Abstract
White lesions both physiologic as well as pathologic are relatively frequent in the oral cavity, the most common pathology being oral leukoplakia (OL). There are many variants of OL, one of which is oral proliferative verrucous leukoplakia (OPVL). OPVL is a rare clinico-pathological entity, which is slow growing, long-term progressive lesion, but remains an enigmatic and difficult to define. The etiology of OPVL remains still unclear. Tobacco use does not seem to have a significant influence on the appearance of OPVL. These lesions may occur both in smokers and non-smokers. It is observed more frequently in women and elderly patients over 60 years at the time of diagnosis. The buccal mucosa and tongue are the most frequently involved sites. It develops initially as a white plaque of hyperkeratosis that eventually becomes a multifocal disease with confluent, exophytic and proliferative features. Various published case series have presented OPVL as a disease with aggressive biological behavior due to its high probability of recurrence and a high rate of malignant transformation. Prognosis is poor for this seemingly harmless-appearing white lesion of the oral mucosa. This article describes the clinical aspects and histologic features of an OPVL case that demonstrated the typical behavior pattern in a long-standing, persistent lesion and discusses this relatively rare entity in light of current information.
Keywords: Carcinoma, hyperkeratosis, leukoplakia, malignant transformation, oral, proliferative verrucous leukoplakia
Introduction
White lesions are relatively frequent in the oral cavity with prevalence of approximately 24.8%.[1] Among them oral leukoplakia (OL) is quite prevalent (0.2-3.6%).[1] In a retrospective study, Hansen et al.,[2] reported that 26 of the 30 lesions initially diagnosed as OL became oral carcinomas in patients followed for 1-20 years (average, 6.1 years). After this study, these lesions were named oral proliferative verrucous leukoplakia (OPVL).[3] According to the latest World Health Organization nomenclature, OPVL conforms to the new terminology of “potentially malignant disorders” given that it is neither a delimited lesion nor a condition.[1] It is best-defined as a continuum of oral epithelial disease with hyperkeratosis at one end of a clinical and microscopic spectrum and verrucous carcinoma or squamous cell carcinoma at the other.[4] It is a long-term progressive condition, which develops initially as a white plaque of hyperkeratosis that eventually becomes a multifocal disease with confluent, exophytic and proliferative features[1] and behaves in a more aggressive and relentless manner than the more innocuous white oral lesions that it can resemble clinically.[5]
Etiopathogenesis
Many potential etiologies have been hypothesized, but little has been proved about the origin of this disease process.[6] The disease seems to be idiopathic.[6] Tobacco is frequently absent as a known risk factor as OPVL occurs both in smokers and non-smokers.[1] An association has been reported between human papillomavirus (HPV) and OPVL. Between 0% and 89% of OPVL are reported to be HPV positive,[7] especially for HPV types 16 and 18.[8] Apparently, there is no unequivocal pathogenetic link between HPV and OPVL[9] and it has also been reported in association with Epstein-Barr virus[8] or candida infection.[7] Despite such extensive works, the etiology of OPVL is still as enigmatic as the disease itself.[10]
Kresty et al.,[7] reported for the first time that there is a notable difference in the mode and incidence of Inhibitor of cyclin-dependent kinase 4a/alternate reading frame (INK4a/ARF) inactivation events in OPVL compared with non-OPVL high-risk premalignant lesions. The authors hypothesized that significant rates of concomitant alterations in the INK4a/ARF locus as well as increases in p16INK4a and p14ARF homozygous deletion rates, contribute to the extremely aggressive nature of OPVL. In addition to that, transforming growth factor-alpha expression, up-regulation of cyclooxygenase-2, deoxyribonucleic acid ploidy, p53 mutation and tumor suppressor loci for loss of heterozygosity have been studied in OPVL.[9] The proliferative effect of OPVL was explained on the basis of the high rate of field cancerization existing in OPVL patients.[10] However, none of these studies have produced a clear insight in the etiopathogenesis of OPVL.[9]
Clinical features
Two of the largest studies of OPVL patients reported a predilection for this lesion in elderly women, with a ratio as high as 4:1 for women to men[7] unlike other forms of OL. The mean age at the time of diagnosis is slightly over 60 years.[1] It has been shown that almost all lesions occur bilaterally, mainly affecting the lower alveolar ridge and buccal mucosa.[3] Clinically, it generally presents as a simple benign form, which tends to spread and become diffuse.[4] In time, OPVL develops exophytic, wart-like or erythroplakic areas that become oral carcinomas.[11]
Histopathological features
The microscopic findings associated with OPVL are dependent on the stage of the disease and the adequacy of the biopsy.[4] Hansen et al.,[2] suggested histologic stages in the continuum of OPVL with intermediates.
