Abstract
Context:
Chronic periodontitis is an inflammatory disease with an aberrant response characterized by exaggerated inflammation, involving the release of excess proteolytic enzymes and reactive oxygen species (ROS). Diabetes mellitus is a group of complex multisystem metabolic disorders characterized by a relative or absolute insufficiency of insulin secretion and or concomitant resistance to the metabolic action of insulin on target tissues. Increased production of ROS necessitates elevated requirements for the nutrients involved in antioxidant defenses: Selenium, zinc, and copper. Inflammatory states promote a decrease in the amount of systemic glutathione levels. Catalase is a central antioxidant enzyme constituting the primary defense against oxidative stress.
Aims:
This study has been designed to evaluate the comparison of glutathione, catalase, and selenium levels in the serum of diabetes mellitus type 2 patients and healthy individuals with and without periodontal disease.
Settings and Design:
This study is a case control study.
Materials and Methods:
The study was designed as a case - control study comprising of 150 subjects, inclusive of both sexes and were divided into three groups of 50 patients each. Group I: 50 subjects with type 2 diabetes mellitus and chronic periodontitis. Group II: 50 subjects who are systemically healthy with the chronic periodontitis. Group III: 50 subjects who are systemically healthy and not suffering from
Periodontitis:
Serum samples were taken for estimation of glutathione, catalase, and selenium from all groups, and Subjected to biochemical analysis after which atomic absorption spectrophotometry method was used to obtain their levels in serum.
Statistical Analysis Used:
ANOVA and Tukey HSD.
Results:
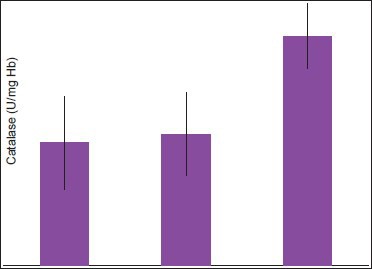
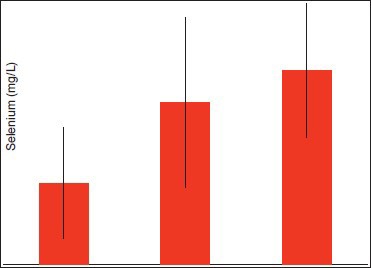
The serum levels of glutathione in diabetic patients with periodontitis were significantly lower with a mean of 61.36 + 8.054 when compared to healthy individuals with and without periodontitis with a mean of 56.93 + 6.874 and 90.36 + 6.564 respectively (P ≤ 0.005). The serum levels of catalase were significantly lower in diabetic patients with periodontitis with a mean of 19.30 + 7.355 when compared to healthy individuals with and without periodontitis with a mean of 20.71 + 6.472 and 36.09 + 5.108 respectively (P ≤ 0.005). The serum levels of selenium were significantly lower in diabetic patients with periodontitis with a mean of 81.41 + 55.419 when compared to healthy individuals with and without periodontitis with a mean of 161.44 + 84.787 and 193.84 + 66.713 respectively (P ≤ 0.005).
Conclusions:
The findings from the study suggest that the levels of glutathione, catalase, and selenium are significantly lower in diabetic patients with periodontitis and also in healthy individuals with periodontitis, but are highest in healthy controls, showing that the serum levels are inversely proportional to inflammation and tissue destruction.
Keywords: Catalase, diabetes mellitus type 2, glutathione, periodontitis, selenium
Introduction
Periodontitis is a term used to describe an inflammatory process, initiated by the plaque biofilm, that leads to loss of periodontal attachment to the root surface and adjacent alveolar bone and which ultimately results in tooth loss.
The inflammatory and immune responses to the bacteria and also viruses that colonize the periodontal and associated tissues involve the systemic circulation and ultimately the peripheral systems of the body, which creates a complex bi-directional series of host-microbial interactions involving cellular and humoral factors.[1]
More specifically, a loss of homeostatic balance between reactive oxygen species (ROS) and the antioxidant defense systems that protect and repair vital tissue, cell, and molecular components are believed to be responsible.[2]
Under normal conditions, antioxidant systems of the cell minimize oxidative stress.[3]
Diabetes mellitus is a group of complex multisystem metabolic disorders characterized by a relative or absolute insufficiency of insulin secretion and or concomitant resistance to the metabolic action of insulin on target tissues. The more prevalent form of diabetes is type 2 in which oxidative stress reduces insulin secretion and increases insulin resistance.
