Abstract
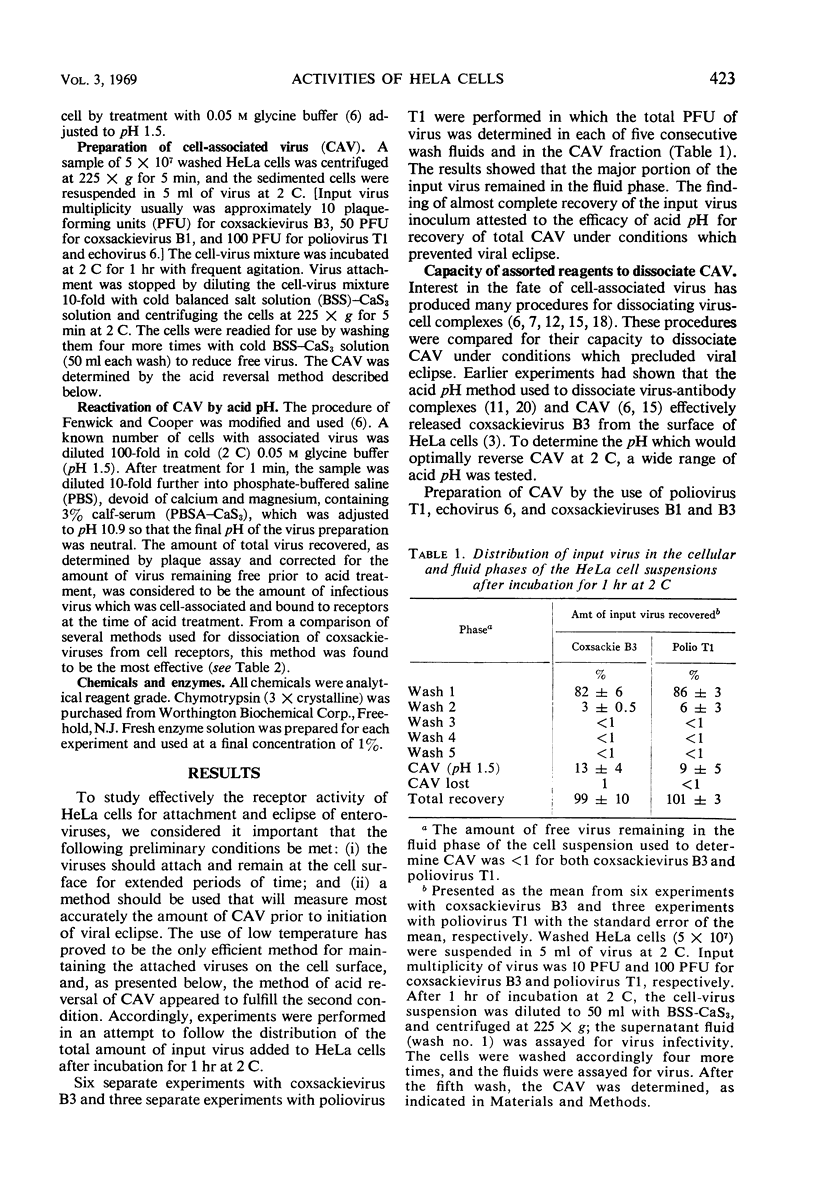
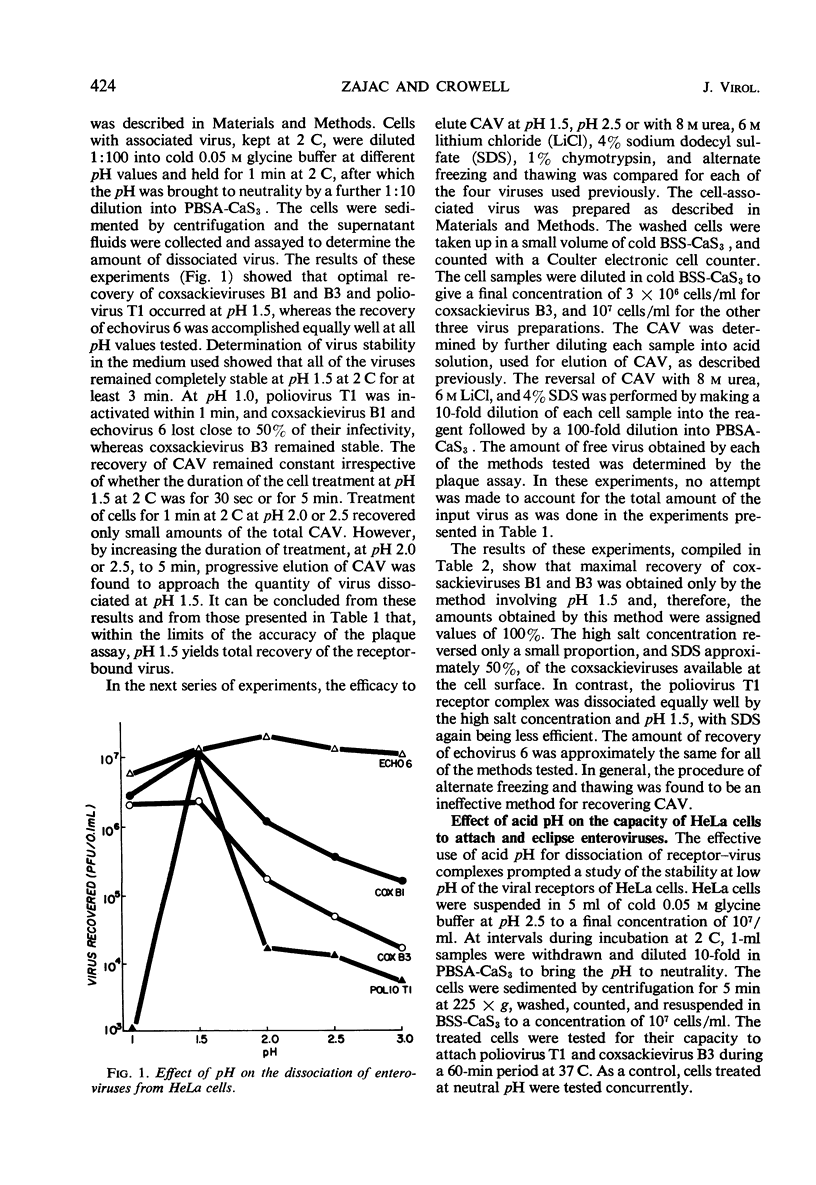
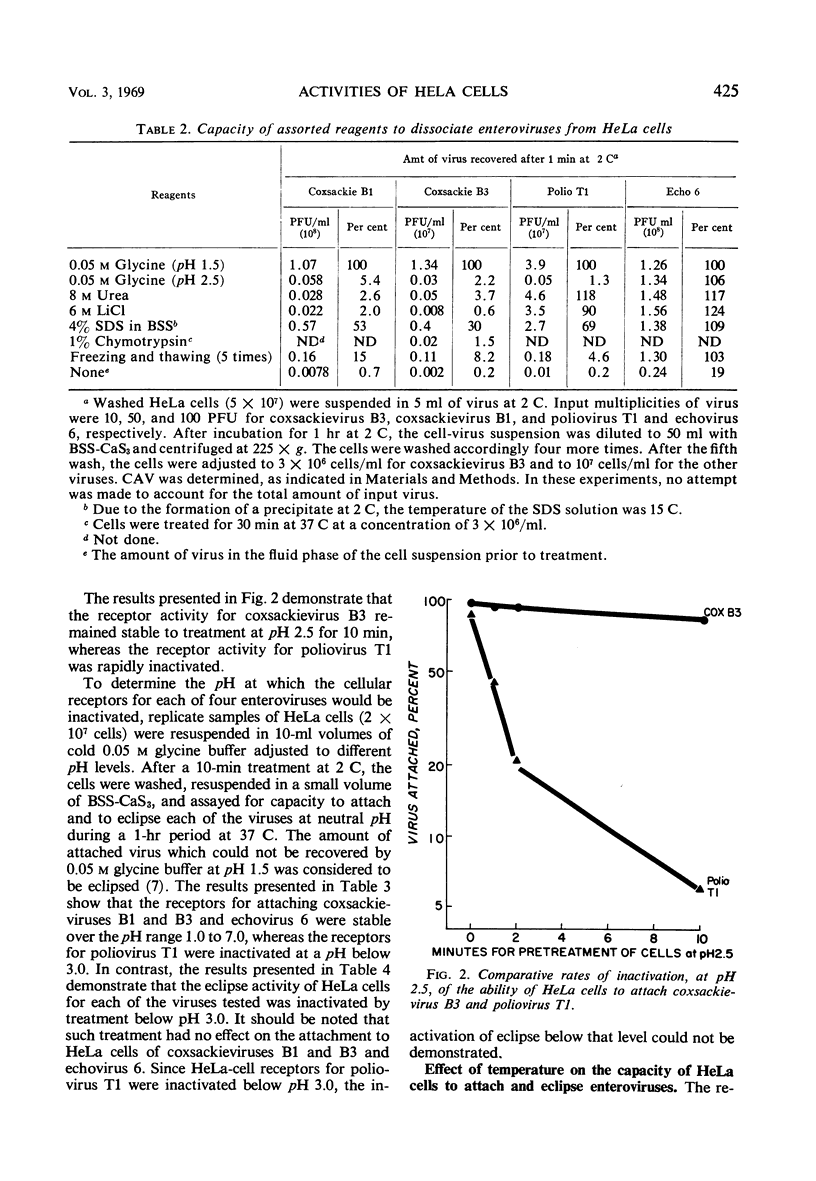
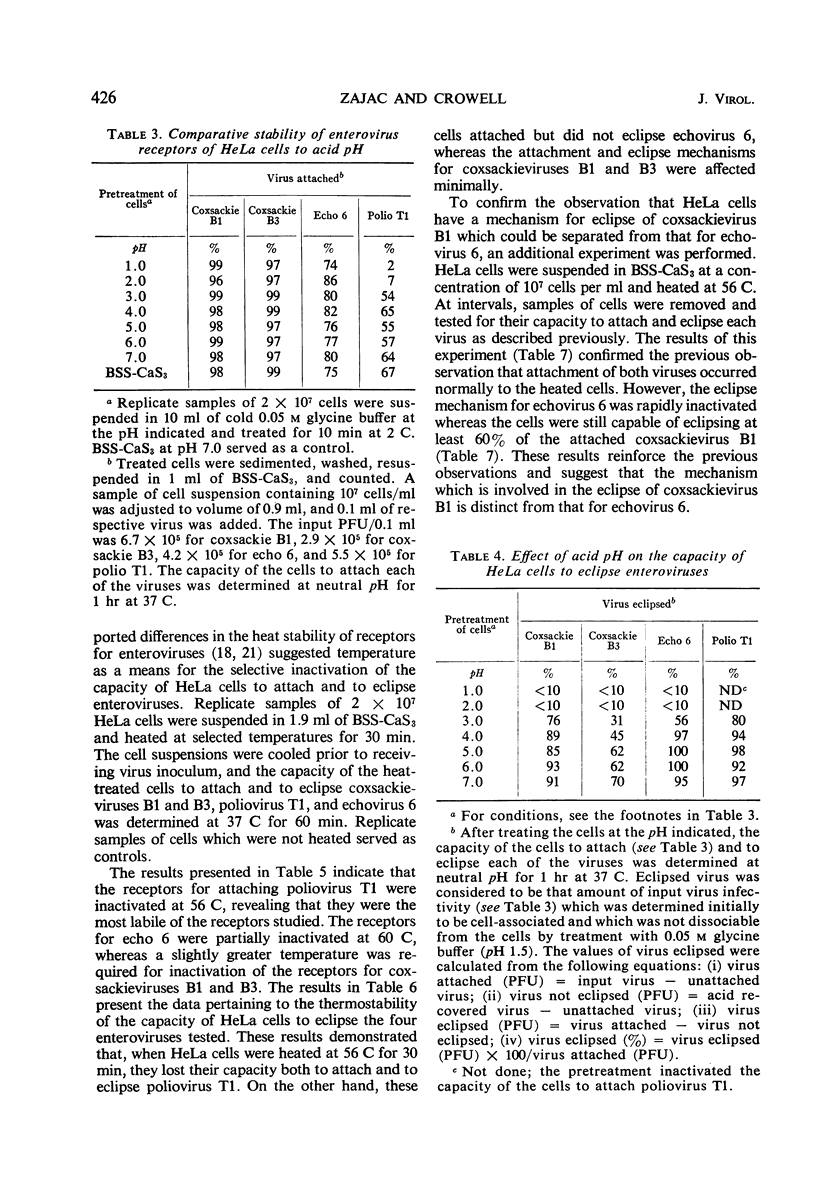
Receptor activities of HeLa cells were evaluated for ability to both attach and eclipse enteroviruses after exposure of cells to acid or heat. A modified procedure of acid (pH 1.5) elution of cell-associated virus, as compared with other procedures, provided a general method for the optimal recovery of receptor-bound enteroviruses. With this procedure, eclipse of virus operationally was considered to be that amount of virus infectivity which was determined initially to be cell-associated and which was not dissociable from the cells. HeLa cells killed by heating at 56 C for 30 min could not attach or eclipse poliovirus T1, but they attached and eclipsed coxsackieviruses B1 and B3, and they attached echovirus 6 but did not eclipse it. HeLa cells treated at pH 2.5 for 10 min at 2 C could not attach or eclipse poliovirus T1, but they attached coxsackieviruses B1 and B3 and echovirus 6, although these viruses were not eclipsed. These results showed that, within the operational definition of virus eclipse, the