Abstract
Filariasis is an endemic disease in tropical and subtropical countries. Filarial nematodes can infect humans through vectors, commonly mosquitoes. Human infection can manifest as lymphatic filariasis, subcutaneous or pulmonary nodules and with eye involvement. Intra-oral presentation is very rare and often poses a diagnostic dilemma to the dentist. We report a case of intra-oral Dirofilaria repens infection in a 54-year-old female patient, involving the buccal mucosa. History was unremarkable and on clinical examination, a diffuse swelling with no significant signs and symptoms was seen. Laboratory investigations and radiographs were non-contributory to diagnosis. Ultrasound findings revealed a hypo-echoic lesion in the muscular layer of the left cheek. Differential diagnoses considered were minor salivary gland tumor, parotid sialolith, and cysticercosis among others. The presence of a Dirofilaria worm in the excised nodule confirmed the diagnosis. Medical awareness of the risk of intra-oral nematode infection is essential. A detailed travel history, awareness of endemic status of certain diseases, proper diagnosis and management helps in better prognosis for the patient.
Keywords: Dirofilaria, filariasis, nematode infection, oral cavity
Introduction
Filariasis is an endemic disease in most tropical and subtropical countries and is known to infect humans with various clinical manifestations. However, intra-oral presentation is very rare. Few cases have been reported in the buccal mucosa with patients being totally asymptomatic. Knowledge of endemic areas with poor vector control due to improper sanitation and overpopulation is often helpful in diagnosis.
Case Report
A 54-year-old female school teacher reported with a complaint of intermittent swelling inside the mouth on the left side. Repeated exacerbations of the swelling occurred 4-5 times in a span of 8 months. Itching of the cheek always preceded the swelling, which got resolved within 1-2 days. There was no change in sensation over the affected area. She had consulted many clinicians prior to reporting to our hospital and had taken medications. However, the patient's symptoms had persisted. The detail of past consultations or medications regarding presenting complaint was obscure. She gave a medical history of hypertension and dyslipidemia for which she was on medication since 5 years. General physical examination did not show any relevant findings. No cervical or generalized lymphadenopathy was found.
Extra-orally, diffuse swelling of left cheek was noted with no color changes. Intra-orally, examination of the left buccal mucosa revealed a hard movable mass of 1 cm × 0.5 cm anterior to the anterior border of ramus almost at the level of 27 [Figure 1]. Slight tenderness was present on palpation of the mass. Although the parotid papilla was prominent, it was not tender on palpation. Blood investigations were unremarkable. The erythrocyte sedimentation rates were mildly raised, Mantoux test was normal. Blood smear for staining malarial and filarial parasites were negative.
Figure 1.
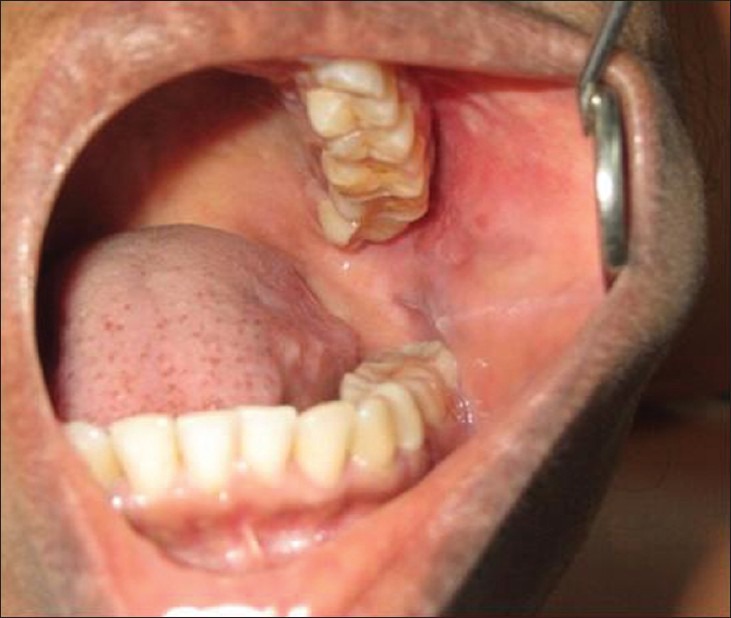
Swelling on the left buccal mucosa without any inflammatory signs
An occlusal radiograph placed vertically in the upper gingival sulcus in contact with the buccal mucosa to check for any soft-tissue calcifications did not reveal any findings.
