Abstract
Paget disease of bone (PDB) is a chronic progressive disease of the bone of uncertain etiology, characterized initially by an increase in bone resorption, followed by a disorganized and excessive formation of bone, leading to pain, fractures, and deformities. It can manifest as a monostotic or polyostotic disease. The prevalence of PDB is common in the Anglo-Saxon population, but relatively rare in India. The disease is often asymptomatic and commonly seen in an aging population. The diagnosis of the disease is mostly based on radiological examination and on biochemical markers of bone turnover. Markedly elevated serum alkaline phosphatase (SAP) is a constant feature while calcium and phosphate levels are typically within normal limits. It is being successfully treated by biphosphonates, a group of anti-resorptive drugs, thereby decreasing the morbidity and mortality associated with the disease. We report a classic case of PDB with craniofacial involvement resulting in Leontiasis Ossea (lion like face), cotton wool appearance of the skull and elevated SAP.
Keywords: Cotton wool appearance, leontiasis ossea, Paget's disease of bone, serum alkaline phosphatase
Introduction
Paget disease of bone (PDB) was first described in 1877 by Sir James Paget, a British surgeon who named the condition “osteitis deformans,” as he believed the disease was caused by chronic inflammation. It is an osseous dysplasia seen in the middle-aged and elderly. PDB can be monostotic or polyostototic, the former being the more common form of the disease, affecting the axial skeleton. All bones of the craniofacial complex however, may be affected to varying degrees.[1] It is characterized by a focal alteration of bone remodeling, which leads to a bone with an anomalous structure and altered mechanical properties, associated with pain and pathological fractures. Complications such as neurological deficits and cardiac failure may also occur.
Case Report
A 58-year-old male patient reported to the dental hospital with the complaint of difficulty in chewing food as his teeth were not in contact during mastication.
History revealed that, there were swellings in the maxillary right and left posterior teeth region, which started 6 years back. Similar swellings were also seen bilaterally in the malar region and in the forehead of the right side. All swellings gradually increased in size.
On examination, extra-orally bilateral diffuse swellings were seen in the malar and zygomatic region bilaterally, obliterating the nasolabial sulcus and in the right frontal region [Figures 1], which were bony hard in consistency on palpation. The swellings were immobile and attached to the underlying bone. Intra-orally, diffuse swelling of the maxillary alveolus was seen bilaterally, extending from 18 to 28 region [Figure 2] with obliteration of the buccal sulcus. Bicortical expansion was seen in the maxillary premolar-molar region, which were non-tender and hard in consistency, attached to the underlying bone. The occlusion was deranged and on occluding, the teeth were not in contact.
Figure 1.
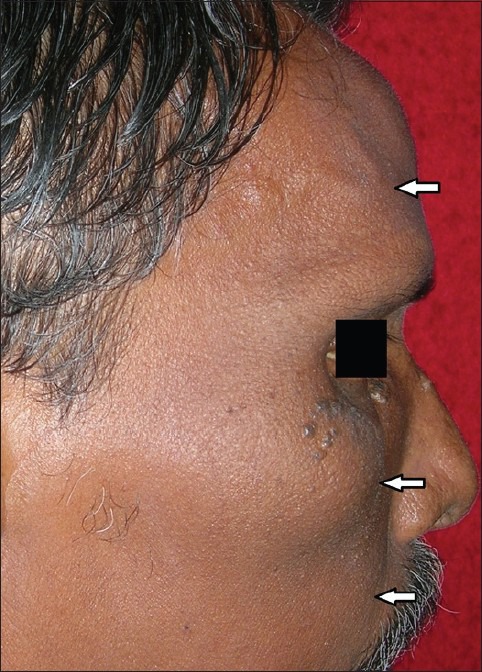
Facial profile of the patient, right side showing swelling in the frontal, malar and maxillary region, relative maxillary prognathism with apparent leontiasis ossea
Figure 2.
