Abstract
Aim:
The aim of this study is to evaluate the efficacy of 1% curcumin (CU) solution and compare it with conventional irrigant i.e., 0.2% chlorhexidine (CHX) gluconate and a positive control (saline) as an adjunct to thorough scaling and root planing.
Materials and Methods:
A total of 23 patients with non-adjacent probing pocket depths (PPDs) ≥5mm were randomly assigned to CHX, CU and positive control irrigation groups and subjected to randomized single blinded clinical control trial. The clinical parameters bleeding on probing, redness, plaque index, PPD and microbiological parameter N-benzoyl-DL-arginine-2-naphthylamide (BANA) test were evaluated at baseline, 1, 3 and 6 months interval.
Results:
At 1 month evaluation, CU group showed better results compared with the other groups. However, by the end of the study period CHX group showed the best results with as light recurrence in the CU group. The results of BANA test showed similar results for both CU and CHX group throughout the study period.
Conclusion:
The results of this study show a mild to moderate beneficiary effect of CU irrigation when used as an adjunct to Scaling and root planing. Further studies may be required using varied concentrations of the drug to improve the substantivity of the drug and also to prevent early re-colonization of periodontal pathogens.
Keywords: Curcumin, chlorhexidine digluconate, chronic periodontitis, irrigation, periodontal pocket
Introduction
Periodontitis is an inflammatory disease that causes pathological alterations in the tooth supporting tissues, leading to loss of periodontal structures and tooth loss gradually in later stages. Thus, periodontal disease points to the need form or effective and efficient management of this condition, in an attempt to restrict its impact on general health and patient quality-of-life. Because the major cause for periodontal disease is dental plaque, much of the research should be directed toward a more effective and economic means of plaque control that can prevent further disease progression. Thus, more effective methods of management of shallow periodontal pockets are essential for the prevention of disease progression to the later stages.
From the past four decades, sub-gingival irrigation has been used as a possible and effective adjunct for treatment of periodontal diseases. Sub-gingival delivery of antimicrobial agents was shown to be potent both as in office procedures as well as home oral hygiene regime. Various anti bacterial agents such as chlorhexidine (CHX),[1,2,3] metronidazole,[4] tetracyclines,[5] and povidone-iodine[6] and herbal products such as propolis[7] and dentol,[8] have been tried in the recent past and were proven to be effective as sub-gingival irrigants in the management of shallow periodontal pockets. However, research still progresses toward identification of drugs, which are more efficient than those currently used.
Curcumin (CU) is a diferuloyl methane present in extracts of the plant (turmeric or rhizome). Curcuminoids are responsible for the yellow color of turmeric. They are derived from turmeric by ethanol extraction. CU longa contains three major curcuminoids (approximately 77% CU, 17%demethoxycurcumin, and 3% bisdemethoxycurcumin).[9] Various studies showed that CU has antioxidant and anti-inflammatory properties. Plummer et al., and Chen et al., in 1999 reported that CU down-regulates the expression of CoX-2 protein,[10,11] most likely through the down-regulation of nuclear factor-κβ (NF-κβ) activation, which is needed for CoX-2 expression.[11]
While the efficacy of oral irrigants on the clinical and microbiological parameters remain inconclusive this study aims at evaluating the efficacy of 1% CU solution and compare it with conventional irrigant i.e., 0.2% CHX, and a positive control (saline) as an adjunct to thorough scaling and root planing.
Materials and Methods
A total of 26 patients (12 male and 14 female) in the age range of 30-55 years were selected from the outpatient pool after obtaining ethical clearance from Institutional review board. The sample size was calculated after doing a pre-study power analysis. Patients who were diagnosed as having chronic Periodontitis and at least three sites with probing pocket depth (PPD)≥5 mm in three different quadrants and radiographic evidence (intraoral periapical radiographs) of horizontal bone loss were considered as eligible for the study. Exclusion criteria were the presence of overhanging restorations, antibiotics or any form of periodontal treatment in the previous 6 months, smoking habits, history of systemic disease that could influence the course of periodontal disease or would require prophylactic antibiotics prior to dental treatment, allergy to the medicaments used and pregnancy. Three sites in three different quadrants in each subject were randomly assigned to each group using simple repeated fair coin tossing method.