Differential diagnoses of a parotid sialolith, minor salivary gland tumor, cysticercosis, calcified facial node, and lipoma were made.
Ultrasound scan of the cheek revealed a well-defined round hypo-echoic lesion in the muscular layer of the left cheek measuring 0.8 cm × 0.5 cm, with small specks of calcification. There were no obvious vascularity within the lesion and no perilesional edema [Figure 2]. The parotids appeared normal bilaterally. The probability of inflammatory pathology was considered.
Figure 2.
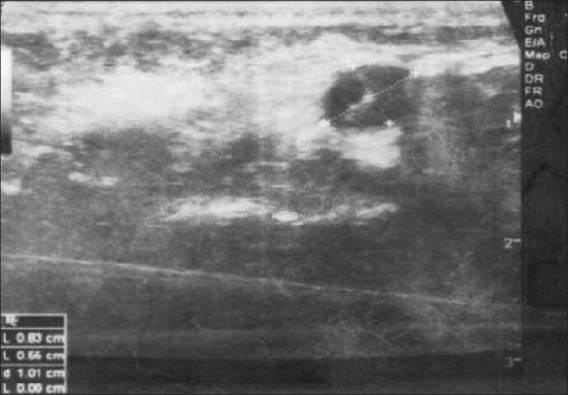
Ultrasound scan of cheek showing a small well-defined round hypo-echoic lesion
Excisional biopsy of the nodule was carried out. Histological examination revealed a dense fibrous capsule showing infiltrate of mixed inflammatory cells consisting of lymphocytes, macrophages, eosinophils, and neutrophils. Within the capsule, a single female filarial worm showing “double uterus” appearance containing numerous larval forms was seen [Figure 3]. Further, magnification revealed a thick layed cuticle with multiple longitudinal ridges and centrally placed intestinal tubule within the well-developed musculature [Figures 4 and 5]. A definitive diagnosis of Dirofilariasis repens was arrived at.
Figure 3.
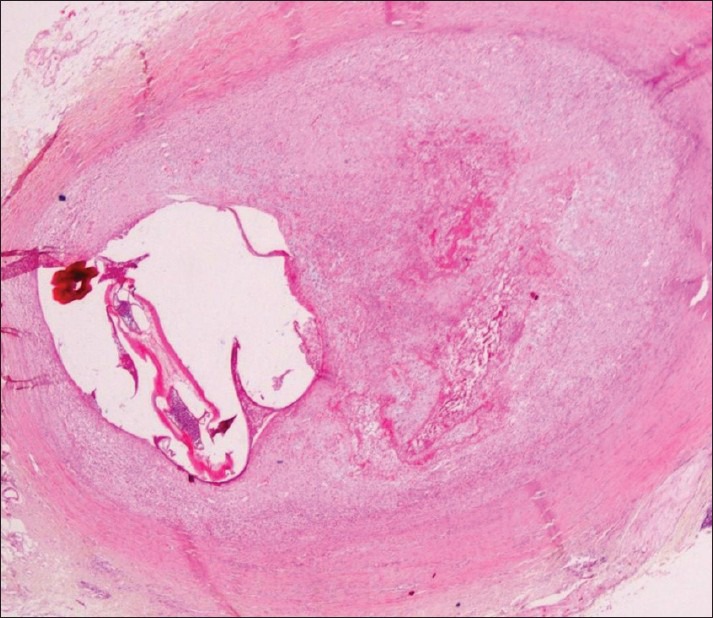
Histopathological section showing filarial worm with “double uterus” appearance: Scanner view
Figure 4.
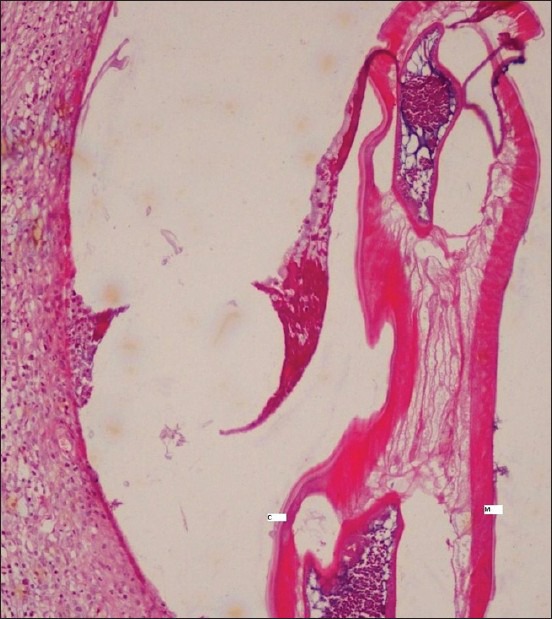
Centrally placed intestinal tubule in the worm with thick cuticle (c) and muscular layer (m): H and E, ×10
Figure 5.