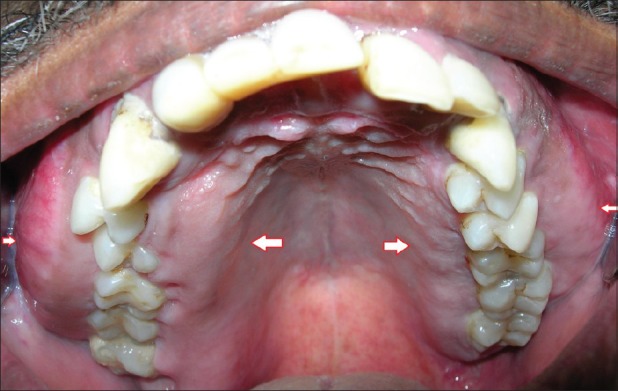
Intra-oral picture of the maxillary arch showing bilateral enlargement and bicortical expansion
Since, the swellings were multiple, bilateral, gradually increasing in size, asymptomatic, and bony hard in consistency a provisional diagnosis of disorder of bone was made; radiologic, biochemical and histopathologic investigations were performed.
Periapical, occlusal, and panoramic radiographs reveal diffuse radiopacities and hypercementosis of roots of the maxillary teeth [Figures 3]. Postero-anterior and Lateral view of the skull reveal generalized flecks of irregularly shaped radiopacities involving the entire skull, giving a cotton wool appearance [Figures 4].
Figure 3.
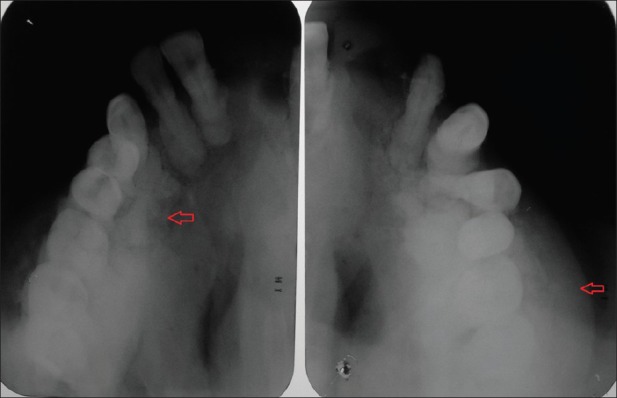
Maxillary lateral occlusal radiographs showing osteosclerosis and cotton wool appearance
Figure 4.
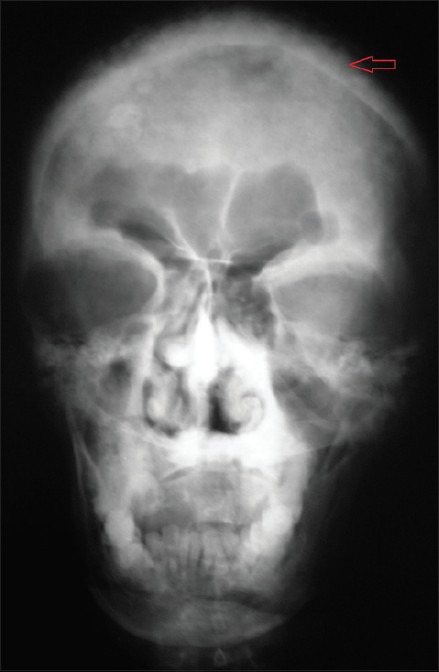
Postero-anterior view of the skull reveal opacification of the maxillary antra, generalized flecks of irregularly shaped radiopacities involving the entire skull, giving a cotton wool appearance
Computed tomography scan reveals osteosclerosis of right maxillary, ethmoid, frontal, and sphenoid sinuses with widening of diploic space with sclerotic and lytic areas involving inner and outer tables of bony calvarium [Figure 5]. There is an expansion, with sclerosis of all walls of right maxillary, right ethmoid, and floor of left maxillary sinuses.
Figure 5.
