The dendritic spine as a fundamental player in CNS plasticity and its potential role in pain plasticity
Since the time of Ramon y Cajal, the dendritic spine, or “espina” as Cajal first named it, has been an enigmatic figure in the area of neuroscience. At first a prime character in Cajal's neuron doctrine (Ramon y Cajal, 1899), the last two decades have led to remarkable advances in our understanding of the role of dendritic spines in the form and function of the nervous system. Many, but not all, principle neurons in the brain have spines decorating their dendrites and these spines are almost always the postsynaptic site for an excitatory synapse (Arellano et al., 2007). We now know that spines demonstrate remarkable plasticity in response to increases in presynaptic activity and that this plasticity is orchestrated by a complex symphony of signaling localized to the spine (Yuste, 2010; Yuste and Bonhoeffer, 2001). In terms of function, recent studies have shown clear changes in spine morphology in several important neurological developmental disorders and changes in spine shape are linked to major functions of the brain like learning and memory. This latter function appears to be linked to the establishment of long-term potentiation (LTP) suggesting that changes in spine morphology, in particular toward a mushroom-shaped morphology, are a critical component of amplification of synaptic signaling in the CNS (De Roo et al., 2008).
Despite this explosion of interest and understanding in the dendritic spine biology, the role of spine plasticity in chronic pain and/or pain amplification has been largely ignored until relatively recently. Three papers by Andrew Tan, Bryan Hains, Stephen Waxman and colleagues (Tan et al., 2008b, 2009, 2011), the most recent of which was published in this issue of Experimental Neurology (Tan et al., 2011), shed considerable light on the role of spine plasticity in the development and maintenance of neuropathic pain. These papers also open up exciting new areas for pain researchers, affording opportunities to gain more extensive insight into mechanisms underlying chronic pain conditions. To understand these opportunities fully, we should first consider some of the fundamentals of spine plasticity in the brain and then relate these findings to our current understanding of pain amplification mechanisms in the spinal dorsal horn.
Molecular mechanisms of spine plasticity
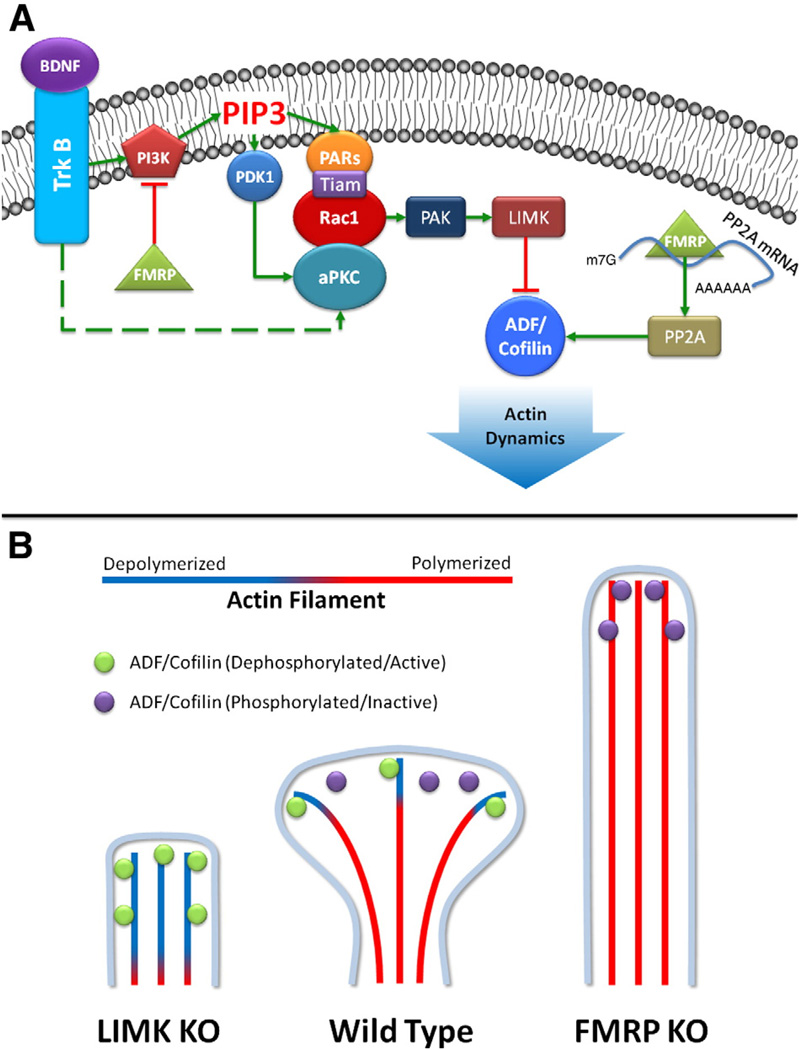
The revelation that the same conditions that induce LTP also induce morphological changes in dendritic spines mediated by actin polymerization was one of the earliest observations that links structural and functional plasticity (Fifkova, 1985). Many of these changes appear to be linked to brain derived neurotrophic factor (BDNF), which is critical both for the development of dendritic spines and expression of LTP (An et al., 2008; Shimada et al., 1998; Tanaka et al., 2008). BDNF signals, among some other pathways (Yoshii and Constantine-Paton, 2010), through tyrosine receptor kinase B (TrkB) to activate the Phosphatidylinositol-3-kinases (PI3K) pathway (Huang and