Abstract
Purpose.
To determine whether ultrasound treatment can promote the permeation of topical riboflavin into the corneal stroma.
Methods.
Fresh cadaveric rabbit eyes with intact epithelium were left for 45 minutes in riboflavin 0.1% solution and divided in the following groups: A – untreated, epithelium-on; B – ultrasound-treated (1 W/cm2 at 880 kHz for 6 minutes) with epithelium-on; and C – epithelium-off (no ultrasound). Eyes were removed from the riboflavin solution, corneas were excised, and group B was divided into B1 (with epithelium maintained) and B2 (epithelium removed for the fluorescence analysis). Confocal microscopy was performed to quantify the fluorescence intensity in the cornea according to the distance from the surface (with epithelium in groups A and B1; without epithelium in groups B2 and C).
Results.
The average fluorescence intensity of riboflavin at a depth of 100, 150, 200, and 250 μm was 69.97, 58.83, 49.23, and 41.72 arbitrary units (A.U.) in group A, respectively; 255.26, 206.01, 159.81, 124.20 A.U. in group B1; 218.90, 177.90, 141.43, 110.45 A.U. in group B2; and 677.64, 420.10, 250.72 and 145.07 A.U. in group C. The difference in fluorescence was statistically significant between groups A and B1 (P = 0.001) and groups B2 and C (P < 0.0001).
Conclusions.
Ultrasound treatment increased the entry of topical riboflavin into the corneal stroma despite the presence of a previously intact epithelial barrier. This approach may offer a means of achieving clinically useful concentrations of riboflavin within the cornea with minimum epithelial damage, thereby improving the risk profile of corneal cross-linking procedures.
Keywords: ultrasonics, drug delivery systems, phonophoresis, riboflavin, cornea
Ultrasound treatment can promote the corneal stromal uptake of topical riboflavin, potentially offering a means of performing cross-linking treatment without epithelial removal.
Introduction
During the last decade corneal collagen crosslinking (CXL) techniques have gained widespread successful use in the treatment of corneal ectasias.1–4 Though the mechanism of action is still not fully understood, it is believed that when riboflavin is excited with UV-A radiation, it can interact directly, or through generation of singlet oxygen with the surrounding collagen molecules, generating new bonds and, consequently, increasing corneal stiffness.5,6 Riboflavin is a large molecule with poor penetration through the intact corneal epithelium, so to achieve a satisfactory stromal concentration of riboflavin, the corneal epithelium is removed over the area to be treated in the classical CXL technique.1 Although CXL is considered a safe procedure, infectious complications related to epithelial removal have been reported.7–10 Aiming to make CXL even more safe and comfortable for patients, methods of increasing the stromal delivery of riboflavin without removing the epithelium are being studied.11,12 The use of ultrasound waves to enhance the movement of drugs through intact skin (phonophoresis) has been reported since the 1950s.13 Transcorneal phonophoresis of hydrocortisone, papain, and hypotensive agents has been reported since the 1970s in Europe.14,15 More recently, phonophoresis was reported to increase up to 10-fold the concentration of topically applied sodium fluorescein in the aqueous humor.16 Mechanisms by which phonophoresis enhances drug delivery include radiation forces, acoustic streaming, and acoustic cavitation.17–19 We investigated the ability of phonophoresis to augment the penetration of riboflavin into the corneal stroma over an intact epithelium.
Methods
Seventy-two fresh cadaveric rabbit eyes (Pel-Freez Biologicals, Rogers, AR) with intact corneal epithelium were used in this study. The eyes were used within 30 hours of enucleation, and to confirm epithelial integrity, two drops of fluorescein 1% solution (Sigma-Aldrich, St. Louis, MO) were applied over the corneas, and a portable slit lamp (SL-RVP; Ray Vision, Beijing, China) with a cobalt blue filter was used to check the corneal surface for the existence of scratches or epithelial damage. The eyes with intact epithelium were gently washed with saline solution and randomly assigned to one of the experimental groups. The groups were as follows: group A – untreated epithelium-on eyes (n = 15); group B – ultrasound-treated epithelium-on eyes (n = 31); group C – untreated epithelium-off eyes (n = 16). In order to allow a depth-related fluorescence comparison among these groups, group B was subdivided in two groups: B1 – confocal analysis done with epithelium maintained (n = 15); and B2 – confocal analysis done after removal of epithelium (n = 16). In a second part of the experiment, we assessed the temperature variation in ultrasound-treated and untreated corneas.