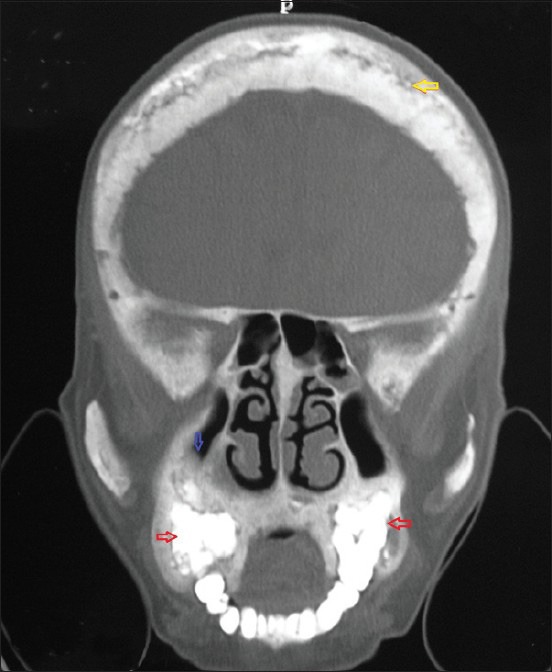
Coronal view of computed tomography scan showing widened diploic space with sclerotic and lytic areas involving inner and outer tables of bony calvarium giving a cotton wool appearance (yellow arrow) with obliteration of the maxillary antrum on right side (blue arrow) and osteosclerosis of maxillary alveoli (red arrows)
Biochemical tests revealed normal serum calcium: 9.2 mg/dL and serum phosphorus: 4 mg/dl, but markedly raised serum alkaline phosphatase (SAP): 1251 IU and urinary hydroxy-proline: Creatine ratio: 243.6 mmol.
An incisional biopsy of the swelling was carried out in the left maxillary alveolus. Histopathologic examination revealed a cellular proliferating inter-trabecular fibroblastic marrow with many clusters of osteoclasts separating spicules of bone, which appear lamellar in some of the bits with numerous cement lines forming a mosaic pattern. Trabecula of bone with basophilic reversal lines in connective tissue stroma are seen [Figure 6].
Figure 6.
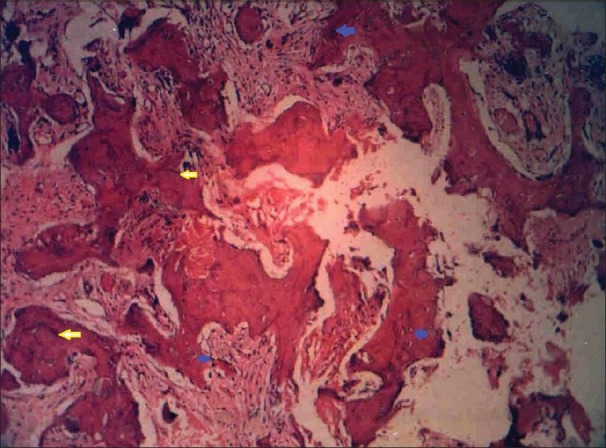
H and E section in low power magnification (×10) showing jig-saw puzzle or mosaic pattern of bony trabeculae with osteocytes (blue arrows) and basophilic reversal lines (yellow arrows)
Correlating the clinical, radiologic, biochemical, and histopathologic findings a final diagnosis of PDB was established. Patient was referred to the Rheumatologist for treatment.
Discussion
Although originally described by Sir James Paget in 1877 as “osteitis deformans”, it is a misnomer. PDB is a chronic, non-inflammatory, localized bone-remodeling disorder that affects widespread, non-contiguous areas of the skeleton and so the term “osteodystrophia deformans” would be more apt. PDB is the second most common bone disease after osteoporosis. The prevalence of Paget disease shows wide geographical variation, common in Western Europe, Americas, and Australia, but rare in Asia and Africa. However, recent studies have reported an unexplained reduction in both prevalence and disease severity.[2] This disease is relatively common in older people, occurs in approximately 3-4% of the population aged over 50 years with a slight male gender predilection. Juvenile Paget disease or idiopathic hyperphosphatasia is a rare metabolic bone dysplasia that is inherited as an autosomal recessive trait characterized by increased bone turnover secondary to enhanced osteoclastic activity and unrelated to classic PDB, which is seen in the elderly.[3]
The cause of PDB is currently an area of intensive investigation, with both genetic and environmental factors being implicated in the pathogenesis of this disease. Genetic factors are clearly an important component of the etiology of PDB since 15-40% of affected patients have a first-degree relative with PDB. Inclusion bodies resembling paramyxovirus nucleocapsid particles have been observed and canine distemper virus has been localized from the pagetic osteoclasts.[4]
Clinically pagetic bone may appear to be perfectly normal if the changes are early or mild, or the bone may be enlarged and grossly deformed with more advanced disease. Pagetic bone and overlying skin may be warm to the touch in regions like the tibia or skull due to increased vascularity when bone turnover is high. Although most patients are asymptomatic, symptoms resulting directly from bony involvement (bone pain, secondary arthritis, and fractures) or secondarily from the expansion of bone causing compression of surrounding neural tissue may be encountered.