Impressions of the upper and lower arches were made and acrylic stents were prepared on each patients cast to fit over the occlusal one-third of the teeth selected for the study. A groove was cut in the acrylic stent so that the probe could be inserted at a standardized point of entry into the pocket at recall visits.
During the first visit (day 0) after obtaining informed consent, all the clinical parameters including plaque index (silness and loe), bleeding on probing, redness (presence/absence) and PPD were measured. The pocket depth was measured using a pressure sensitive probe (Aesculap periodontometer, UNC15, Aesculap Dental). The microbiological parameter assessed was N-benzoyl-DL-arginine-2-naphthylamide (BANA) enzymatic test. After recording the clinical parameters, BANA test was used to verify the presence of Tannerella forsythia, Treponema denticola, Porphyromonas gingivalis and certain strains of Capnocytophaga species. These anaerobic microorganisms possess a trypsin like enzyme capable of hydrolyzing the synthetic substrate, BANA, resulting in a color reaction. The subgingival plaque samples were taken from the study sites using a curette after removal of supragingival deposits and transferred to the BANA card, which was incubated at 55°C for 15 min.[12] The card was subsequently examined for the presence (positive), absence (negative) or mild presence (weak positive) of a blue color.
Thorough scaling and root planing were performed. 1% CU solution was prepared from 1mg of CU extract dissolved in 5 ml of ethanol, with the addition of 95 ml of glycerol. A 2 ml syringe was used with a 24 gauze needle for irrigation. The needle was bent in the center at an angle of 110°, which was then inserted subgingivally and irrigation was done for 20s. A total of 10 ml of the solution was irrigated in each group and at each time interval. Patients were emphasized on the importance of routine plaque control and maintenance.
During the second appointment (day 7), plaque index was recorded to analyze the patient's oral hygiene before repeating subgingival irrigation. Three patients were eliminated from the study due to their improper oral hygiene maintenance with a plaque index score ranging from 2.0 to 3.0. Repeated sub gingival irrigation was done at baseline, 7, 14, and 21 days. The clinical parameters assessed include clinical parameters were recorded at baseline, 1, 3 and 6 month intervals.
Statistical analysis
Statistical analysis was performed using SPSS software. Analysis of variance (ANOVA) was performed for evaluating plaque index and PPD. Intra group comparison was performed by paired t-test and inter group comparison by one way ANOVA followed by post-hoc Tukey's test. Chi-square test was carried out to evaluate bleeding on probing, redness and BANA test results.
Results
After the thorough sub-gingival irrigation at 4 different time intervals including the baseline, results were evaluated at periods of 1, 3 and 6 months. In the present study, the mean bleeding scores [Table 1, Graph 1] decreased drastically in all the groups. At 1 month interval, the test groups (CHX-87% and CU-95.7%) showed significantly (P < 0.01) better reduction in bleeding scores compared with control group (positive control-52.2%). CU group showed the best results with 95.7% of reduction in bleeding scores. In the intergroup comparison of the bleeding scores at the end of the study period, there was no significant difference between any of the groups. However, CHX group showed the best results with about 82.6% of reduction in bleeding scores compared to baseline.
Table 1.
Clinical results and statistical analysis of bleeding on probing for all sites
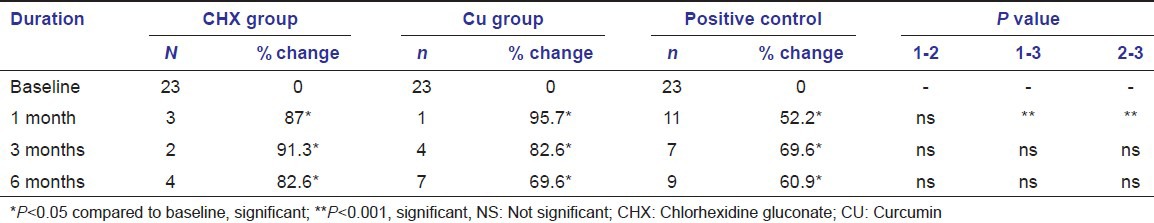
Graph 1.