Grade 0: Normal mucosa
Grade 2: Hyperkeratosis (clinical leukoplakia)
Grade 4: Verrucous hyperplasia
Grade 6: Verrucous carcinoma
Grade 8: Papillary squamous cell carcinoma
Grade 10: Less well-differentiated squamous cell carcinoma
Batsakis et al.,[12] reduced the number of histologic stages to four with intermediates:
Grade 0: Clinical flat leukoplakia without dysplasia
Grade 2: Verrucous hyperplasia
Grade 4: Verrucous carcinoma
Grade 6: Conventional squamous cell carcinoma with intermediates
It is of interest that the early phase of these lesions usually exhibits an interface lymphocytic infiltrate that may have a pronounced lichenoid pattern characterized by basal vacuolar degeneration containing apoptotic cells and eosinophilic bodies, similar to types of oral lichenoid stomatitis such as lichen planus.[9] Therefore, OPVL has no single defining histopathologic feature.[9]
Diagnosis
Because of the lack of specific histological criteria, the diagnosis of OPVL is based on combined clinical and histopathologic evidence of progression.[9] In previously published series, diagnosis of OPVL was made according to Hansen's et al., definition.[1] There are few studies that apply a set of diagnostic criteria that are mentioned as follows
-
Ghazali et al.,[13] established the following criteria:
- The lesion starts as homogenous leukoplakia without evidence of dysplasia at the first visit
- With time, some areas of leukoplakia become verrucous
- The disease progresses to the development of multiple isolated or confluent lesions at the same or a different site
- With time, the disease progresses through the different histopathological stages reported by Hansen et al.[2]
- The appearance of new lesions after treatment
- A follow-up period of no less than 1 year.
-
Gandolfo et al.,[14] establish the following criteria:
- An initially innocuous lesion characterized by a homogenous plaque that progresses over time to an exophytic, diffuse, usually multifocal, lesion with a verrucous epithelial growth pattern
- Histopathologically, proliferative verrucous leukoplakia (PVL) changes gradually from a simple plaque of hyperkeratosis without dysplasia to verrucous hyperplasia, verrucous carcinoma or oral squamous cell carcinoma (OSCC).
Cerero-Lapiedra et al.,[1] established the following major and minor criteria:
Major criteria
A leukoplakia lesion with more than two different oral sites, which is most frequently found in the gingiva, alveolar processes and palate
The existence of a verrucous area
That the lesions have spread or engrossed during development of the disease
That there has been a recurrence in a previously treated area
Histopathologically, there can be from simple epithelial hyperkeratosis to verrucous hyperplasia, verrucous carcinoma or OSCC, whether in situ or infiltrating.
Minor criteria
An OL lesion that occupies at least 3 cm when adding all the affected areas
That the patient be female
That patient (male or female) be a non-smoker
A disease evolution higher than 5 years.
In order to make the diagnosis of PVL, it was suggested that one of the two following combinations of the criteria mentioned before were met.
Three major criteria (being E among them) or
Two major criteria (being E among them) + two minor criteria.
Nevertheless, at present, there is no criterion that will allow for the early diagnosis of the disease.[14]
Treatment
Advise patients with OPVL to avoid other known factors associated with development of oral carcinoma, such as tobacco, alcohol and betel.