Jaw involvement is seen with the ratio of maxilla to mandible 2:1. Maxilla exhibits progressive enlargement, alveolar ridge becomes widened and palate is flattened as observed in the present case. Teeth may become mobile and migrate, producing some spacing. Edentulous patients with dentures commonly complain of difficulty in using dentures due to increasing tightness as a result of expansion of the jaws. When the maxilla increases in size it may encroach to orbit, the nasal cavity and the antra.[5] “Leontiasis Ossea” or lion like face, characterized by an overgrowth of the facial and cranial bones is seen in PDB, but it may also be seen in patients who have advanced lepromatous leprosy. In the present case, due to the swelling of the frontal, maxillary and zygomatic regions the patient had a lion like appearance.
Mandible and maxilla involved with PDB are highly susceptible to infection, particularly by the organisms found in the normal oral flora. Dental extractions are likely to be more difficult, because there may be hypercementosis and/or ankylosis of the roots. The healing of the extraction sites is often slow and localized osteitis is common. A significant number of patients develop osteomyelitis secondary to the locally infected extraction sites.[6]
Pagetic bone deformity may also include such findings as an enlarged skull, kyphosis, and bowing of affected limbs include bone pain; gait disturbances due to a shortened bowed femur or tibia; headache or hearing loss with extensive skull involvement; and lumbar spinal stenosis or other syndromes of neural compression with attendant sensory or motor deficits. Heart failure risk is significantly increased due to increased vascularity of pagetic bone. Osteosarcoma arising in the affected bone is a rare and dreaded complication, occurring in about 0.3% of patients.
PDB is diagnosed primarily by radiological examination. Early in the course of the disease, lytic activity predominates, causing focal osteolytic lesions. Subsequently, areas of sclerosis develop, leading to the characteristic appearances of mixed lytic and sclerotic areas, thickened trabecula, bone expansion, cortical thickening, and deformity. The radiological appearances are usually characteristic, but occasionally differential diagnosis of sclerotic or lytic metastases needs to be considered.
A radioisotope bone scan may be recommended in all patients as part of the initial diagnostic assessment to determine the distribution of the disease, in particular the involvement of sites with the potential for complications, such as the base of the skull, spine and long bones.[7] Extensive involvement of the mandible may occasionally reveal the uptake of the radiotracer from one condyle to another giving rise to “Lincoln sign” or “Black beard” sign. Computed tomography scanning is helpful to assess skull-base involvement especially for patients with deafness, spinal stenosis or other neurological complications.
On skull radiographs, advancing osteolysis is noted as large areas of radiolucency usually in the frontal and occipital bones and is designated “osteoporosis circumscripta”. Later marked thickening of the inner calvarial table produces marked enlargement of the diploic space known as a “TamO'shanter” skull. Classic “cotton wool” appearance is caused by irregular areas of focal osteosclerosis as seen in the present case.[8]
Radiographs of the jaw bones and teeth may show loss of lamina dura, hypercementosis, resorption, and replacement of roots. In advanced cases, like the present case, cotton wool appearance of the trabecula is seen.
The “blade of grass” or “flame sign” in the long bones, thickened pelvic brim known as the “Brim sign” and the “picture frame,” “double contour” or “windowed” vertebral body are other classic radiographic appearances seen in various bones affected by PDB, which were absent in our case. Pathologic fracture is the most common complication of PDB, which can be crippling because of the high frequency of non-union in diseased bone.
Different biochemical markers of bone remodeling that are increased in PDB and play a useful role in the diagnosis of the disease. The markers of bone resorption which are increased are: Urinary hydroxyproline, serum N-telopeptide of type I collagen, Serum C-telopeptide of type I collagen and serum deoxypyridinoline cross-links of type I collagen. Markers of bone formation that are elevated are: SAP, serum bone-specific alkaline phosphatase, osteocalcin, serum N-terminal propeptide of type I collagen.[9] In the present case, the SAP and urinary hydroxyproline: Creatinine ratio were abnormally elevated.