eclipse activity of HeLa cells for some viruses can be irreversibly inactivated without impairing the activity of the receptors for attaching these viruses. The data provided additional evidence that HeLa cells possess specific receptors for the different enteroviruses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axler D. A., Crowell R. L. Effect of anticellular serum on the attachment of enteroviruses to HeLa cells. J Virol. 1968 Aug;2(8):813–821. doi: 10.1128/jvi.2.8.813-821.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWELL R. L. SPECIFIC VIRAL INTERFERENCE IN HELA CELL CULTURES CHRONICALLY INFECTED WITH COXSACKIE B5 VIRUS. J Bacteriol. 1963 Sep;86:517–526. doi: 10.1128/jb.86.3.517-526.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWELL R. L., SYVERTON J. T. The mammalian cell-virus relationship. VI. Sustained infection of HeLa cells by Coxsackie B3 virus and effect on superinfection. J Exp Med. 1961 Feb 1;113:419–435. doi: 10.1084/jem.113.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell R. L. Specific cell-surface alteration by enteroviruses as reflected by viral-attachment interference. J Bacteriol. 1966 Jan;91(1):198–204. doi: 10.1128/jb.91.1.198-204.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARNELL J. E., Jr, SAWYER T. K. The basis for variation in susceptibility to poliovirus in HeLa cells. Virology. 1960 Aug;11:665–675. doi: 10.1016/0042-6822(60)90113-6. [DOI] [PubMed] [Google Scholar]
- FENWICK M. L., COOPER P. D. Early interactions between poliovirus and ERK cells: some observations on the nature and significance of the rejected particles. Virology. 1962 Oct;18:212–223. doi: 10.1016/0042-6822(62)90007-7. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J. ENTEROVIRUS ENTRANCE INTO SPECIFIC HOST CELLS, AND SUBSEQUENT ALTERATIONS OF CELL PROTEIN AND NUCLEIC ACID SYNTHESIS. Bacteriol Rev. 1964 Mar;28:2–13. doi: 10.1128/br.28.1.2-13.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. Irreversible eclipse of poliovirus by HeLa cells. Virology. 1962 Feb;16:163–176. doi: 10.1016/0042-6822(62)90292-1. [DOI] [PubMed] [Google Scholar]
- KUNIN C. M. Virus-tissue union and the pathogenesis of enterovirus infections. J Immunol. 1962 May;88:556–569. [PubMed] [Google Scholar]