Treatment
Eyes in group A were placed in a solution of 0.1% riboflavin (Sigma-Aldrich) for 45 minutes without receiving any additional treatment. Eyes in group B were treated with continuous-wave ultrasound 880 kHz at 1 W/cm2, applied to the central cornea for the first 6 minutes, and then remained in the riboflavin solution for an additional 39 minutes (total 45 minutes). Eyes in group C had the epithelium removed with a surgical blade (N24; Feather, Osaka, Japan) before placement in the riboflavin solution, where they were left for 45 minutes. A water bath system was used to maintain the temperature at 34°C during the experiment (Fig. 1). After the 45 minute immersion, all eyes were removed from the riboflavin solution, corneas were excised, epithelium was removed from corneas in group B2, and a fluorescent analysis using confocal microscopy was immediately performed in all samples. The 458-nm wavelength was chosen as the excitation wavelength, and the emission was collected from wavelengths 560 to 615 nm through a Zeiss LD Plan-Neofluar 40×/0.6 objective (Carl Zeiss, Jena, Germany) on a Zeiss LSM510 confocal microscope (Carl Zeiss). The microscope excitation and detection settings were selected based on pilot data and fixed for all experiments (pinhole = 1.0 airy units; optical section thickness = 3 μm; detector gain = 691; amplifier gain = 1; laser transmission 10%). The experiment was repeated more than three times, always with at least one eye of each group. The anterior corneal surface was marked as the starting point (0-μm depth), and mean fluorescence of the entire image field (440 μm2) was measured in a 12 bit dynamic range in arbitrary units, at 100-, 150-, 200-, and 250-μm depth in the cornea. The results were compared between groups A and B1 (with epithelium) and between groups B2 and C (without epithelium).
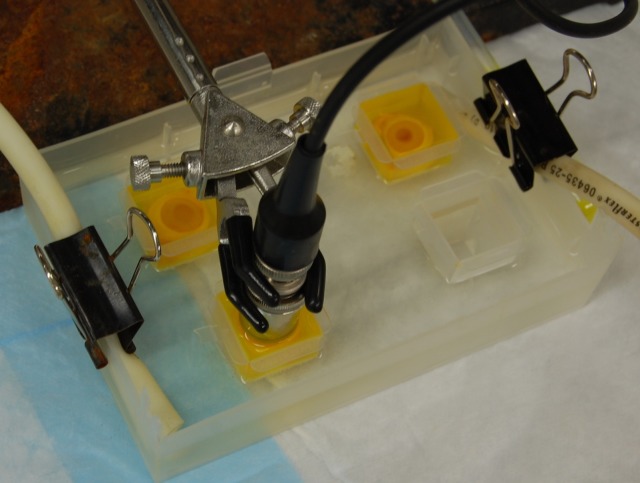
Figure 1. .
Two cadaveric rabbit eyes submerged in riboflavin 0.1% solution (top) and one eye being treated with ultrasound (1 W/cm2) over the cornea (bottom). The experiment was done inside a temperature-controlled water bath.
Temperature Variation
As described before, a water bath system was used to warm up the eyes and maintain the experiment temperature at 34 ± 1°C. Riboflavin solution was applied at 34°C in group D (n = 4) and at room temperature (24°C) in group E (n = 6). All eyes in group D and three eyes from group E were treated for 6 minutes with continuous wave ultrasound 880 Khz at 1 W/cm2; the remaining three eyes from group E were left untreated as controls. To assess the variation in corneal temperature, a thermocouple (HYP1; Omega, Stamford, CT) was inserted in the superficial corneal stroma of the preheated eyes immediately after its submersion in riboflavin solution (T0) and after 6 minutes (T6) with or without ultrasound treatment.
Statistical Analysis
The numerical data were initially entered into a spreadsheet (Microsoft Excel XP; Microsoft, Redmond, WA) and then exported to the R statistical package (version 2.12; R Foundation for Statistical Computing, Vienna, Austria). The difference in the mean vectors between groups was assessed using the Two-sample Hotelling test. An alpha level of P less than or equal to 0.05 was chosen as the criterion of significance.