Reichardt, 2003). PI3K mediates the conversion of PI (4,5)P2 to PI(3,4,5)P3. In cultured hippocampal neurons, PI3K and PI(3,4,5)P3 concentrates at the tip of protrusions that will eventually differentiate into an axon. Moreover, the activation of the PI3K pathway is important for polarization during axonal development and its inhibition mislocalizes the polarity proteins PAR3 and PAR6, indicating the importance of the PAR complex as downstream effectors of PI3K induced neuronal polarization (Shi et al., 2003). A conserved signaling pathway comprised of the Rho GTPase ras-related C3 botulinum toxin substrate 1 (Rac1), PAR3, PAR6 and atypical protein kinase C (aPKC) mediates cellular polarity in various biological contexts (Macara, 2004). PAR3 spatially restricts the Rac1 guanine exchange factor T-cell lymphoma invasion and metastasis 1 (Tiam1) to dendritic spines which allows for localized activation of Rac1 and actin-dependent dendritic spine morphogenesis (Zhang and Macara, 2006). Moreover, evidence suggests the activation of atypical protein kinase C (aPKC) may be dependent on the activity of PI3K and Rac1 (Ghosh et al., 2008). Ectopic expression of constitutively active aPKC results in increased density of dendritic spines while the expression of the kinase dead mutant fails to produce mature spines (Zhang and Macara, 2008). Collectively, current evidence suggests that Rac1-PARs-aPKCs may act as complex that mediates the genesis and maturation of dendritic spines (Fig. 1A).
Fig. 1.
A) BDNF signals through the TrkB receptor to activate PI3K. PI3K in turn mediates the conversion of PIP2 to PIP3, both PI3K and PDK1 accumulate at the leading tip of growing protrusions that would differentiate into a dendritic spine. PIP3 recruits the polarity protein PARs which can activate Tiam, leading to the activation of Rac1. This allows for a localized activation of Rac1 and prevents its inappropriate activation. aPKCs function in a complex which include PARs and Rac1. The activity of aPKCs is dependent on Rac1 and PI3K-PDK1. Rac1 activates PAK which in turn activates LIMK. ADF/cofilin, a key regulator of actin polymerization, is phosphorylated by LIMK and dephosphorylated by PP2A. FMRP represses the function of PI3K and recruits PP2A mRNA. B) Systematic spatially and temporally regulated activation of various pathway leads to the sculpting of mushroom-shaped dendritic spines. ADF/cofilin activation and inactivation at the tip of a growing spine depolymerizes actin, which in concert with other pathways allows for the formation of mushroom shaped spine (wild type). Genetic loss of LIMK, which causes Williams Syndrome, leads to excessive ADF/cofilin activation due to dephosphorylation leading to increased depolymerization of actin and production of “stubby” spines (LIMK KO). Loss of FMRP function causes Fragile X Syndrome which leads to increased PI3K activity and ADF/cofilin inactivation, causes increased polymerization of actin and long dendritic spines. Loss of FMRP may also prevent the localized expression of PP2A leading to dysregulation in local ADF/cofilin activation and actin depolymerization (FMRP KO).