- Levitt N. H., Crowell R. L. Comparative studies of the regeneration of HeLa cell receptors for poliovirus T1 and coxsackievirus B3. J Virol. 1967 Aug;1(4):693–700. doi: 10.1128/jvi.1.4.693-700.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDEL B. Reversibility of the reaction between polio-virus and neutralizing antibody of rabbit origin. Virology. 1961 Jul;14:316–328. doi: 10.1016/0042-6822(61)90317-8. [DOI] [PubMed] [Google Scholar]
- MANDEL B. The use of sodium dodecyl sulfate in studies on the interaction of poliovirus and HeLa cells. Virology. 1962 Jun;17:288–294. doi: 10.1016/0042-6822(62)90119-8. [DOI] [PubMed] [Google Scholar]
- Mandel B. The relationship between penetration and uncoating of poliovirus in HeLa cells. Virology. 1967 Apr;31(4):702–712. doi: 10.1016/0042-6822(67)90198-5. [DOI] [PubMed] [Google Scholar]
- McLAREN L. C., HOLLAND J. J., SYVERTON J. T. The mammalian cell-virus relationship. I. Attachment of poliovirus to cultivated cells of primate and non-primate origin. J Exp Med. 1959 May 1;109(5):475–485. doi: 10.1084/jem.109.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren L. C., Scaletti J. V., James C. G. Isolation and properties of enterovirus receptors. Wistar Inst Symp Monogr. 1968;8:123–135. [PubMed] [Google Scholar]