Results
Untreated Epithelium-On Eyes Versus Ultrasound-Treated Epithelium-On Eyes
Ultrasound treatment increased the permeation of riboflavin through the corneal epithelium and into the corneal stroma, compared with untreated controls. The average fluorescence intensity of riboflavin at a depth of 100, 150, 200, and 250 μm was 69.97, 58.83, 49.23, and 41.72 A.U. in group A, respectively, and 255.26, 206.01, 159.81, and 124.20 A.U. in group B1, respectively (Fig. 2). The difference between groups was statistically significant (P = 0.001).
Figure 2. .
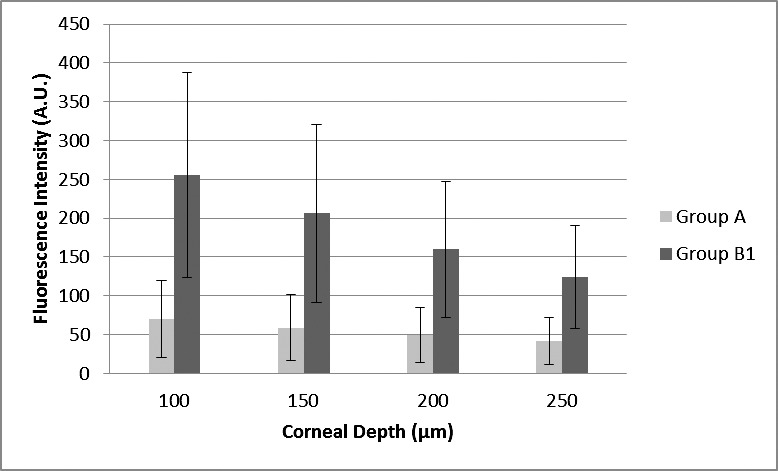
Fluorescence intensity (in arbitrary units; with SD bars) according to corneal depth (micrometers) measured from anterior surface (with epithelium) in groups A (untreated epithelium-on eyes; n = 15) and B1 (ultrasound-treated epithelium-on eyes; n = 15).
Untreated Epithelium-Off Eyes Versus Ultrasound-Treated Epithelium-On Eyes
Eyes that underwent epithelial removal, but had no ultrasound treatment had more riboflavin penetration into the stroma compared with ultrasound-treated eyes. The average fluorescence intensity of riboflavin at a depth of 100, 150, 200, and 250 μm was 218.90, 177.90, 141.43, 110.45 A.U. in group B2, respectively, and 677.64, 420.10, 250.72 and 145.07 A.U. in group C, respectively (Fig. 3). The difference between groups was statistically significant (P < 0.0001).
Figure 3. .
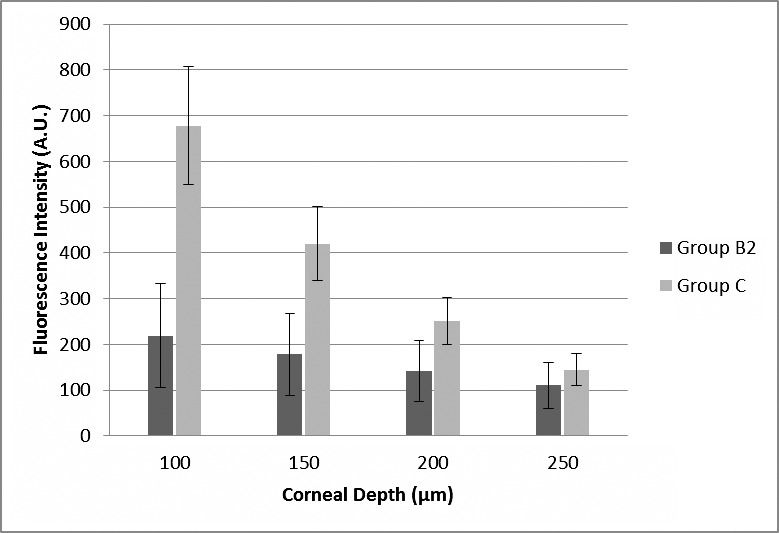
Average fluorescence intensity (in arbitrary units; with SD bars) according to corneal depth (micrometers) measured from anterior surface (without epithelium) in groups B2 (ultrasound-treated epithelium-on eyes, epithelium was removed for the fluorescence analysis; n = 16) and C (epithelium-off eyes, n = 16).