Substantial evidence demonstrates that Rac1 activity is essential for the maturation of dendritic spines (Zhang and Macara, 2006). Rac1 activates p21-associated kinase (PAK), which activates LIM kinase. This cascade results in the phosphorylation of ADF/cofilin, a key regulator of actin polymerization (Yang et al., 1998). ADF/cofilin is dephosphorylated by protein phosphatase (PP)2Ac (Castets et al., 2005) and Slingshot (Van Troys et al., 2008). ADF/cofilin phosphorylation (inactivation) and dephosphorylation (activation) correlate with spine growth and shrinkage during LTP and long-term depression (LTD) respectively (Chen et al., 2007; Fedulov et al., 2007; Rex et al., 2009; Zhou et al., 2004). Interestingly, this process may be modulated by the RNA binding protein and translation repressor, fragile X mental retardation protein (FMRP), because Rac1 activation recruits FMRP to actin-containing processes thereby repressing translation of PP2Ac mRNA, a known FMRP target, and preventing dephosphorylation of ADF/cofilin holding it in its inactive state (Castets et al., 2005). Because the inactivation of ADF/cofilin is essential for the development of mushroom-shaped spines, this provides a potential mechanism linking local regulation of translation by FMRP with spine morphology (Fig. 1B).
While links between Rac1 and aPKCs in spine morphology are emerging, one isoform of aPKC, called PKMζ, has been demonstrated to play a key role in synaptic plasticity in the CNS. PKMζ is transcribed from the PKCζ gene such that it lacks the regulatory subunit but retains the catalytic portion of the kinase. This kinase has emerged as a molecular candidate for maintenance of long term memory storage and is both sufficient and necessary for the induction and maintenance of LTP (Sacktor, 2011). Signaling pathway analysis reveals several pathways, most notably the PI3K-mTOR pathway, which mediates the synthesis of PKMζ while the persistent phosphorylation of T410 by phosphoinositide-dependent kinase 1 (PDK1) is critical for the activity of PKMζ (Kelly et al., 2007a; Le Good et al., 1998). Actin polymerization has also been demonstrated to be critical for the induction of LTP and de novo synthesis of PKMζ (Kelly et al., 2007b). PKMζ mediated potentiation of AMPA responses and the expression of late LTP is, however, not influenced by actin dynamics (Kelly et al., 2007b) and the role of Rac1 in regulation of PKMζ is not known. Moreover, the specific role of PKMζ in remodeling of dendritic spines and the sequelae of molecular events that govern this process, remain to be elucidated. Despite these gaps in knowledge concerning connections between Rac1 signaling, PKMζ and spine morphology, we have recently demonstrated that spinal PKMζ is essential for the maintenance of persistent nociceptive sensitization (Asiedu et al., 2011). These findings are consistent with those by Tan and colleagues demonstrating a critical role for Rac1-mediated changes in spine dynamics in neuropathic pain evoked by spinal cord or peripheral nerve injury (Tan et al., 2008b, 2011).
Spine plasticity, Rac1, PKMζ and FMRP: an emerging picture of pain plasticity
The first findings linking spine plasticity to neuropathic pain came from a study by Tan et al. (2008b). This important paper demonstrated that spinal cord injury, which creates a long-lasting neuropathic pain state in rats, was accompanied by changes in spine morphology in lamina IV/V neurons characterized by increased mushroom-shaped spines with a higher frequency of spines located at close proximity to the soma. Importantly, these effects were reversed by a Rac1 inhibitor, NSC23766, suggesting that these changes occurred in a Rac1-dependent fashion. Moreover, administration of the Rac1 inhibitor led to a reversal of wide dynamic range (WDR) neuron hyperexcitability after spinal cord injury providing a functional link between altered spine morphology and distribution and neurophysiological changes thought to underlie neuropathic pain. In a follow-up study, the same group utilized a computational modeling approach to better understand the ramifications of spine plasticity for neuropathic pain caused by spinal cord injury (Tan et al., 2009). Their findings indicated that altered spine morphology and a redistribution of mature spines toward the soma led to a reduction in inhibitory gating, increased synaptic efficacy, improved frequency following ability and a greater probability of action potential generation for an identical input. Hence, this computation approach reveals large-scale neurophysiological changes as a result of Rac1-mediated spine plasticity that are likely to underlie aspects of neuropathic pain that are the most challenging to treat clinically.