Medical care
Owing to the progressive nature of OPVL, many forms of therapy used for the management of traditional leukoplakia have been disappointing. Carbon dioxide laser, radiation, topical bleomycin solution, oral retinoids, beta-carotene and systemic chemotherapy have all failed at achieving permanent cure. Methisoprinol is a synthetic agent capable of inhibiting viral ribonucleic acid synthesis and replication and of stimulating antiviral cell–mediated reactions that has been shown to have some clinical efficacy in HPV-induced lesions. Although improvements have been noted with some of these modalities, recurrence rates after cessation of therapy are high, often within months of discontinuation of treatment.[15]
Laser ablation reportedly has been successful in a very small group of patients followed for 6-178 months. Topical photodynamic therapy also may prove useful; it causes relatively low morbidity and no scarring and multiple mucosal sites can be treated simultaneously. However, multiple treatments over the course of the diseases progression may be required.[15]
Surgical care
This lesion is resistant to the presently available treatment modalities; therefore, total excision with free surgical margins is critical combined with a lifelong follow-up.[9]
Malignant transformation and recurrences
OPVL is known for its aggressive[3] pathology, given its multifocal involvement, high malignant transformation rates (60-100%), frequent recurrences (87-100%) and high mortality rates (30-50%).[7] The gingiva and palate represented the areas with the highest frequency of these multiple malignant tumors.[1] Given the high tendency for (OSCCs) to appear in these patients, they should be checked for life at least once every 6 months.[16]
Clinical presentation
A 60-year-old male patient [Figure 1] reported to the Department of Oral Medicine and Radiology with the chief complaint of missing teeth in upper and lower jaw since 2 years and wanted replacement. There was a history of reduced mouth opening since last 2 years and his past medical history, including his family history was unremarkable. Patient's dental history revealed extraction 2 years back. Patient gave a history of tobacco chewing since childhood 2-3 times/day but has quit the habit completely since last 2 years.
Figure 1.
Extra-oral photograph of the patient
Clinical examination
Extra-oral examination revealed right and left submandibular lymphadenopathy, which was non- tender and mobile. Intra-oral examination revealed white verrucous, slightly raised lesion with a granular texture measuring approximately 4 cm × 4 cm in size on maxillary anterior alveolus in relation to tooth number 12-21, extending to upper vestibule including labial frenum [Figure 2]. Similar lesion was present on left mandibular alveolus in relation to tooth number 31-35 including vestibule and labial mucosa, crossing the midline [Figure 3]. Superioinferiorly the lesion presented as a thin linear raised band on the left side of the oral cavity extending from left upper vestibule to lower vestibule [Figure 4]. On palpation, the growth was firm, non-tender, non-fluctuant and non-compressible. Based on the history and clinical examination, a provisional clinical diagnosis of OPVL was made.
Figure 2.
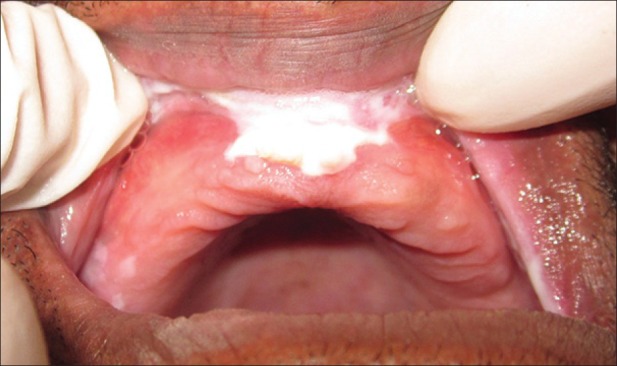
Intra-oral photograph showing white verrucous, slightly raised lesion with a granular texture in maxillary anterior alveolus in relation to tooth number 12-21 extending to upper vestibule including labial frenum
Figure 3.
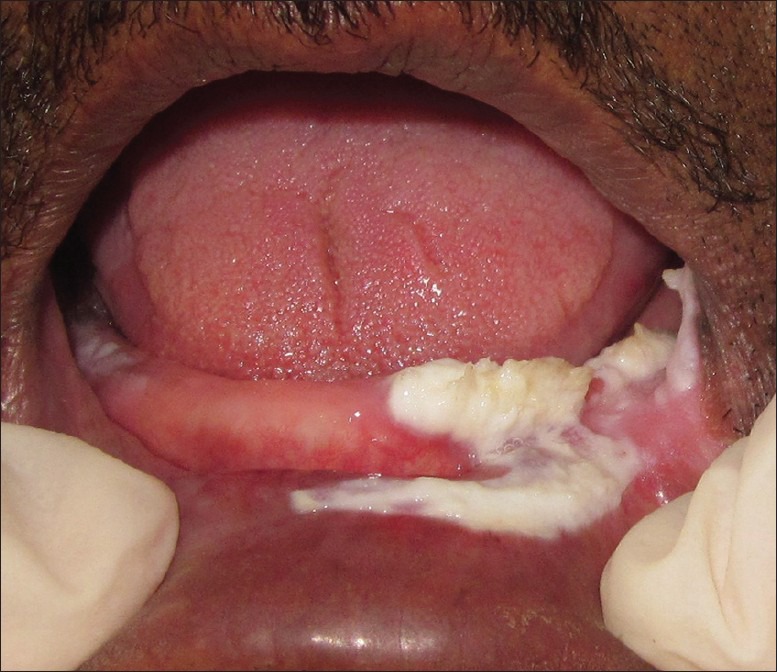
Intra-oral photograph showing lesion in left mandibular alveolus in relation to tooth number 31-35 including vestibule and labial mucosa, crossing the midline
Figure 4.