Temperature Assessment
The mean increase in corneal temperature in eyes from group D was 6.1°C, from 34.1°C in T0 to 40.2°C in T6. Group E eyes were initially submerged in a solution of riboflavin at room temperature, therefore, the corneal temperature immediately decreased (T0) and then increased due to the surrounding water bath and the effect of the ultrasound if treated. The mean difference in group E comparing the final corneal temperature between treated and untreated eyes was 7.5°C (Table).
Table.
Temperature Variation
|
Eye Group |
Sample |
Cornea T0§, °C |
US-Treated‡ |
Cornea T6||, °C |
| D* | 1 | 34 | Yes | 40.3 |
| 2 | 34.4 | Yes | 40.0 | |
| 3 | 34.1 | Yes | 40.3 | |
| 4 | 33.8 | Yes | 40.3 | |
| E† | 1 | 27.5 | Yes | 38.5 |
| 2 | 25.5 | Yes | 39.1 | |
| 3 | 25.6 | Yes | 38.8 | |
| 4 | 27.5 | No | 33.0 | |
| 5 | 25.5 | No | 30.0 | |
| 6 | 25.6 | No | 31.0 |
Group D = Eyes initially at 34°C, submerged in riboflavin solution at 34°C.
Group E = Eyes initially at 34°C, submerged in riboflavin solution at 24°C.
US, Ultrasound.
T0 = Temperature in the cornea at time 0 minutes, immediately after eye submersion in riboflavin solution.
T6 = Temperature in the cornea at time 6 minutes.
Discussion
We sought to determine whether the use of phonophoresis could enhance the permeation of topical riboflavin into the corneal stroma. We used the inherent fluorescent property of the riboflavin as an indirect signal to detect its presence and make comparisons in between groups under similar conditions. This study showed that the use of phonophoresis (1 W/cm2 at 880 kHz) increased up to 3.6-fold the fluorescence intensity within the corneal stroma of treated eyes compared with untreated eyes. This result is comparable to what Zderic et al.19 achieved using dissected rabbit corneas in a two-chamber setup to study the transcorneal permeation of fluorescein, showing up to 4.2-fold increase in the permeation in corneas treated with ultrasound at 0.56 W/cm2 at 880 kHz.
We designed our experiment in order to permit a more accurate comparison of the results among groups using confocal microscopy. Comparing samples with and without epithelium in the same setup would not be desirable due to the partial absorption of light by the corneal epithelium, potentially attenuating the levels of fluorescence measured. Using a different approach than previously reported,20 we presented fluorescence values in arbitrary units, which represents the true output of the equipment. Performing a mathematical conversion of these values to a calculated riboflavin concentration introduces error and was not necessary for our purposes, which were to obtain relative comparisons among groups and determine whether fluorescence would be increased in ultrasound-treated groups.
Corneal collagen crosslinking is currently accepted as a safe technique in many countries. The complications reported are usually secondary or related to the epithelial removal step of the procedure. Koppen et al.21 observed a prevalence of sterile keratitis and scarring of 3.4% of 117 treated eyes and Koller et al.22 reported a complication rate (eyes losing 2 or more Snellen lines) of 2.9% in a similar series.