Corroborating evidence for the role of spine plasticity in chronic pain disorders may come from a surprising area of research, developmental neurological disorders. These disorders, which often result in intellectual disabilities, are frequently characterized by abnormalities in dendritic spine morphology (Kaufmann and Moser, 2000). Perhaps the best example of this is Fragile X Mental Retardation, which is caused by silencing of FMRP. Loss of FMRP leads to an immature spine morphology in mice and humans (Hinton et al., 1991; Irwin et al., 2001; Rudelli et al., 1985) and aberrant synaptic plasticity including enhanced LTD and impaired LTP in some brain regions. As mentioned above, there is evidence linking FMRP regulation of translation to Rac1-mediated control of actin dynamics that may be relevant for spine abnormalities and deficits in plasticity in the human disease. Moreover, increased PI3K activity is observed in Fmr1 KO mice (Gross et al., 2010) further suggesting deficits in signaling via pathways associated with spine plasticity in this neurological disorder. Significantly, loss of FMRP in mice leads to a striking pain phenotype wherein these mice are deficient in a variety of pain amplification assays, including a profound reduction in neuropathic pain (Asiedu et al., 2011; Peebles and Price, 2011; Price et al., 2007). While no studies have examined the morphology of dendritic spines in the dorsal horn following loss of FMRP, Rac1-mediated spine plasticity in neuropathic pain models (Tan et al., 2008b, 2009, 2011) strongly suggests that defective pain amplification in the absence of FMRP may be functionally connected to altered spine plasticity.
The findings published by Tan et al., in this issue of Experimental Neurology extend their previous findings using models of spinal cord injury in several important ways. First, they demonstrate that changes in spine morphology, including increases in mushroom-shaped spines, and spine redistribution also occur in response to peripheral nerve injury using the chronic constriction injury (CCI) model (Tan et al., 2011). Second, they find that these changes are similarly sensitive to Rac1 inhibition. Third, their findings demonstrate that Rac1 inhibition over a three-day period leads to a reversal of WDR neuron hyperexcitability and relief of CCI-induced mechanical allodynia, but not thermal hyperalgesia. Collectively, these findings firmly establish spine plasticity as a novel mechanism for neuropathic pain (originating from central or peripheral injury) and point to Rac1 as a potential target for the alleviation of neuropathic pain. We will argue below, that these findings may also have a profound impact on solving some current controversies in pain research, a somewhat ironic statement considering that the technique used by Tan et al., to discover these changes in spine morphology after nervous system injury (Golgi embedding) is little changed since the time of Cajal's early discoveries on the structure of the nervous system.
Power of spines for addressing current questions facing chronic pain research
As mentioned above, much current work on dendritic spines is focused on understanding the role of dendritic spine plasticity in synaptic plasticity, in particular, LTP and LTD. In this regard, several important themes have emerged: 1) maturation of spines into a mushroom-shaped morphology appears to coincide with the consolidation of late-LTP, 2) persistent changes in spine morphology are associated with learning events and may represent memory storage mechanisms and 3) changes in spine morphology are likewise associated with AMPA-receptor trafficking events that are molecularly linked to signaling pathways that may play a key role in changing spine morphology via the regulation of actin dynamics (De Roo et al., 2008; Yuste and Bonhoeffer, 2001). How then, do these findings connect to our current understanding of pain plasticity in the CNS and do they offer insight into current controversies?