- PHILIPSON L., LIND M. ENTEROVIRUS ECLIPSE IN A CELL-FREE SYSTEM. Virology. 1964 Jul;23:322–332. doi: 10.1016/0042-6822(64)90254-5. [DOI] [PubMed] [Google Scholar]
- PINHEIRO F., HSIUNG G. D. A STUDY ON DISSOCIATION OF COXSACKIE B4 VIRUS-ANTIBODY COMPLEX. Virology. 1963 Jul;20:457–463. doi: 10.1016/0042-6822(63)90094-1. [DOI] [PubMed] [Google Scholar]
- Philipson L. Attachment and eclipse of adenovirus. J Virol. 1967 Oct;1(5):868–875. doi: 10.1128/jvi.1.5.868-875.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloviev V. D., Krispin T. I., Zaslavsky V. G., Agol V. I. Mechanism of resistance to enteroviruses of some primate cells in tissue culture. J Virol. 1968 Jun;2(6):553–557. doi: 10.1128/jvi.2.6.553-557.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAJAC I., CROWELL R. L. EFFECT OF ENZYMES ON THE INTERACTION OF ENTEROVIRUSES WITH LIVING HELA CELLS. J Bacteriol. 1965 Mar;89:574–582. doi: 10.1128/jb.89.3.574-582.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAJAC I., CROWELL R. L. LOCATION AND REGENERATION OF ENTERIOVIRUS RECEPTORS OF HELA CELLS. J Bacteriol. 1965 Apr;89:1097–1100. doi: 10.1128/jb.89.4.1097-1100.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]