Multiple alternatives have been investigated to perform transepithelial CXL, which would make the treatment more comfortable for patients and reduce the risk of infection related to scarification.11,23–27 The use of iontophoresis was investigated and a 1.7-fold increase was reported in the concentration of riboflavin in the corneal stroma compared with controls (Ziebarth NM, et al. IOVS 2011;52:E-Abstract 2544).. Other groups are modifying the solution of riboflavin by adding substances such as benzalkonium chloride, tromethamine, and EDTA, which can enhance the penetration of riboflavin partially due to their toxicity to the corneal epithelium.12,24,25 The two main factors that probably contributed to the inferior results obtained in many studies applying transepithelial techniques24–26 are the reduced levels of riboflavin in the corneal stroma due to poor penetration, and also the partial absorption of UV-A by the epithelium saturated with riboflavin. In a cohort study, Filippello et al. concluded that transepithelial CXL using a solution of riboflavin containing trometamol and EDTA appeared to halt keratoconus progression; however, they also reported that when these enhancers were used in the riboflavin solution, 40% of the patients developed transient hyperemia and foreign body sensation.12
In our study, the mean fluorescence intensity in the group of ultrasound-treated eyes at a 150 μm-depth was 42.34% of the intensity measured in the group of epithelium-off eyes. Raiskup et al.27 reported that using a riboflavin solution containing benzalkonium chloride 0.01% and NaCl 0.44% for 30 minutes, the coefficient of absorption of UV-A was 33% in the eyes with epithelium compared with the standard epithelium off procedure. Hypotonic solutions can partially damage the epithelial cells due to osmolysis.28 Flynn and Hill showed that a mere splash with distilled water could impair epithelial corneal function for up to 15 minutes.29 Krutsinger et al. have shown that exposure to hypotonic 0.19% saline caused loss of desmosomal contacts between epithelial cells, cell deformation, and an increased incidence of surface cell detachment in rabbit corneas.30 Rylander et al. reported that in order to reduce or even eliminate the irritating properties of tap water, concentrations of NaCl above 0.5% are needed,31 and Stevenson et al. found measurable corneal swelling in human corneas after exposure to salt concentrations of 0.7% or less.32
In other words, most of the proposed methods to increase the epithelial permeability to riboflavin depend, to some degree, on superficial epithelial damage. The main mechanisms attributed to phonophoresis are acoustic streaming, representing the development of unidirectional flow currents in fluid that are the result of the presence of sound waves, and cavitation, which is the formation of gaseous cavities that can collapse and cause structural alteration in the surrounding tissue upon ultrasound exposure.18 In a histologic investigation of corneal changes after ultrasound application over dissected flattened corneas, Zderic et al.19 found no evidence of stromal damage, but an increased number of damaged epithelial cells per millimeter in the treated group when compared with untreated corneas. Once future studies determine the best ultrasound device settings, a dedicated and more specific safety study should be performed in order to evaluate the extension of the phonophoresis effects in the corneal structures, including any unexpected effect upon the endothelium. While the extent of the damage remains unknown for our experiment, one of the advantages of phonophoresis is that we can aim and limit the damage to a defined geographic area of the cornea (i.e., central), and since the limbal stem cells are not affected, we do not expect to have any irreversible effect on the epithelial layers of the treated central zone. On the contrary, when administering a solution of riboflavin containing toxic “enhancers” multiple times to the eye, even though the damage to the surface of the cornea is considered to be minor, the possibility of untoward effects resulting from the diffuse exposure of these toxic enhancer chemicals over the surface of the limbus and conjunctiva has to be taken into account. For this reason, targeted corneal therapy with ultrasound may offer a safer means of enhancing riboflavin penetration through an intact corneal epithelium.
In order to better understand the impact of the phonophoresis upon corneal permeability, in this study we did not add any enhancers to the riboflavin solution, and the solution was prepared using saline 0.85%. However, we do believe that the association of phonophoresis with a riboflavin solution containing enhancers and less NaCl might be the answer to reducing the time necessary to load the cornea with riboflavin and reduce the potential of damage outside the area aimed in the corneal surface by using a cup to reduce the exposure of the surrounding surface to the “enhanced” riboflavin solution.
The mean increase in the corneal temperature attributed to the ultrasound treatment was 6.1°C with a maximum temperature of 40.3°C measured immediately after treatment in an eye where the solution of riboflavin had been previous warmed to 34°C. According to Spoerl et al. the biomechanical properties of the pig cornea remained unchanged in the temperature range of 30°C to 50°C.33 Goldblatt et al. found that up to 45°C for 15 minutes was well tolerated by healthy rabbit corneas and 52°C was a transitional temperature for keratocyte damage, where exposure times inferior to 5 minutes produced no evidence of histologic damage at the 1-week follow-up examination.34 Therefore, the increase in temperature observed in our study is safe and below the threshold necessary to change biomechanical properties or to damage the keratocytes.
In the last 10 years, multiple groups have proposed different approaches for transepithelial treatment, but so far the literature suggests that the clinical results are not as good as those obtained with the standard method proposed by Wollensak et al.1 In the future, new medical devices may help to determine the amount of increase in stiffness needed by each patient and what would be the best relationship of “riboflavin concentration versus UV-A irradiance/dose” to achieve that goal in a more personal way, but currently this is not possible. Ultrasound technology is broadly used in medicine, and we have shown it to be effective in enhancing the intrastromal delivery of riboflavin. Further studies should aim to confirm the efficacy of intrastromal phonophoresis in vivo, find the best device settings, and confirm the safety of the proposed settings. Moreover, most of the literature regarding phonophoresis was done with transdermal delivery, and the improvement in the method for corneal intrastromal delivery is important not only for riboflavin but also for antibiotics, antifungals, and other drugs of poor corneal penetration.