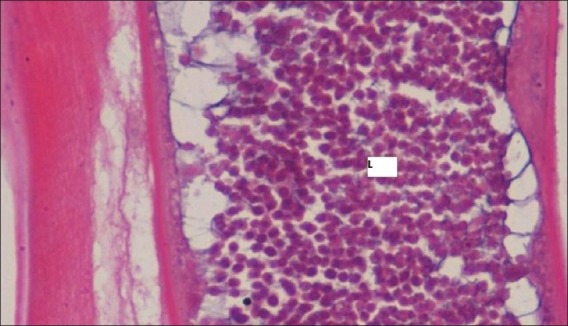
Filarial worm with numerous larval forms (l): H and E, ×45
Patient was treated with diethylcarbamazine (DEC) 100 mg tid for 3 weeks, albendazole 400 mg hs and cetrizine 5 mg Once daily for 5 days.
Following medication, patient was reviewed regularly for 8 months and found to be completely asymptomatic.
Discussion
Dirofilariasis is an endemic disease in certain parts of Africa, Asia, Europe and America. Although nearly forty species of Dirofilaria have been identified, only a few have been reported to cause human infection; the most common being Dirofilaria immitis, a parasite of dogs, D. tenuis a parasite of raccoons, Dirofilaria repens, a parasite of dogs and cats, and D. ursi a parasite of bears.[1,2]
Dirofilaria repens infection is endemic in India and Srilanka[3,4,5] and in the present case, the infection was localized intra orally in the buccal mucosa.
Human dirofilariasis is most often caused by D. repens that belongs to subgenus Nochtiella. These are nematodes that have a long, thin, filariform appearance and a rounded anterior end with an oral cavity. Occasional transmission of Dirofilaria larvae to man occurs via the mosquito vector. Mosquito species of the Anopheles, Aedes variety are the common vectors. Once the infected larvae penetrate through the wound into the human body, they migrate into the subcutaneous tissue and develop into the adult form in 6 months.[6,7]
In humans, a strong inflammatory reaction in the surrounding tissue is seen. Patients with skin involvement present with subcutaneous lumps found in the trunk, breast conjunctiva, and joints. Respiratory infection results in solitary or multiple pulmonary lesions called “coin lesions,” seen on chest radiographs.[6,8]
Dirofilariasis of the oral cavity is extremely rare, but when present, is usually seen in the buccal mucosa as sub mucosal nodules. It occurs in individuals over the age of 40 with a female predilection. Tilakaratne and Pitakotuwage has reported the most common site to be the buccal mucosa in his study of 7 cases.[9] Swelling of the lips and interdental papillae have been reported in a young patient with microfilaraemia.[10]
Diagnosis is confirmed with biopsy, where the pathognomonic nematode is found in the center of dense inflammatory response. Worms belonging to the genus Dirofilaria are identified by their thick laminated cuticle, broad lateral ends and large muscle cells. D. immitis can be differentiated from Dirofilaria repens by the absence of ridges. D. immitis also causes microfilaremia in human, presents clinically as pulmonary lesions and requires anti-helminthic drugs, whereas microfilaremia is absent in human cases of infection caused by Dirofilaria repens and mostly presents intra-ocularly.[11]
Polymerase chain reaction based diagnosis of human dirofilariasis on samples embedded in paraffin were first studied in pulmonary lesions in 2006. This was useful in cases of uncertain identification of dirofilarial species due to poor preservation of the worm. Earlier in 2002, studies using the PCR was carried out on ocular samples.[12]
The definitive treatment of Dirofilaria infection in humans is surgical removal of the adult worm. Medications such as DEC, ivermectin and albendazole are routinely administered. The safety and tolerability of these treatments have been evaluated through many clinical and laboratory assessments. Both the DEC and albendazole are well-tolerated when given alone or in combination with no adverse events observed and these drugs are rapidly absorbed from the gastro-intestinal tract.[13]