Periodontitis has been recognized as the 6th major complication of diabetes.[2] There is also a clearly defined and substantial role for ROS in periodontitis.[4] Antioxidants are substances that delay or prevent the oxidation of cellular oxidizable substrates. The various antioxidants exert their effect by scavenging ROS (hydrogen peroxide, superoxide, etc.).
Catalase protects cells from hydrogen peroxide generated within them.[5] Glutathione is essential to the glutathione peroxidase anti-oxidant enzyme system, which also removes hydrogen peroxide. Selenium, an essential trace element, is involved in the complex system of defense against oxidative stress through selenium-dependent glutathione peroxidases and other selenoproteins.
Objective
Hence, this study has been designed to evaluate the comparison of glutathione; catalase and selenium levels in the serum of diabetes mellitus type 2 patients and healthy individuals with and without periodontal disease.
Materials and Methods
A total of 150 patients reporting to the Department of Periodontics, A. B. Shetty Memorial Institute of Dental Sciences and Department of Medicine, KSHEMA who have given their informed consent to participate in the study were selected.
Method of collection of data
Group I: 50 subjects with type 2 diabetes mellitus and periodontal disease
Group II: 50 subjects who are systemically healthy with periodontal disease
Group III: 50 subjects who are systemically healthy and without periodontal disease.
Inclusion criteria
Patients categorized as type 2 diabetes mellitus who are under treatment for at least 6 months and are on oral hypoglycemic drugs, in the age group of 30-60 years
Patients with clinical attachment loss >4 mm in more than 30% of the site for Group I and II
Subjects who have a gingival index score of <2 in Group III
Subjects with minimum complement of 20 teeth
All measurements and samples are taken before starting any periodontal therapy.
Exclusion criteria
History of any antibiotic/anti-inflammatory therapy for 3 months prior to study
History of any systemic diseases for Group II and III
History of any systemic disease other than diabetes mellitus type 2 for the Group I
Pregnancy/lactation
Subjects with a history of smoking and any form of tobacco consumption
Subjects with a history of use of mouth wash within 3 months prior to study
Subjects with a history of vitamins/minerals or antioxidant supplements intake during the last 3 months
Subjects who had undergone any periodontal treatment for at least 3 months prior to study.
Results
A total of 150 subjects were selected for the study. The aim of this study was to estimate the levels of glutathione, catalase, and selenium in the serum of diabetic patients with periodontitis and healthy individuals with and without chronic periodontitis. The study group comprised of 50 diabetic patients with chronic periodontitis, 50 healthy subjects with chronic periodontitis and 50 healthy subjects without chronic periodontitis.
Quantitative evaluation of glutathione, catalase, and selenium levels in serum was carried out using analysis of variance test. Comparison was performed using the Tukey HSD test. Results were tabulated, mean values, and standard deviations were calculated [Table 1, 2, 3, 4, 5 and 6].
Table 1.
Mean and standard deviation of glutathione serum levels between diabetics with periodontitis (Group 1) and healthy individuals with and without periodontitis (Group 2 and Group 3 respectively)
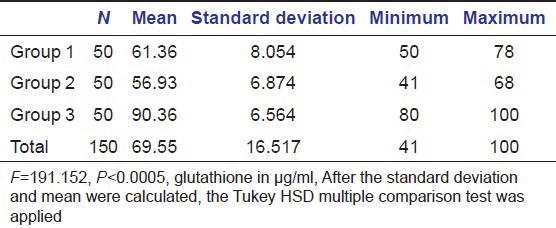
Table 2.
Tukey HSD multiple comparison test
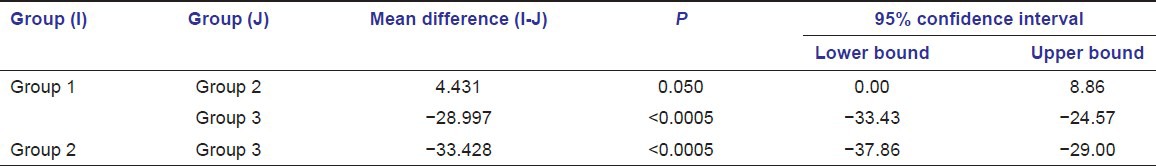
Table 3.