In conclusion, ultrasound treatment facilitated the entry of topical riboflavin into the corneal stroma despite the presence of a previously intact epithelial barrier. This approach may offer a means of achieving clinically useful concentrations of riboflavin in the cornea with minimum epithelium damage, thereby improving the risk profile of corneal cross-linking procedures. Further studies need to be done to investigate the safety and efficacy of this technique in vivo.
Acknowledgments
The authors thank Geoffrey Lambright of Carl Zeiss Microscopy for providing technical assistance with the confocal microscope.
Supported by grants from That Man May See, Inc., Research to Prevent Blindness, and the National Institutes of Health-National Eye Institute (EY002162) - Core Grant for Vision Research.
Disclosure: R. Lamy, None; E. Chan, None; H. Zhang, None; V.A. Salgaonkar, None; S.D. Good, None; T.C. Porco, None; C.J. Diederich, None; J.M. Stewart, None
References
- 1. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003; 135: 620–627 [DOI] [PubMed] [Google Scholar]
- 2. Vinciguerra P, Albè E, Trazza S, et al. Refractive, topographic, tomographic, and aberrometric analysis of keratoconic eyes undergoing corneal cross-linking. Ophthalmology. 2009; 116: 369–378 [DOI] [PubMed] [Google Scholar]
- 3. Caporossi A, Baiocchi S, Mazzotta C, Traversi C, Caporossi T. Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A rays induced cross-linking of corneal collagen: preliminary refractive results in an Italian study. J Cataract Refract Surg. 2006; 32: 837–845 [DOI] [PubMed] [Google Scholar]
- 4. Lamy R, Netto CF, Reis RG, et al. Effects of corneal cross-linking on contrast sensitivity, visual acuity, and corneal topography in patients with keratoconus. Cornea. 2013; 32: 591–596 [DOI] [PubMed] [Google Scholar]
- 5. McCall AS, Kraft S, Edelhauser HF, et al. Mechanisms of corneal tissue cross-linking in response to treatment with topical riboflavin and long-wavelength ultraviolet radiation (UVA). Invest Ophthalmol Vis Sci. 2010; 51: 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kamaev P, Friedman MD, Sherr E, Muller D. Photochemical kinetics of corneal cross-linking with riboflavin. Invest Ophthalmol Vis Sci. 2012; 53: 2360–2367 [DOI] [PubMed] [Google Scholar]
- 7. Sharma N, Maharana P, Singh G, Titiyal JS. Pseudomonas keratitis after collagen crosslinking for keratoconus: case report and review of literature. J Cataract Refract Surg. 2010; 36: 517–520 [DOI] [PubMed] [Google Scholar]
- 8. Zamora KV, Males JJ. Polymicrobial keratitis after a collagen cross-linking procedure with postoperative use of a contact lens: a case report. Cornea. 2009; 28: 474–476 [DOI] [PubMed] [Google Scholar]
- 9. Pollhammer M, Cursiefen C. Bacterial keratitis early after corneal crosslinking with riboflavin and ultraviolet-A. J Cataract Refract Surg. 2009; 35: 588–589 [DOI] [PubMed] [Google Scholar]
- 10. Pérez-Santonja JJ, Artola A, Javaloy J, Alió JL, Abad JL. Microbial keratitis after corneal collagen crosslinking. J Cataract Refract Surg. 2009; 35: 1138–1140 [DOI] [PubMed] [Google Scholar]
- 11. Kanellopoulos AJ. Collagen cross-linking in early keratoconus with riboflavin in a femtosecond laser-created pocket: initial clinical results. J Refract Surg. 2009; 25: 1034–1037 [DOI] [PubMed] [Google Scholar]
- 12. Filippello M, Stagni E, O'Brart D. Transepithelial corneal collagen crosslinking: bilateral study. J Cataract Refract Surg. 2012; 38: 283–291 [DOI] [PubMed] [Google Scholar]
- 13. Fellinger K, Schmid J. Klinik und Therapie des Chronischen Gelenkrheumatismus. Vienna, Austria: Maudrich; 1954: 549–552 [Google Scholar]