Comparison of % of sites with bleeding on probing between the groups at different time intervals
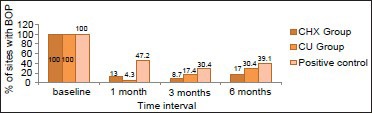
The percentage of sites with redness showed highly significant difference from baseline in all three groups [Table 2, Graph 2]. Inter group analysis showed significant change (P < 0.001) in the percentage of sites with redness between the test groups (CHX-95.7%, CU-95.7%) and the control group (65.2%) at 1month interval. However, intergroup comparisons within the test groups did not show any significant difference throughout the study period (P < 0.01). Although not statistically significant percentage of sites with redness in the CU (73.9%) and positive control (69.6%) groups increased drastically as compared with CHX group (17.4%) by the end of the study period.
Table 2.
Clinical results and statistical analysis of redness for all sites
Graph 2.
Comparison of % of sites with redness between the groups at different time intervals
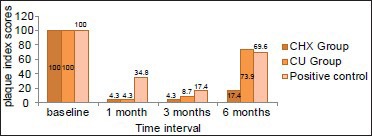
The mean plaque index scores showed a significant reduction in all the 3 groups when compared with the baseline [Table 3, Graph 3]. The CHX (0.85 + 0.22, 0.98 + 0.18, 0.93 + 0.15 at 1, 3 and 6 months respectively) and CU (0.81 + 0.22, 0.89 + 0.17, 0.97 + 0.16 at 1,3 and 6 months respectively) groups showed equivalent reduction in plaque index scores at anytime interval, where as the positive control group showed higher plaque index scores at all the time intervals compared with the test groups (1.10 + 0.20, 1.14 + 0.20, 1.17 + 0.14 at 1, 3 and 6 months respectively).
Table 3.
Clinical results and statistical analysis of plaque index scores for all the groups
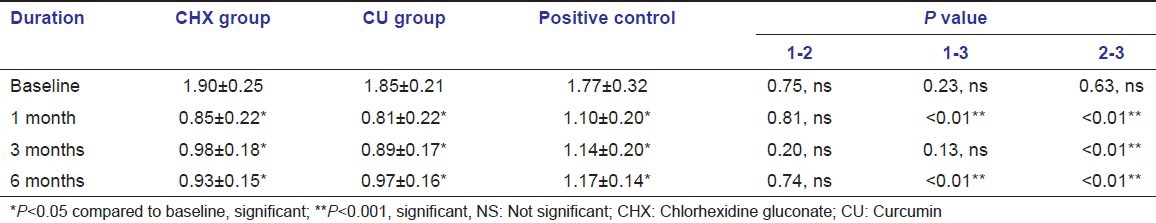
Graph 3.
Comparison of plaque index scores between the groups at different time intervals
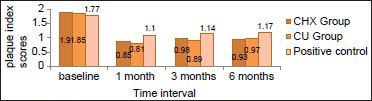
The PPDs [Table 4, Graph 4] showed a significant reduction in all three groups. The highest reduction in the PPD scores occurred in the CU group (2.70 + 0.63) at 1 month evaluation period when compared to CHX (3.61 + 0.78) and positive control group (4.30 + 0.76), with the least reduction occurring in the positive control group. However, the evaluation of the PPD scores at the end of the study period showed similar results in all the 3 groups with an average of 2.35 mm, 1.87 mm and 1.70 mm reduction in the CHX, CU and positive control groups respectively. Intergroup comparison of pocket depth scores showed a significant difference between the CHX and CU groups at 1 month period with CU showing greater reduction. However, at 6 month interval though there was also statistically significant difference between the two tests groups, CHX group showed greater reduction in pocket depths (i.e., CHX-2.35 mm, CU-1.9 mm, positive control-1.72 mm reduction).
Table 4.
Clinical results and statistical analysis of probing pocket depth scores for all the groups
Graph 4.