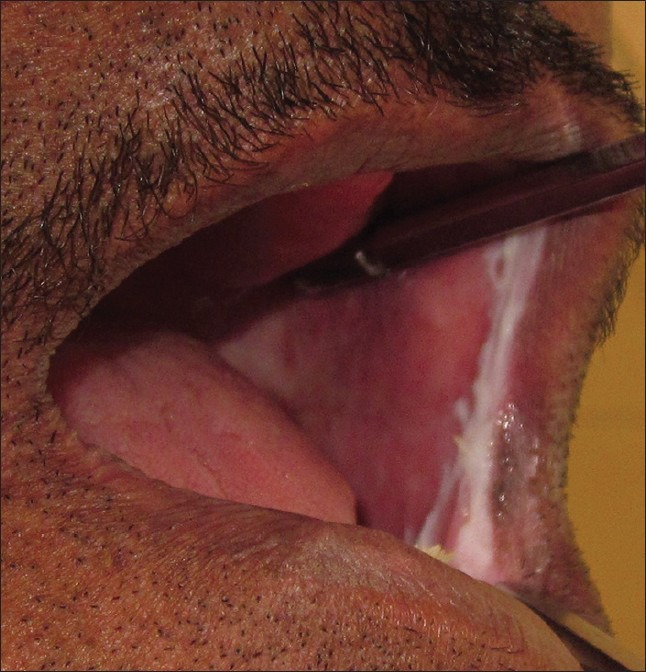
Intra-oral photograph showing lesion as a thin linear raised band on the left side of the oral cavity extending from left upper vestibule to lower vestibule
Differential diagnosis
The following entities were considered as clinical differential diagnosis:
Oral leukoplakia,
Chronic hyperplastic candidiasis,
Verrucous hyperplasia, and
Verrucous carcinoma.
Other dental findings included completely edentulous maxillary and mandibular arches. Fibrotic bands were palpable in right and left buccal mucosa along with blanching, shrunken uvula and reduced mobility of the soft palate. Mouth opening was 3.2 cm, tongue protrusion of 3.8 cm and cheek flexibility was 0.8 cm. These features were suggestive of oral sub mucous fibrosis grade II (Lai et al.,[17] 1995) [Figure 5].
Figure 5.
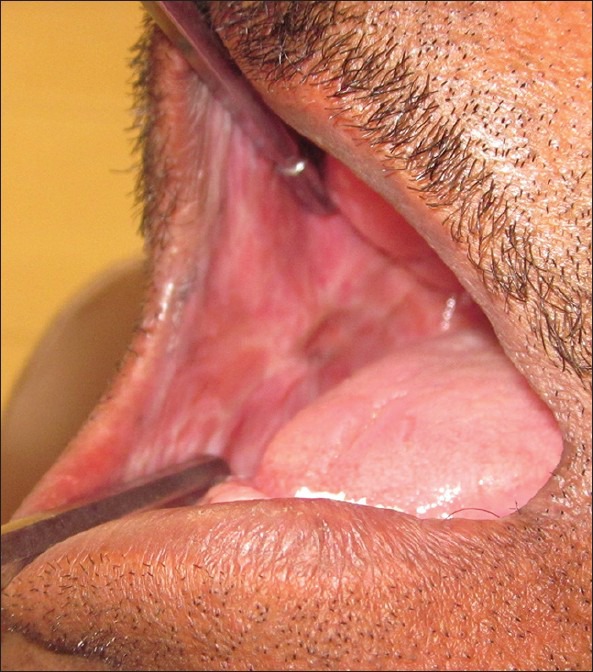
Intra-oral photograph showing blanching of right buccal mucosa
Patient was subjected to following investigations to reach to a probable diagnosis:
Toluidine blue staining used as routine staining was positive in this area, which was then completely excised
A complete hemogram was performed and all the values were in the normal range
An excisional biopsy was performed at left mandibular alveolus region and the excised tissue was sent for histopathological analysis.