Microscopically, a benign fibro-osseous pattern typically is seen. In the initial osteolytic phase, disordered areas of resorption with large multinucleated osteoclasts are seen. In the osteoblastic phase, haphazardly laid new bone matrix and formation of woven bone is seen. Irregularly shaped trabecula of woven bone are scattered throughout the stroma. The bone often is lined by osteoblasts and osteoclasts, indicating simultaneous resorption and formation of bone. Repeated episodes of bone removal and formation result in small irregularly shaped bone fragments that appear to be joined in a jigsaw or mosaic pattern with deeply staining hematoxyphilic (basophilic) reversal lines.
Many medications have been used in the effort to treat patients with symptomatic PDB. Calcitonin was used earlier as it inhibits bone resorption and provides timely pain relief. Unpleasant side-effects of calcitonin therapy were frequent such as flushing, nausea, and vomiting. The current mainstay of treatment in PDB; however, is the second-generation nitrogen containing bisphosphonates (disodium pamidronate, alendronate, risedronate), which are potent inhibitors of bone resorption and provide prolonged remission of the disease in addition to a more dramatic decrease in the parameters of bone turnover compared with calcitonin. New bone formation after such treatment has a more normal, lamellar pattern, and mineralization abnormalities are rare to absent with the newer compounds prompting a more aggressive management philosophy in which both symptomatic disease and also asymptomatic disease at sites with a risk of progression and future complications are viewed as clear indications for treatment.[10] Another therapeutic agent that has been used to treat Paget disease is Mithramycin, a cytotoxic antibiotic, reserved for those cases resistant to other forms of medical treatment.
Conclusion
Paget disease is a bone disease which may be asymptomatic initially and it can be diagnosed by its classic radiologic features and an early diagnosis can help prevent potential complications such as pathological fracture, arthritis, hearing loss, other neurological complications, heart failure, and rarely, osteosarcoma. Advances in treatment have resulted in the availability of several potent bisphosphonate compounds, which produce normal or near normal bone turn-over indices in a majority of patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Eversole R, Su L, ElMofty S. Benign fibro-osseous lesions of the craniofacial complex. A review. Head Neck Pathol. 2008;2:177–202. doi: 10.1007/s12105-008-0057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastin S, Bird H, Gamble G, Cundy T. Paget's disease of bone: Becoming a rarity? Rheumatology (Oxford) 2009;48:1232–5. doi: 10.1093/rheumatology/kep212. [DOI] [PubMed] [Google Scholar]
- 3.Bae KB, Kwon JH, Kim YH, Jung TY, Cho JH. Juvenile Paget's disease with paranasal sinus aplasia. Clin Exp Otorhinolaryngol. 2008;1:224–6. doi: 10.3342/ceo.2008.1.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karunakaran K, Murugesan P, Rajeshwar G, Babu S. Paget's disease of the mandible. J Oral Maxillofac Pathol. 2012;16:107–9. doi: 10.4103/0973-029X.92984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sekar B, Augustine D, Murali S. Paget's disease of mandible: A case report and review of literature. J Orofac Sci. 2010;2:13–17. [Google Scholar]
- 6.Theodorou DJ, Theodorou SJ, Kakitsubata Y. Imaging of Paget disease of bone and its musculoskeletal complications: Review. AJR Am J Roentgenol. 2011;196:S64–75. doi: 10.2214/AJR.10.7222. [DOI] [PubMed] [Google Scholar]
- 7.Butt ST, Fatima S, Butt R, Nasir W, Jameel G, Irfan J. Polyostotic Paget's disease. J Coll Physicians Surg Pak. 2012;22:461–3. [PubMed] [Google Scholar]
- 8.Bhargava P, Maki JH. Images in clinical medicine. “Cotton wool” appearance of Paget's disease. N Engl J Med. 2010;363:e9. doi: 10.1056/NEJMicm0912945. [DOI] [PubMed] [Google Scholar]
- 9.Roodman GD, Windle JJ. Paget disease of bone. J Clin Invest. 2005;115:200–8. doi: 10.1172/JCI24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michou L, Brown JP. Emerging strategies and therapies for treatment of Paget's disease of bone. Drug Des Devel Ther. 2011;5:225–39. doi: 10.2147/DDDT.S11306. [DOI] [PMC free article] [PubMed] [Google Scholar]