Comparison of probing pocket depth scores between the groups at different time intervals
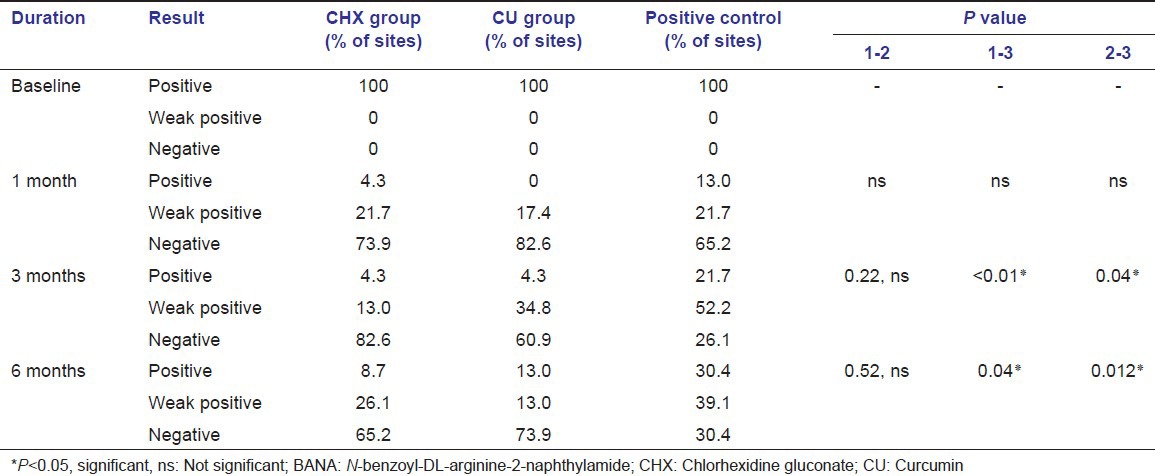
Microbiological results
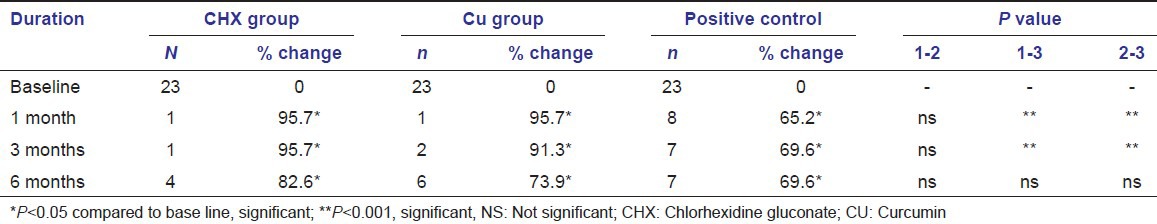
The results of the BANA test [Table 5] showed a significant shift in the BANA positive sites to negative sites in all three groups by the end of study period. At 1 month interval, the CU group showed less number (17.4%) of BANA positive sites, when compared to the CHX (21.7%) and positive control groups (21.7%). Similar results were also found at the 6 months interval i.e., CU group showed less number (13.0%) of BANA positive sites when compared to the CHX (26.1%) and positive control (39.1%) groups. This shows that the number of BANA positive sites remained stable by the end of study period in both CHX and CU groups, whereas there is a slight increase in the positive control group.
Table 5.
Statistical analysis of BANA test results for all groups
Discussion
CU has been widely studied throughout literature for its anti-inflammatory,[13,14] anti-oxidant,[15] antibacterial[16] and wound healing,[17] properties. However, its application in dentistry has been reported only in the last decade.[18,19,20] Though few studies have been performed to evaluate the clinical and microbiological efficacy of cucumin irrigation as an adjunct to scaling and root planing, none of the randomized clinical control trials evaluated the long-term treatment outcome over a period of 6 months.[21] Several authors have evaluated the efficacy of antiseptics as an adjunct to improving the clinical outcome and the biocide of the disease in moderate periodontitis patients, but none of them have shown a complete reduction and long term efficacy of the drug.[6,22,23] Therefore, this randomized clinical control trial aims at evaluating the efficacy of CU as an adjunct to scaling and root planing and to compare it with the gold standard CHX solution over a period of 6 months.