The pioneering studies of Mendell and Wall (1965) and Woolf (1983) were the first to demonstrate amplification of pain signaling in the CNS. These forms of amplification, termed “wind-up” in the case of the work of Mendell and Wall and “central sensitization” in the case of the work of Woolf, are widely thought to be dependent on afferent input and to resolve following long-term blockade or termination of this afferent input (Latremoliere and Woolf, 2009; Woolf, 2011). On the other hand, LTP, which is a long-lasting form of plasticity that does not require ongoing afferent input (at least in the brain), can be evoked in the spinal dorsal horn and clearly consolidates into a late form of LTP as is observed in other brain regions (Sandkuhler, 2007). An important finding in this regard is the discovery that, at certain lamina I/II dorsal horn neurons, LTP can be evoked by low-frequency stimulation or by natural stimulation of C-fibers (Ikeda et al., 2006). Moreover, this low-frequency stimulation-induced late-LTP consolidation requires intact BDNF signaling (Zhou et al., 2008) (as HFS-induced LTP does in brain) and LTP initiation is dependent on signaling pathways that also regulate LTP initiation in the hippocampus and cortex (Ikeda et al., 2006; Sandkuhler, 2007). Does stimulation of C-fibers in humans result in late-LTP consolidation? A recent study suggests that, at least in some individuals, the answer to this question is yes. Presumptive induction of LTP via stimulation of C-fibers in humans leads to a perceptual form of LTP characterized by primary hyperalgesia. In most individuals this perceptual LTP decays over the course of 24–48 h, however, in a significant subset of individuals this hyperalgesia persists for weeks or even months (Pfau et al., 2011). Hence, a transition to late-LTP consolidation in the spinal dorsal horn (and other CNS regions) likely occurs both in preclinical models of pain and in humans.
What molecular mechanisms underlie this long-term plasticity? The encoding of memory engrams in CNS structures is separated into initiation and maintenance phases with distinct molecular mechanisms. Initiation of engram encoding requires protein synthesis and an atypical protein kinase C (aPKC) called PKMζ. Maintenance of the engram is solely dependent on PKMζ, which represents the only known kinase whose activity is required for the maintenance of late-long-term potentiation (LTP) and long-term memory (Sacktor, 2011). We have recently demonstrated that the pharmacology of a chronic pain state in mice parallels memory engram encoding in the CNS (Asiedu et al., 2011). In this model, an allodynic state is initiated by a “priming” event: hindpaw interleukin 6 (IL-6) injection or plantar incision. After the resolution of the allodynic state, a subsequent nociceptive hypersensitivity can be revealed by hindpaw injection of prostaglandin E2 (PGE2), causing a prolonged allodynia, or spinal administration of the metabotropic glutamate receptor 1/5 (mGluR1/5) agonist dihydroyphenyglycine (DHPG), causing pronounced nocifensive behaviors. In naïve animals, PGE2 and DHPG only elicit transient allodynia or nocifensive behaviors, respectively. Hence, this model establishes a persistent nociceptive sensitization that can be clearly divided into an initiation (induction of priming) and maintenance phase that persists for long periods of time. Consistent with concepts governing memory encoding and the pharmacology of late-LTP maintenance, these findings demonstrate that persistent nociceptive sensitization initiation requires spinal protein synthesis and PKMζ whereas maintenance is solely dependent on PKMζ. These results strongly suggest the encoding of a permanent (or at least semi-permanent) form of synaptic plasticity in the spinal dorsal horn that may underlie susceptibility to chronic pain (Asiedu et al., 2011).
Monitoring changes in spine morphology in the dorsal horn affords an excellent opportunity to gain insight into the mechanisms underlying these changes and whether they do, in fact, persist for long periods of time. For instance, we would predict that changes in dendritic spine morphology would be observed in a wide variety of preclinical pain models including models linked to PKMζ, such as hyperalgesic priming (Reichling and Levine, 2009) and plantar incision (Asiedu et al., 2011). We would further predict that these changes in spine morphology would, at least in part, persist even after the resolution of allodynia or hyperalgesia induced by the original injury. In this regard, the CCI model presents an excellent opportunity for testing this hypothesis because it is one of the few neuropathic pain models wherein hyperalgesia and allodynia eventually resolve. Hence, the findings of Tan et al. could readily be extended into later stages after CCI to examine this question rigorously. Examining changes in spine morphology in the context of preclinical pain models could also provide important evidence linking Rac1 signaling, PAR complex proteins and aPKCs, especially PKMζ, to persistent changes in spine morphology.