- 14. Cherkasov IS, Marmur RK, Radkovskaia AI, Loskova LM. Phonophoresis of hypotensive agents in the treatment of simple glaucoma [in Russian]. Oftalmol Zh. 1974; 29: 114–118 [PubMed] [Google Scholar]
- 15. Zobina LV, Proskurova GI. Phonophoresis of hydrocortisone through the cornea [in Russian]. Oftalmol Zh. 1970; 25: 502–506 [PubMed] [Google Scholar]
- 16. Zderic V, Clark JI, Vaezy S. Drug delivery into the eye with the use of ultrasound. J Ultrasound Med. 2004; 23: 1349–1359 [DOI] [PubMed] [Google Scholar]
- 17. Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery-a general review. Expert Opin Drug Deliv. 2004; 1: 37–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lavon I, Kost J. Ultrasound and transdermal drug delivery. Drug Discov Today. 2004; 9: 670–676 [DOI] [PubMed] [Google Scholar]
- 19. Zderic V, Clark JI, Martin RW, Vaezy S. Ultrasound-enhanced transcorneal drug delivery. Cornea. 2004; 23: 804–811 [DOI] [PubMed] [Google Scholar]
- 20. Søndergaard AP, Hjortdal J, Breitenbach T, Ivarsen A. Corneal distribution of riboflavin prior to collagen cross-linking. Curr Eye Res. 2010; 35: 116–121 [DOI] [PubMed] [Google Scholar]
- 21. Koppen C, Vryghem JC, Gobin L, Tassignon MJ. Keratitis and corneal scarring after UVA/riboflavin cross-linking for keratoconus. J Refract Surg. 2009; 25: 819 [DOI] [PubMed] [Google Scholar]
- 22. Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009; 35: 1358–1362 [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y, Sukthankar P, Tomich JM, Conrad GW. Effect of the synthetic NC-1059 peptide on diffusion of riboflavin across an intact corneal epithelium. Invest Ophthalmol Vis Sci. 2012; 53: 2620–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koppen C, Wouters K, Mathysen D, Rozema J, Tassignon MJ. Refractive and topographic results of benzalkonium chloride-assisted transepithelial crosslinking. J Cataract Refract Sur. 2012; 38: 1000–1005 [DOI] [PubMed] [Google Scholar]
- 25. Wollensak G, Iomdina E. Biomechanical and histological changes after corneal crosslinking with and without epithelial debridement. J Cataract Refract Surg. 2009; 35: 540–546 [DOI] [PubMed] [Google Scholar]
- 26. Leccisotti A, Islam T. Transepithelial corneal collagen cross-linking in keratoconus. J Refract Surg. 2010; 26: 942–948 [DOI] [PubMed] [Google Scholar]
- 27. Raiskup F, Pinelli R, Spoerl E. Riboflavin osmolar modification for transepithelial corneal cross-linking. Curr Eye Res. 2012; 37: 234–238 [DOI] [PubMed] [Google Scholar]
- 28. Shin YJ, Wee WR, Kim M, Lee JH. Corneoscleral cyst treated with distilled water injection. Korean J Ophthalmol. 2002; 16: 110–113 [DOI] [PubMed] [Google Scholar]
- 29. Flynn W, Hill R. Hypotonic exposures. J Am Optom Assoc. 1984; 55: 221–222 [PubMed] [Google Scholar]
- 30. Krutsinger B, Bergmanson J. Corneal epithelial response to hypotonic exposure. Int Eyecare. 1985; 1: 440–443 [Google Scholar]
- 31. Rylander R, Victorin K, Sorensen S. The effect of saline on the eye irritation caused by swimming pool water. J Hyg. 1973; 71: 587–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stevenson R, Vaja N, Jackson J. Corneal transparency changes resulting from osmotic stress. Ophthalmic Physiol Opt. 1983; 3: 33–39 [PubMed] [Google Scholar]
- 33. Spörl E, Genth U, Schmalfuss K, Seiler T. Thermomechanical behavior of the cornea. Ger J Ophthalmol. 1996; 5: 322–327 [PubMed] [Google Scholar]
- 34. Goldblatt W, Finger P, Perry H, et al. Hyperthermic treatment of rabbit corneas. Invest Ophthalmol Vis Sci. 1989; 30: 1778–1783 [PubMed] [Google Scholar]