Mean and standard deviation of catalase serum levels between diabetics with periodontitis (Group 1) and healthy individuals with and without periodontitis (Group 2 and Group 3 respectively)
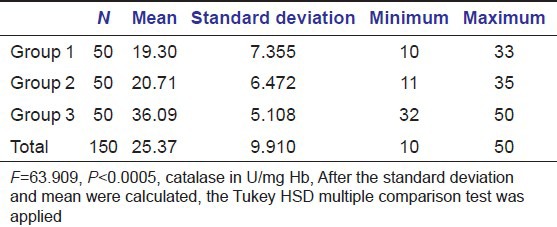
Table 4.
Tukey HSD multiple comparison test

Table 5.
Mean and standard deviation of selenium serum levels between diabetics with periodontitis (Group 1) and healthy individuals with and without periodontitis (Group 2 and Group 3 respectively)

Table 6.
Tukey HSD multiple comparison test

The mean values of glutathione, catalase and selenium levels in these three conditions were correlated and the results were statistically analyzed.
Discussion
Inflammatory and immune reactions to microbial plaque are the predominant features of gingivitis and periodontitis. The inflammatory reaction is visible both microscopically and clinically in the affected periodontium and represents the host's response to the plaque microbiota and its products.
These immune reactions will result in further release of cytokines and proinflammatory mediators, which will be more harmful to the host.
Chronic inflammatory conditions are generally associated with increased oxidative stress during the phagocytosis and killing.
ROS are produced during normal cellular function.
Superoxide, hydrogen peroxide, and hydroxyl radicals are involved in a large number of degenerative changes, often associated with an increase in peroxidative processes and linked to low antioxidant concentration.[5]
Enzymes that detoxify ROS include glutathione peroxidase, and catalase (the two enzymes that detoxify hydrogen peroxide). Selenium is a co-factor for enzyme glutathione peroxidase.
Catalase protects cells from hydrogen peroxide generated within them.
Glutathione peroxidase shares the substrate, H2 O2, with catalase.[5]
Micronutrient deficiency leads to dysregulation of the balanced host response. Selenium is essential for optimum immune response and influences the innate and acquired immune systems. It plays a key role in the redox regulation and antioxidant function through glutathione peroxidases.
Hence, level of glutathione, catalase, and selenium is expected to be inversely related to periodontal disease severity.
Gutteridge and Halliwell reported that the extent of tissue damage could be assessed by measuring the concentrations of lipid peroxidation products and antioxidants. Guttaridge et al. reported that the products of lipid peroxidation diffuse from the site of inflammation, and can therefore be measured in the plasma.[6]
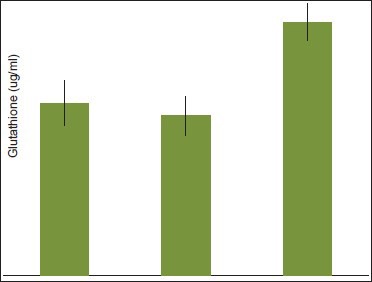
In this study, an attempt was made to evaluate the levels of glutathione, catalase, and selenium in the serum of healthy patients with and without periodontal disease using atomic absorption spectrophotometry. The results of this study demonstrated lower levels of glutathione, catalase, and selenium in serum of patients with periodontitis as Compared to control group [Graphs 1, 2 and 3]. The results of the levels of glutathione and catalase were found to be significant, which are consistent with the outcomes of other studies.[1,2,3,4,7]
Graph 1.
Comparison of glutathione serum levels between diabetics with periodontitis (Group 1) and healthy individuals with and without periodontitis (Group 2 and Group 3 respectively)
Graph 2.
Comparison of Catalase serum levels between diabetics with periodontitis (Group 1) and healthy individuals with and without periodontitis (Group 2 and Group 3 respectively)
Graph 3.