CHX irrigation has been used in previous studies and was shown to have good antibacterial properties. CHX in an in vivo study when used with a minimum inhibitory concentration of 250 μg/ml showed inhibition of 25 bacterial strains from sub gingival samples.[24] However, few studies have shown that the substantivity of CHX in human root dentin is relatively low and decreases with time.[25]
The results of our study show a significant improvement in all the clinical and microbiological parameters in both test and control groups. This can be explained by the fact that thorough good quality SRP has a greater impact on the clinical and microbiological findings irrespective of the adjunctive treatment procedures. The evaluation of the bleeding on probing, and redness showed that there was no significant difference between CHX and CU groups at 1 month interval, and the results were better than the positive control group. This reduction in the bleeding on probing and redness can be attributed to the anti-inflammatory property of CU via (a) reducing the inflammatory edema and vascular engorgement in the connective tissue,[26] (b) reduction in vascularization by fibrosis of the connective tissue.[26] However, by the end of study period there was a slight increase in the number of sites with bleeding on probing in the CU group, though not statistically significant. This shows that the concentration of CU used is not sustained and thus does not have long-term effects.
The mean plaque index scores did not show any significant difference between the CHX and CU groups at any time interval. The CHX group showed similar results in plaque index scores as that of studies done by Vinholis et al.,[27] and Paolantonio et al.[28]
The inter group comparison of PPD scores between the test groups showed better result with CU group (3.0 mm reduction) at 1 month interval. However, by the end of study period all the 3 groups showed no significant differences, with as lightly greater reduction in pocket depth in the CHX group. These results are in accordance with the study done by Nandini et al.[21] Mizrak et al.[29] Soskolne et al.[30] Lander et al.[31] Faveri et al.[32] The increase in the pocket depth scores in CU group from 1 months to 6 months interval might be due to its reduced substantivity on the root surface over a period of time.
The result of the microbiological study shows that, the CU group has a better reduction in the number of BANA positive sites at both 1 month interval and by the end of study period. However, this difference is not statistically significant. These results can be attributed to the anti-inflammatory and antioxidant properties of CU. This proves that though there is no statistically significant difference when compared with CHX group, CU shows good potential as a sub gingival irrigant. CHX showed similar percentage of BANA positive sites as that of studies by Vinholis et al.[27]
In consideration of all the clinical and microbiological parameters CU irrigation group showed good results at the first recall visit i.e.,1 month interval. However, the results are not stable by the end of study period. Thus, to obtain better long standing results a long-term regime (routine irrigation) or a change in the concentration of the drug can add to the benefits of the drug. As CU acts by suppression of NF-κβ activation which helps in decreasing the antibiotic resistance,[33] it can be undoubtedly used as a routine professional or home care regime, which improves its antibacterial properties and thus shows better results.
Conclusion