Histopathologic features
Histopathological examination revealed hyperkeratotic epithelium showing dysplastic features like basilar hyperplasia and hyperchromatic cells extending up to the lower third of epithelium. The stroma was made up of collagen fibers with plump to spindle shaped fibroblasts along with patchy distribution of inflammatory cells predominately lymphocytes and plasma cells seen in the juxta-epithelial region [Figure 6]. Histologically, the lesion was diagnosed as hyperkeratosis with mild dysplasia (Hansen et al.,[2] microscopic grade 1-4).
Figure 6.
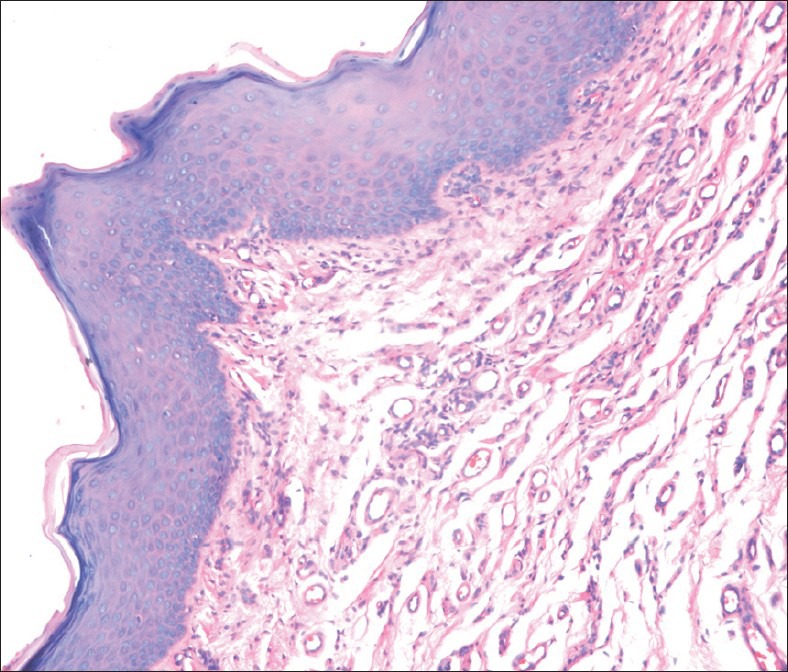
Photomicrograph of the lesion showing hyperkeratotic epithelium and dysplastic features like basilar hyperplasia and hyperchromatic cells (×40)
The overall clinical and histopathological findings were considered diagnostic for OPVL; hence, the final diagnosis.
Treatment and follow-up
Subsequent to histological diagnosis, the entire lesion was surgically excised using electrocautery [Figure 7a] and the region was sutured [Figure 7b and c]. The tissue excised was sent for histopathological re-evaluation, which confirmed the previous histopathological diagnosis. Later the follow-up was not possible as patient did not report back.
Figure 7.
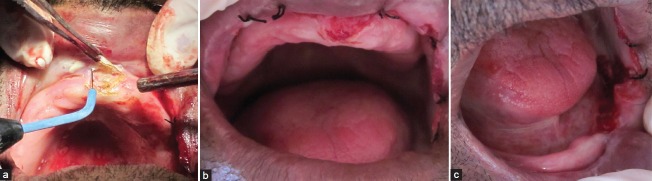
(a) Surgical excision of lesion in maxillary anterior region using electrocautery. (b) Sutures in maxillary anterior region. (c) Sutures in mandibular anterior region
Conclusions
OPVL is a rare, but highly aggressive form of OL, which requires special awareness on the part of the clinician. Therefore, it is recommended to have the earliest possible diagnosis and total excision of this lesion. The aim/intention of reporting this case was to report a case with typical clinical and histologic features of OPVL so as to sensitize the oral physicians. The care should be taken to follow-up these cases for a long time even after surgical management as they have higher recurrence rate and are also known to undergo malignant transformation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Cerero-Lapiedra R, Baladé-Martínez D, Moreno-López LA, Esparza-Gómez G, Bagán JV. Proliferative verrucous leukoplakia: A proposal for diagnostic criteria. Med Oral Patol Oral Cir Bucal. 2010;15:e839–45. [PubMed] [Google Scholar]
- 2.Hansen LS, Olson JA, Silverman S., Jr Proliferative verrucous leukoplakia. A long-term study of thirty patients. Oral Surg Oral Med Oral Pathol. 1985;60:285–98. doi: 10.1016/0030-4220(85)90313-5. [DOI] [PubMed] [Google Scholar]
- 3.Navarro CM, Sposto MR, Sgavioli-Massucato EM, Onofre MA. Transformation of proliferative verrucous leukoplakia to oral carcinoma: A ten years follow-up. Med Oral. 2004;9:229–33. [PubMed] [Google Scholar]
- 4.Greer RO, McDowell JD, Hoernig G. Proliferative verrucous leukoplakia: Report of two cases and a discussion of clinicopathology. J Calif Dent Assoc. 1999;27:300–5. 308. [PubMed] [Google Scholar]
