus and Nephropotection
REFERENCES
- 1.Jawetz H. Aminoglycosides and polymyxins. In: Katzung BG, editor. Basic and Clinical Pharmacology. New York: Appleton and Lang; 1992. pp. 645–52. [Google Scholar]
- 2.Chow JW, Fine MJ, Shlaes DM, Quinn JP, Hooper DC, Johnson MP, et al. Enterobacter bacteremia: Clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115:585–90. doi: 10.7326/0003-4819-115-8-585. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert DN, Bennett WM. Polyaspartic acid for the prevention of aminoglycoside nephrotoxicity. Prospectus. 1993 [Google Scholar]
- 4.Negrelle RR, Gomes EC. C. citratus chemical composition and biological activities. Rev Bras Pl Med. 2007;9:80–92. [Google Scholar]
- 5.Bassolé IH, Lamien-Meda A, Bayala B, Obame LC, Ilboudo AJ, Franz C, et al. Chemical composition and antimicrobial activity of Cymbopogon citratus and Cymbopogon giganteus essential oils alone and in combination. Phytomedicine. 2011;18:1070–4. doi: 10.1016/j.phymed.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Tiwari M, Dwivedi UN, Kakkar P. Suppression of oxidative stress and pro-inflammatory mediators by Cymbopogon citratus D. Stapf extract in lipopolysaccharide stimulated murine alveolar macrophages. Food Chem Toxicol. 2010;48:2913–9. doi: 10.1016/j.fct.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Khan MS, Ahmad I. In vitro antifungal, anti-elastase and anti-keratinase activity of essential oils of Cinnamomum-, Syzygium- and Cymbopogon- species against Aspergillus fumigatus and Trichophyton rubrum. Phytomedicine. 2011;19:48–55. doi: 10.1016/j.phymed.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Wright SC, Maree JE, Sibanyoni M. Treatment of oral thrush in HIV/AIDS patients with lemon juice and lemon grass (Cymbopogon citratus) and gentian violet. Phytomedicine. 2009;16:118–24. doi: 10.1016/j.phymed.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad M, Mahmood Q, Gulzar K, Akhtar MS, Saleem M, Qadir MI. Antihyperlipidemic and hepatoprotective activity of Dodonaea viscose leaves extracts in alloxan-induced diabetic rabbits (Oryctolaguscuniculus) Pak Vet J. 2012;32:50–4. [Google Scholar]
- 10.Sofowora EA. Medicinal Plants and Traditional Medicine in Africa. 3rd ed. Nigeria: Spectrum Books Limited; 1993. Phytochemical Assays; pp. 150–3. [Google Scholar]
- 11.Smith ST. Non protein nitrogen. In: Bishop ML, Duben-Von Laufen JL, Fody EP, editors. Clinical Chemistry: Principles, Procedures, Correlations. Philadelphia: JB Lippincott Company; 1985. pp. 411–23. [Google Scholar]
- 12.Blosser N. Electrolytes. In: Bishop ML, Duben-Von Laufen JL, Fody EP, editors. Clinical Chemistry: Principles, Procedures, Correlations. Philadelphia: JB Lippincott Company; 1985. pp. 263–89. [Google Scholar]
- 13.Johnson AM, Rohlfs EM, Silverman LM. Proteins. In: Burtis CA, Ashwood ER, editors. Tietz Textbook of Clinical Chemistry. 3rd ed. Philadelphia: WB Saunders; 1999. pp. 477–540. [Google Scholar]
- 14.Deutsch GK. Standardisierung von Methoden zur Bestimmung von Enzymaktivitaten in biologischen flussigkeiten (Recommendation of the German Society of Clinical Chemistry. Standardization of methods for measurement of enzymatic activities in biological fluids) Z klin Chem Klin Biochem. 1972;10:182–92. [Google Scholar]
- 15.Kosek JC, Mazze RI, Cousins MJ. Nephrotoxicity of gentamicin. Lab Invest. 1974;30:48–57. [PubMed] [Google Scholar]
- 16.Bennett WM, Elzinga LW, Porter GA. Tubulointerstitial disease and toxic nephropathy. In: Brenner BM, Rector FC, editors. The Kidney. 4th ed. Philadelphia: WB Saunders Company; 1991. pp. 1430–96. [Google Scholar]
- 17.Tavafi M, Ahmadvand H, Toolabi P. Inhibitory effect of olive leaf extract on gentamicin-induced nephrotoxicity in rats. Iran J Kidney Dis. 2012;6:25–32. [PubMed] [Google Scholar]
- 18.Brinker KR, Bulger RE, Dobyan DC, Stacey TR, Southern PM, Henrich WL, et al. Effect of potassium depletion on gentamicin nephrotoxicity. J Lab Clin Med. 1981;98:292–301. [PubMed] [Google Scholar]
- 19.Guyton AC. Text Book of Medical Physiology. 8th ed. Philadelphia: WB Saunders Company; 1991. Renal disease, Diuresis and micturition; pp. 344–54. [Google Scholar]
- 20.Solez K. International Review of Experimental Pathology. New York: Academic Press; 1983. Pathogenesis of acute renal failure; pp. 321–6. [PubMed] [Google Scholar]
- 21.Meena MK, Kushwah HK, Rajagopala M, Ravishankar B. An experimental evaluation on nephroprotective activity of Nagaradi Kashaya. AYU. 2009;30:55–61. [Google Scholar]
- 22.Thompson JR, Simonsen R, Spindler MA, Southern PM, Cronin RE. Protective effect of KCl loading in gentamicin nephrotoxicity. Am J Kidney Dis. 1990;15:583–91. doi: 10.1016/s0272-6386(12)80530-0. [DOI] [PubMed] [Google Scholar]
- 23.Cronin RE, Bulger RE, Southern P, Henrich WL. Natural history of aminoglycoside nephrotoxicity in the dog. J Lab Clin Med. 1980;95:463–74. [PubMed] [Google Scholar]
- 24.Cronin RE, Nix KL, Ferguson ER, Southern PM, Henrich WL. Renal cortex ion composition and Na+-K+-ATPase activity in gentamicin nephrotoxicity. Am J Physiol. 1982;242:F477, 83. doi: 10.1152/ajprenal.1982.242.5.F477. [DOI] [PubMed] [Google Scholar]
- 25.Adelman RD, Conzelman G, Spangler w, Ishizaki G. Comparative nephrotoxicity of gentamicin and netilmicin: Functional and morphological correlations with urinary enzyme activities. Curr Probl Clin Biochem. 1979;9:166–82. [PubMed] [Google Scholar]
- 26.Furuta N, Nakada J. Study on gamma-GTP activity in urine and renal tissue of drug-induced nephrotoxicity in rats. Nihon Hinyokika Gakkai Zasshi. 1993;84:1197–205. doi: 10.5980/jpnjurol1989.84.1197. [DOI] [PubMed] [Google Scholar]
- 27.Blais A, Morvan Baleynaud J, Friedlander G, Le Grimellec C. Primary culture of rabbit proximal tubules as a cellular model to study nephrotoxicity of xenobiotics. Kidney Int. 1993;44:13–8. doi: 10.1038/ki.1993.206. [DOI] [PubMed] [Google Scholar]
- 28.Kadkhodaee M, Khastar H, Faghihi M, Ghaznavi R, Zahmatkesh M. Effects of co-supplementation of vitamins E and C on gentamicin-induced nephrotoxicity in rat. Exp Physiol. 2005;90:571–6. doi: 10.1113/expphysiol.2004.029728. [DOI] [PubMed] [Google Scholar]