The results of this study show a mild to moderate beneficiary effect of CU irrigation when used as an adjunct to SRP. Though CU and CHX have equivalent efficacy as sub-gingival irrigants, in consideration of the fewer side effects and better patient acceptance CU can be used as effective alternative for CHX irrigation. However, further studies may be required using varied concentrations of the drug to improve the substantivity of the drug and also to prevent early recolonization of periodontal pathogens.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Walsh TF, Glenwright HD, Hull PS. Clinical effects of pulsed oral irrigation with 0.2% chlorhexidine digluconate in patients with adult periodontitis. J Clin Periodontol. 1992;19:245–8. doi: 10.1111/j.1600-051x.1992.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 2.Asari AM, Newman HN, Wilson M, Bulman JS. 0.1/0.2% commercial chlorhexidine solutions as sub gingival irrigants in chronic periodontitis. J Clin Periodontol. 1996;23:320–5. doi: 10.1111/j.1600-051x.1996.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds MA, Lavigne CK, Minah GE, Suzuki JB. Clinical effects of simultaneous ultrasonic scaling and sub gingival irrigation with chlorhexidine. Mediating influence of periodontal probing depth. J Clin Periodontol. 1992;19:595–600. doi: 10.1111/j.1600-051x.1992.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 4.Linden GJ, Newman HN. The effects of sub gingival irrigation with low dosage metronidazole on periodontal inflammation. J Clin Periodontol. 1991;18:177–81. doi: 10.1111/j.1600-051x.1991.tb01130.x. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes LA, Martins TM, Almeida JM, Nagata MJ, Theodoro LH, Garcia VG, et al. Experimental periodontal disease treatment by sub gingival irrigation with tetracycline hydrochloride in rats. J Appl Oral Sci. 2010;18:635–40. doi: 10.1590/S1678-77572010000600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krück C, Eick S, Knöfler GU, Purschwitz RE, Jentsch HF. Clinical and microbiologic results 12 months after scaling and root planing with different irrigation solutions in patients with moderate chronic periodontitis: A pilot randomized trial. J Periodontol. 2012;83:312–20. doi: 10.1902/jop.2011.110044. [DOI] [PubMed] [Google Scholar]
- 7.Gebaraa EC, Pustiglioni AN, de Lima LA, Mayer MP. Propolis extract as an adjuvant to periodontal treatment. Oral Health Prev Dent. 2003;1:29–35. [PubMed] [Google Scholar]
- 8.Shahab A, Haghighati F, Baeeri M, Jamalifar H, Abdollahi M. A clinical, microbiological and immunological comparison between sub gingival irrigation with Dentol and chlorhexidine in advanced periodontitis. Arch Med Sci. 2011;7:154–60. doi: 10.5114/aoms.2011.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee RK. Turmeric and curcumin: Biological actions and medicinal applications. Curr Sci. 2004;87:44–50. [Google Scholar]
- 10.Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemo preventive agent curcumin involves inhibition of NF-kappa B activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–20. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Zhang ZS, Zhang YL, Zhou DY. Curcumin inhibits cell proliferation by interfering with the cell cycle and inducing apoptosis in colon carcinoma cells. Anticancer Res. 1999;19:3675–80. [PubMed] [Google Scholar]
- 12.Loesche WJ, Bretz WA, Lopatin D, Stoll J, Rau CF, Hillenburg KL, et al. Multi-center clinical evaluation of a chair side method for detecting certain periodontopathic bacteria in periodontal disease. J Periodontol. 1990;61:189–96. doi: 10.1902/jop.1990.61.3.189. [DOI] [PubMed] [Google Scholar]
- 13.Chan MM, Huang HI, Fenton MR, Fong D. In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties. Biochem Pharmacol. 1998;55:1955–62. doi: 10.1016/s0006-2952(98)00114-2. [DOI] [PubMed] [Google Scholar]
- 14.Chainani- Wu N. Safety and anti- inflammatory activity of curcumin: A component of turmeric (Curcuma longa) J Altern Complement Med. 2003;9:161–8. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 15.Osawa T, Sugiyama Y, Inayoshi M, Kawakishi S. Antioxidative activity of tetra hydro curcuminoids. Biosci Biotechnol Biochem. 1995;59:1609–12. doi: 10.1271/bbb.59.1609. [DOI] [PubMed] [Google Scholar]
- 16.Singh R, Chandra R, Bose M, Luthra PM. Antibacterial activity of curcumin longa rhizome extract on periopathogenic bacteria. Curr Sci. 2002;83:737–40. [Google Scholar]
- 17.Sidhu GS, Singh AK, Thaloor D, Banaudha KK, Patnaik GK, Srimal RC, et al. Enhancement of wound healing by curcumin in animals. Wound Repair Regen. 1998;6:167–77. doi: 10.1046/j.1524-475x.1998.60211.x. [DOI] [PubMed] [Google Scholar]