Comparison of selenium serum levels between diabetics with periodontitis (Group 1) and healthy individuals with and without periodontitis (Group 2 and Group 3 respectively)
However, a study performed by Voltarelli et al. reported that in the plasma, erythrocytes, erythrocyte membranes and gingival tissues of the periodontitis sufferers, enzymatic antioxidant activities were found to be significantly higher, whereas the levels of non-enzymatic antioxidants were significantly lower (except for reduced glutathione in the gingival tissues) relative to the parameters found for healthy subjects. The disturbance in the endogenous antioxidant defense system due to over-production of lipid peroxidation products at inflammatory sites and can be related to a higher level of oxidative stress in patients with periodontitis.[8]
Additional factors contributing to this multifaceted local disease process in the oral cavity include a number of systemic diseases, especially diabetes that can exaggerate the host response to the local microbial factors, resulting in unusual destructive periodontal breakdown.
Hyperactive innate immune response may be the antecedent of both diseases, which probably have a synergistic effect when they co-exist in the host.
Advanced glycation end products, are dependent on oxidative mechanisms and are highly prevalent in type 2 diabetes, which is a major risk factor for periodontitis.
In the present study, the serum levels of glutathione and catalase were found to be significant when compared to the healthy controls and whereas, the serum level of selenium was found to be significant when compared to healthy individuals with and without periodontitis.
However, the study conducted by Asayama et al. reported no change in the catalase levels when the effect of vitamin E deficiency, selenium deficiency, and combined deficiency on islet function and free radical scavenging systems were investigated. This was probably because the specimens were selenium deficient so showed significantly lower levels of glutathione.[9]
Conclusion
Serum levels of glutathione, catalase, and selenium is decreased: In diabetic patients and healthy individuals with periodontitis compared to healthy controls.
Serum levels of catalase and selenium is decreased: In diabetic patients with periodontitis compared to healthy controls.
Serum levels of glutathione is decreased: In healthy individuals with periodontal disease compared to diabetic patients with periodontitis.
As periodontitis is a multifactorial disease, there are various mechanisms leading to breakdown of periodontal tissues, like as oxidative stress that is created, which can further be enhanced in the presence of a systemic disease like diabetes mellitus.
To conclude, the reduction in the serum levels of glutathione, catalase, and selenium in diabetes patients and healthy patients with periodontitis has led to an imbalance leading to increased ROS and hence periodontal breakdown.
Hence, focus has now shifted to the development of antioxidant based approaches to periodontal therapy.
Further, studies need to be carried out on the efficacy of antioxidant therapies that target the free radicals that lead to periodontal tissue breakdown.
Acknowledgment
The Department of Biochemistry and Research laboratory, A. B. Shetty Memorial Institute of Dental Sciences.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Chapple IL, Milward MR, Dietrich T. The prevalence of inflammatory periodontitis is negatively associated with serum antioxidant concentrations. J Nutr. 2007;137:657–64. doi: 10.1093/jn/137.3.657. [DOI] [PubMed] [Google Scholar]
- 2.Thomas B, Kumari S, Ramitha K, Ashwini Kumari MB. Comparative evaluation of micronutrient status in the serum of diabetes mellitus patients and healthy individuals with periodontitis. J Indian Soc Periodontol. 2010;14:46–9. doi: 10.4103/0972-124X.65439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akpata E, Otoh E, Enwonwu C, Adeleke O, Joshipura K. Nutrition and inflammatory markers. J Am Dent Assoc. 2007;138:70–3. doi: 10.14219/jada.archive.2007.0023. [DOI] [PubMed] [Google Scholar]
- 4.Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997;24:287–96. doi: 10.1111/j.1600-051x.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 5.Matés JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- 6.Gutteridge IM, Halliwell B. New York: Oxford University Press; 1994. Antioxidants in Nutrition, health, and disease. [Google Scholar]
- 7.West IC. Radicals and Oxidative stress in diabetes. Diabet Med. 2000;17:171–80. doi: 10.1046/j.1464-5491.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- 8.Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568–76. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- 9.Asayama K, Kooy NW, Burr IM. Effect of vitamin E deficiency and selenium deficiency on insulin secretory reserve and free radical scavenging systems in islets: Decrease of islet manganosuperoxide dismutase. J Lab Clin Med. 1986;107:459–64. [PubMed] [Google Scholar]