Despite these exciting opportunities, our understanding of spine plasticity in chronic pain is still in its infancy and several gaps in knowledge need to be filled. Most obviously, the studies of Tan et al. have focused almost entirely on lamina IV/V neurons (Tan et al., 2008b, 2009, 2011) while much other work in the pain plasticity area has focused on lamina I/II neurons. For instance, low frequency stimulation-induced LTP is evoked primary in a subset of lamina I/II neurons that express the substance P receptor, neurokinin receptor type 1 (NK1) (Ikeda et al., 2006). These neurons are also required for the induction of injury induced hyperalgesia and allodynia (Nichols et al., 1999). It will be interesting to examine whether changes in spine morphology observed in lamina IV/V neurons can also be applied to lamina I/II neurons, especially neurons co-expressing the NK-1 receptor. Likewise, it is not known how Rac1 inhibition influences these neurons or whether Rac1 inhibition influences LTP initiation, late-LTP consolidation or late-LTP maintenance in the spinal dorsal horn. In hippocampus Rac1 plays a key role in LTP initiation but not maintenance (Martinez and Tejada-Simon, 2011). Such experiments will be important for advancing our understanding of the role of spine plasticity in pain and the potential for targeting this mechanism for the reversal of chronic pain states. In this regard, questions surrounding the use of Rac1 inhibitors also need to be addressed. While the Tan et al. papers clearly show a strong inhibition of nerve injury induced allodynia and WDR neuron activity by Rac1 inhibition (Tan et al., 2008b, 2011), these experiments fail to address whether this inhibition represents a long-lasting reversal of the chronic pain state. While palliative treatments for chronic pain disorders are clearly useful, it would be important to know whether Rac1 inhibition leads to disease-modifying effects in preclinical pain models.
Concluding remarks
The discovery of changes in dendritic spine morphology and organization following spinal cord or peripheral nerve injury, in a Rac1-dependent fashion (Tan et al., 2008a, 2011), offers several unique insights into the pathophysiology of chronic pain disorders. These structural changes suggest concomitant molecular and neurophysiological events paralleling mechanisms underlying learning and memory in other CNS regions and provide strong evidence for permanent changes in dorsal horn synaptic architecture as a causative factor in chronic pain disorders. While it remains to be seen whether these discoveries can lead to the generation of novel, disease-modifying agents for the reversal of chronic pain disorders, these discoveries shed light on molecular mechanisms that may afford, for the first time, such opportunities.
Acknowledgments
This work was supported by funds from The Rita Allen Foundation (TJP) and NIH grant NS065926 (TJP). TJP is a Rita Allen Foundation Scholar in Pain.
Abbreviations
- AMPA
2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid
- aPKC
atypical protein kinase C
- BDNF
brain derived neurotrophic factor
- CCI
chronic constriction injury
- DHPG
dihydroxyphenyglycine
- FMRP
fragile X mental retardation protein
- IL-6
interleukin 6
- KO
knockout
- LIMK
LIM Kinase
- LTD
long-term depression
- LTP
long-term potentiation
- mGluR1/5
metabotropic glutamate receptor 1/5
- NK1
neurokinin receptor type 1
- PAK
p21-associated kinase
- PDK1
phosphoinositide-dependent kinase 1
- PI3K
phosphatidylinositol-3-kinases
- PAR
polarity complex proteins
- PGE2
prostaglandin E2
- PKCζ
protein kinase Cζ
- PKMζ
protein kinase Mζ
- PP
protein phosphatase
- Rac1
ras-related C3 botulinum toxin substrate 1
- Tiam1
T-cell lymphoma invasion and metastasis 1
- TrkB
tyrosine receptor kinase B
- WDR
wide dynamic range
Contributor Information
Ohannes K. Melemedjian, Email: ohannes@email.arizona.edu.
Theodore J. Price, Email: tjprice@email.arizona.edu.