- 18.Manifar S, Obwaller A, Gharehgozloo A, Kordi HR, Akhondzadeh S. Curcumin gel in the treatment of minor aphthous ulcer: A randomized, placebo-controlled trial. J Med Plants. 2012;11:40–5. [Google Scholar]
- 19.Purushotham K, Vijaybhaskar D, Pratima S. Formulation of topical oral gel for the treatment of oral sub mucous fibrosis (OSMF) Pharm Lett. 2011;3:103–12. [Google Scholar]
- 20.Waghmare PF, Chaudhari AU, Karhadkar VM, Jamkhande AS. Comparative evaluation of turmeric and chlorhexidine gluconate mouthwash in prevention of plaque formation and gingivitis: A clinical and microbiological study. J Contemp Dent Pract. 2011;12:221–4. doi: 10.5005/jp-journals-10024-1038. [DOI] [PubMed] [Google Scholar]
- 21.Nandini N, Vidya D, Komal A. Comparative evaluation of 1% curcumin solution and 0.2% chlorhexidine irrigation as an adjunct to scaling and root planing in management of chronic periodontitis: A clinico-microbiological study. J Pharm Biomed Sci. 2012;14:1–7. [Google Scholar]
- 22.Kamagate A, Kone D, Coulibaly NT, Ahnoux A. Sub gingival irrigation combined with scaling and root planing. Results of a study with chlorhexidine and sodium hypochlorite. Odontostomatol Trop. 2005;28:28–32. [PubMed] [Google Scholar]
- 23.Hoang T, Jorgensen MG, Keim RG, Pattison AM, Slots J. Povidone-iodine as a periodontal pocket disinfectant. J Periodontal Res. 2003;38:311–7. doi: 10.1034/j.1600-0765.2003.02016.x. [DOI] [PubMed] [Google Scholar]
- 24.Stanley A, Wilson M, Newman HN. The in vitro effects of chlorhexidine on sub gingival plaque bacteria. J Clin Periodontol. 1989;16:259–64. doi: 10.1111/j.1600-051x.1989.tb01651.x. [DOI] [PubMed] [Google Scholar]
- 25.Mohammadi Z, Shahriari S. Residual anti bacterial activity of chlorhexidine and MTAD in human root dentin in vitro. J Oral Sci. 2008;50:63–7. doi: 10.2334/josnusd.50.63. [DOI] [PubMed] [Google Scholar]
- 26.Sajithlal GB, Chithra P, Chandrakasan G. Effect of curcumin on the advanced glycation and cross-linking of collagen in diabetic rats. Biochem Pharmacol. 1998;56:1607–14. doi: 10.1016/s0006-2952(98)00237-8. [DOI] [PubMed] [Google Scholar]
- 27.Vinholis AH, Figueiredo LC, Marcantonio E, Júnior, Marcantonio RA, Salvador SL, Goissis G. Sub gingival utilization of a 1% chlorhexidine collagen gel for the treatment of periodontal pockets. A clinical and microbiological study. Braz Dent J. 2001;12:209–13. [PubMed] [Google Scholar]
- 28.Paolantonio M, D’ Ercole S, Pilloni A, D’ Archivio D, Lisanti L, Graziani F, et al. Clinical, microbiologic, and biochemical effects of sub gingival administration of a Xanthan- based chlorhexidine gel in the treatment of periodontitis: A randomized multicenter trial. J Periodontol. 2009;80:1479–92. doi: 10.1902/jop.2009.090050. [DOI] [PubMed] [Google Scholar]
- 29.Mizrak T, Güncü GN, Caglayan F, Balci TA, Aktar GS, Ipek F. Effect of a controlled-release chlorhexidine chip on clinical and microbiological parameters and prostaglandin E2 levels in gingival crevicular fluid. J Periodonto l. 2006;77:437–43. doi: 10.1902/jop.2006.050105. [DOI] [PubMed] [Google Scholar]
- 30.Soskolne WA, Heasman PA, Stabholz A, Smart GJ, Palmer M, Flashner M, et al. Sustained local delivery of chlorhexidine in the treatment of periodontitis: A multi-center study. J Periodontol. 1997;68:32–8. doi: 10.1902/jop.1997.68.1.32. [DOI] [PubMed] [Google Scholar]
- 31.Lander PE, Newcomb GM, Seymour GJ, Powell RN. The anti microbial and clinical effects of a single sub gingival irrigation of chlorhexidine in advanced periodontal lesions. J Clin Periodontol. 1986;13:74–80. doi: 10.1111/j.1600-051x.1986.tb01417.x. [DOI] [PubMed] [Google Scholar]
- 32.Faveri M, Gursky LC, Feres M, Shibli JA, Salvador SL, deFigueiredo LC. Scaling and root planing and chlorhexidine mouth rinses in the treatment of chronic periodontitis: A randomized, placebo-controlled clinical trial. J Clin Periodontol. 2006;33:819–28. doi: 10.1111/j.1600-051X.2006.00994.x. [DOI] [PubMed] [Google Scholar]
- 33.Baldwin AS. Control of on cogenesis and cancer therapy resistance by the transcription factor NF-kappa B. J Clin Invest. 2001;107:241–6. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]