DEC has a highly selective effect on microfilaria. The most important action of DEC appears to be an alteration of microfilariae membranes so that they are readily phagocytosed by tissue fixed monocytes. Albendazole has an adjuvant value in treating filariasis. Ivermectin, a parasiticide, is effective against microfilariae (blocking their transmission) and can be administered annually as a single oral dose with virtually no side-effects.[14]
Cases of intra-oral nematode often pose a diagnostic dilemma leading to improper management and thereby poorer prognosis to the patient. Medical awareness of the risk of infection is essential and very often a detailed travel history is helpful in diagnosis. Hence, oral filariasis should be considered in the differential diagnosis of a recurrent intra oral or facial swelling that may not have overt inflammatory signs and symptoms or swellings that do not respond to routine therapy and those seen in patients from endemic areas.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Orihel TC, Eberhard ML. Zoonotic filariasis. Clin Microbiol Rev. 1998;11:366–81. doi: 10.1128/cmr.11.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seddon SV, Peckitt NS, Davidson RN, Sugar AW. Helminth infection of the parotid gland. J Oral Maxillofac Surg. 1992;50:183–5. doi: 10.1016/0278-2391(92)90368-a. [DOI] [PubMed] [Google Scholar]
- 3.Hira PR, Al-Buloushi A, Khalid N, Iqbal J, Bain O, Eberhard ML. Zoonotic filariasis in the Arabian Peninsula: Autochthonous onchocerciasis and dirofilariasis. Am J Trop Med Hyg. 2008;79:739–41. [PubMed] [Google Scholar]
- 4.Sathyan P, Manikandan P, Bhaskar M, Padma S, Singh G, Appalaraju B. Subtenons infection by Dirofilaria repens. Indian J Med Microbiol. 2006;24:61–2. doi: 10.4103/0255-0857.19899. [DOI] [PubMed] [Google Scholar]
- 5.Dissanaike AS, Abeyewickreme W, Wijesundera MD, Weerasooriya MV, Ismail MM. Human dirofilariasis caused by Dirofilaria (Nochtiella) repens in Sri Lanka. Parassitologia. 1997;39:375–82. [PubMed] [Google Scholar]
- 6.Pampiglione S, Canestri Trotti G, Rivasi F. Human dirofilariasis due to Dirofilaria (Nochtiella) repens: A review of world literature. Parassitologia. 1995;37:149–93. [PubMed] [Google Scholar]
- 7.Ratnatunga N, Wijesundera MS. Histopathological diagnosis of subcutaneous Dirofilaria repens infection in humans. Southeast Asian J Trop Med Public Health. 1999;30:375–8. [PubMed] [Google Scholar]
- 8.Jelinek T, Schulte-Hillen J, Löscher T. Human dirofilariasis. Int J Dermatol. 1996;35:872–5. doi: 10.1111/j.1365-4362.1996.tb05054.x. [DOI] [PubMed] [Google Scholar]
- 9.Tilakaratne WM, Pitakotuwage TN. Intra-oral Dirofilaria repens infection: Report of seven cases. J Oral Pathol Med. 2003;32:502–5. doi: 10.1034/j.1600-0714.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 10.Prabhu SR, Bhatt AP, Viswanathan R. Helminthic diseases. In: Prabhu SR, Wilson DF, Daftary DK, Johnson NW, editors. Oral Diseases in the Tropics. Oxford: Oxford University Press; 1993. p. 138. [Google Scholar]
- 11.Gautam V, Rustagi IM, Singh S, Arora DR. Subconjunctival infection with Dirofilaria repens. Jpn J Infect Dis. 2002;55:47–8. [PubMed] [Google Scholar]
- 12.Rivasi F, Boldorini R, Criante P, Leutner M, Pampiglione S. Detection of Dirofilaria (Nochtiella) repens DNA by polymerase chain reaction in embedded paraffin tissues from two human pulmonary locations. APMIS. 2006;114:567–74. doi: 10.1111/j.1600-0463.2006.apm_423.x. [DOI] [PubMed] [Google Scholar]
- 13.Shenoy RK, Suma TK, John A, Arun SR, Kumaraswami V, Fleckenstein LL, et al. The pharmacokinetics, safety and tolerability of the co-administration of diethylcarbamazine and albendazole. Ann Trop Med Parasitol. 2002;96:603–14. doi: 10.1179/000349802125001663. [DOI] [PubMed] [Google Scholar]
- 14.Richard-Lenoble D, Chandenier J, Gaxotte P. Ivermectin and filariasis. Fundam Clin Pharmacol. 2003;17:199–203. doi: 10.1046/j.1472-8206.2003.00170.x. [DOI] [PubMed] [Google Scholar]