References
- An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano JI, Espinosa A, Fairen A, Yuste R, DeFelipe J. Non-synaptic dendritic spines in neocortex. Neuroscience. 2007;145:464–469. doi: 10.1016/j.neuroscience.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Asiedu MN, Tillu DV, Melemedjian OK, Shy A, Sanoja R, Bodell B, Ghosh S, Porreca F, Price TJ. Spinal protein kinase M zeta underlies the maintenance mechanism of persistent nociceptive sensitization. J. Neurosci. 2011;31:6646–6653. doi: 10.1523/JNEUROSCI.6286-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castets M, Schaeffer C, Bechara E, Schenck A, Khandjian EW, Luche S, Moine H, Rabilloud T, Mandel JL, Bardoni B. FMRP interferes with the Rac1 pathway and controls actin cytoskeleton dynamics inmurine fibroblasts. Hum. Mol. Genet. 2005;14:835–844. doi: 10.1093/hmg/ddi077. [DOI] [PubMed] [Google Scholar]
- Chen LY, Rex CS, Casale MS, Gall CM, Lynch G. Changes in synaptic morphology accompany actin signaling during LTP. J. Neurosci. 2007;27:5363–5372. doi: 10.1523/JNEUROSCI.0164-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roo M, Klauser P, Garcia PM, Poglia L, Muller D. Spine dynamics and synapse remodeling during LTP and memory processes. Prog. Brain Res. 2008;169:199–207. doi: 10.1016/S0079-6123(07)00011-8. [DOI] [PubMed] [Google Scholar]
- Fedulov V, Rex CS, Simmons DA, Palmer L, Gall CM, Lynch G. Evidence that long-term potentiation occurs within individual hippocampal synapses during learning. J. Neurosci. 2007;27:8031–8039. doi: 10.1523/JNEUROSCI.2003-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifkova E. A possible mechanism of morphometric changes in dendritic spines induced by stimulation. Cell. Mol. Neurobiol. 1985;5:47–63. doi: 10.1007/BF00711085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Marquardt T, Thaler JP, Carter N, Andrews SE, Pfaff SL, Hunter T. Instructive role of aPKCzeta subcellular localization in the assembly of adherens junctions in neural progenitors. Proc. Natl. Acad. Sci. U.S.A. 2008;105:335–340. doi: 10.1073/pnas.0705713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Nakamoto M, Yao X, Chan CB, Yim SY, Ye K, Warren ST, Bassell GJ. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J. Neurosci. 2010;30:10624–10638. doi: 10.1523/JNEUROSCI.0402-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton VJ, Brown WT, Wisniewski K, Rudelli RD. Analysis of neocortex in three males with the fragile X syndrome. Am. J. Med. Genet. 1991;41:289–294. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jager T, Sandkuhler J. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659–1662. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, Swain RA, Weiler IJ, Greenough WT. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am. J. Med. Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb. Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- Kelly MT, Crary JF, Sacktor TC. Regulation of protein kinase Mzeta synthesis by multiple kinases in long-term potentiation. J. Neurosci. 2007a;27:3439–3444. doi: 10.1523/JNEUROSCI.5612-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MT, Yao Y, Sondhi R, Sacktor TC. Actin polymerization regulates the synthesis of PKMzeta in LTP. Neuropharmacology. 2007b;52:41–45. doi: 10.1016/j.neuropharm.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J. Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- Macara IG. Parsing the polarity code. Nat. Rev. Mol. Cell Biol. 2004;5:220–231. doi: 10.1038/nrm1332. [DOI] [PubMed] [Google Scholar]
- Martinez LA, Tejada-Simon MV. Pharmacological inactivation of the small GTPase Rac1 impairs long-term plasticity in the mouse hippocampus. Neuropharmacology. 2011;61:305–312. doi: 10.1016/j.neuropharm.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell LM, Wall PD. Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature. 1965;206:97–99. doi: 10.1038/206097a0. [DOI] [PubMed] [Google Scholar]
- Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- Peebles KA, Price TJ. Self-injurious behaviour in intellectual disability syndromes: evidence for aberrant pain signalling as a contributing factor. J. Intellect. Disabil. Res. 2011 doi: 10.1111/j.1365-2788.2011.01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau DB, Klein T, Putzer D, Pogatzki-Zahn EM, Treede RD, Magerl W. Analysis of hyperalgesia time courses in humans after painful electrical high-frequency stimulation identifies a possible transition from early to late LTP-like pain plasticity. Pain. 2011;152:1532–1539. doi: 10.1016/j.pain.2011.02.037. [DOI] [PubMed] [Google Scholar]
- Price TJ, Rashid MH, Millecamps M, Sanoja R, Entrena JM, Cervero F. Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR. J. Neurosci. 2007;27:13958–13967. doi: 10.1523/JNEUROSCI.4383-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal S. La Textura del Sistema Nerviosa del Hombre y los Vertebrados. Madrid: Moya; 1899. [Google Scholar]
- Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, Lynch G. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J. Cell Biol. 2009;186:85–97. doi: 10.1083/jcb.200901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudelli RD, Brown WT, Wisniewski K, Jenkins EC, Laure-Kamionowska M, Connell F, Wisniewski HM. Adult fragile X syndrome. Cliniconeuropathologic findings. Acta Neuropathol. 1985;67:289–295. doi: 10.1007/BF00687814. [DOI] [PubMed] [Google Scholar]
- Sacktor TC. How does PKMzeta maintain long-term memory? Nat. Rev. Neurosci. 2011;12:9–15. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J. Understanding LTP in pain pathways. Mol. Pain. 2007;3:9. doi: 10.1186/1744-8069-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SH, Jan LY, Jan YN. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 2003;112:63–75. doi: 10.1016/s0092-8674(02)01249-7. [DOI] [PubMed] [Google Scholar]
- Shimada A, Mason CA, Morrison ME. TrkB signaling modulates spine density and morphology independent of dendrite structure in cultured neonatal Purkinje cells. J. Neurosci. 1998;18:8559–8570. doi: 10.1523/JNEUROSCI.18-21-08559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Stamboulian S, Chang YW, Zhao P, Hains AB, Waxman SG, Hains BC. Neuropathic pain memory is maintained by Rac1-regulated dendritic spine remodeling after spinal cord injury. J. Neurosci. 2008a;28:13173–13183. doi: 10.1523/JNEUROSCI.3142-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Stamboulian S, Chang YW, Zhao P, Hains AB, Waxman SG, Hains BC. Neuropathic pain memory is maintained by Rac1-regulated dendritic spine remodeling after spinal cord injury. J. Neurosci. 2008b;28:13173–13183. doi: 10.1523/JNEUROSCI.3142-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Choi JS, Waxman SG, Hains BC. Dendritic spine remodeling after spinal cord injury alters neuronal signal processing. J. Neurophysiol. 2009;102:2396–2409. doi: 10.1152/jn.00095.2009. [DOI] [PubMed] [Google Scholar]
- Tan AM, Chang YW, Zhao P, Hains BC, Waxman SG. Rac1-regulated dendritic spine remodeling contributes to neuropathic pain after peripheral nerve injury. Exp. Neurol. 2011;232(2):222–233. doi: 10.1016/j.expneurol.2011.08.028. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Troys M, Huyck L, Leyman S, Dhaese S, Vandekerkhove J, Ampe C. Ins and outs of ADF/cofilin activity and regulation. Eur. J. Cell Biol. 2008;87:649–667. doi: 10.1016/j.ejcb.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev. Neurobiol. 2010;70:304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R. Dendritic Spines. Cambridge: M.I.T. Press; 2010. [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu. Rev. Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- Zhang H, Macara IG. The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nat. Cell Biol. 2006;8:227–237. doi: 10.1038/ncb1368. [DOI] [PubMed] [Google Scholar]
- Zhang H, Macara IG. The PAR-6 polarity protein regulates dendritic spine morphogenesis through p190 RhoGAP and the Rho GTPase. Dev. Cell. 2008;14:216–226. doi: 10.1016/j.devcel.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Zhou LJ, Zhong Y, Ren WJ, Li YY, Zhang T, Liu XG. BDNF induces late-phase LTP of C-fiber evoked field potentials in rat spinal dorsal horn. Exp. Neurol. 2008;212:507–514. doi: 10.1016/j.expneurol.2008.04.034. [DOI] [PubMed] [Google Scholar]