- 5.Cabay RJ, Morton TH, Jr, Epstein JB. Proliferative verrucous leukoplakia and its progression to oral carcinoma: A review of the literature. J Oral Pathol Med. 2007;36:255–61. doi: 10.1111/j.1600-0714.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 6.Shewale VN, Shewale K. Proliferative verrucous leukoplakia: Benignly progressing towards malignancy. Asian J Oral Health Allied Sci. 2011;1:71–3. [Google Scholar]
- 7.Kresty LA, Mallery SR, Knobloch TJ, Li J, Lloyd M, Casto BC, et al. Frequent alterations of p16INK4a and p14ARF in oral proliferative verrucous leukoplakia. Cancer Epidemiol Biomarkers Prev. 2008;17:3179–87. doi: 10.1158/1055-9965.EPI-08-0574. [DOI] [PubMed] [Google Scholar]
- 8.Bagan JV, Jiménez Y, Murillo J, Poveda R, Díaz JM, Gavaldá C, et al. Epstein-Barr virus in oral proliferative verrucous leukoplakia and squamous cell carcinoma: A preliminary study. Med Oral Patol Oral Cir Bucal. 2008;13:E110–3. [PubMed] [Google Scholar]
- 9.Mete O, Keskin Y, Hafiz G, Kayhan KB, Unur M. Oral proliferative verrucous leukoplakia: Underdiagnosed oral precursor lesion that requires retrospective clinicopathological correlation. Dermatol Online J. 2010;16:6. [PubMed] [Google Scholar]
- 10.Bishen KA, Sethi A. Proliferative verrucous leukoplakia: Diagnostic pitfalls and suggestions. Med Oral Patol Oral Cir Bucal. 2009;14:E263–4. [PubMed] [Google Scholar]
- 11.Bagan J, Scully C, Jimenez Y, Martorell M. Proliferative verrucous leukoplakia: A concise update. Oral Dis. 2010;16:328–32. doi: 10.1111/j.1601-0825.2009.01632.x. [DOI] [PubMed] [Google Scholar]
- 12.Batsakis JG, Suarez P, el-Naggar AK. Proliferative verrucous leukoplakia and its related lesions. Oral Oncol. 1999;35:354–9. doi: 10.1016/s1368-8375(99)00007-x. [DOI] [PubMed] [Google Scholar]
- 13.Ghazali N, Bakri MM, Zain RB. Aggressive, multifocal oral verrucous leukoplakia: Proliferative verrucous leukoplakia or not? J Oral Pathol Med. 2003;32:383–92. doi: 10.1034/j.1600-0714.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- 14.Gandolfo S, Castellani R, Pentenero M. Proliferative verrucous leukoplakia: A potentially malignant disorder involving periodontal sites. J Periodontol. 2009;80:274–81. doi: 10.1902/jop.2009.080329. [DOI] [PubMed] [Google Scholar]
- 15.Azfar RS, Elston DM. Proliferative Verrucous Leukoplakia. Medscape Reference-Drugs, Diseases and Procedures Jan 19. 2012 [Google Scholar]
- 16.Bagán JV, Murillo J, Poveda R, Gavaldá C, Jiménez Y, Scully C. Proliferative verrucous leukoplakia: Unusual locations of oral squamous cell carcinomas, and field cancerization as shown by the appearance of multiple OSCCs. Oral Oncol. 2004;40:440–3. doi: 10.1016/j.oraloncology.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Lai DR, Chen HR, Lin LM, Huang YL, Tsai CC. Clinical evaluation of different treatment methods for oral submucous fibrosis. A 10-year experience with 150 cases. J Oral Pathol Med. 1995;24:402–6. doi: 10.1111/j.1600-0714.1995.tb01209.x. [DOI] [PubMed] [Google Scholar]


