Abstract
Objective
To examine the 2 year post-therapy kinetics of change in the composition of subgingival biofilms.
Material and Methods
178 chronic periodontitis subjects were recruited and clinically monitored at baseline, 3, 6, 12, 18 and 24 months after therapy. All subjects received SRP and 156 one or more of periodontal surgery, systemically administered amoxicillin + metronidazole or local tetracycline at pockets ≥5 mm. Subgingival biofilm samples taken from each subject at each time point were analyzed for their content of 40 bacterial species using checkerboard DNA-DNA hybridization. The significance of changes in median species counts over time was sought using the Wilcoxon or Friedman tests and adjusted for multiple comparisons.
Results
Mean counts were significantly reduced from baseline to 2 years for 30 of the 40 taxa. Marked reductions were observed for periodontal pathogens including Tannerella forsythia, Treponema denticola and Eubacterium nodatum. The kinetics of change differed from species to species. When data were subset according to baseline PD, patterns of change in the microbial profiles were generally similar.
Conclusion
Periodontal therapy leads to a rapid reduction in periodontal pathogens, followed by a slower reduction in other taxa that can be sustained for at least 2 years.
Keywords: Chronic periodontitis, Therapy, Microbiology, Kinetics
Introduction
The goals of periodontal therapy are two-fold: to provide long-term improvement in clinical parameters, including reduction in inflammation, gingival bleeding, probing pocket depth and “gain” in attachment level, and to decrease the levels of disease initiating bacteria. Many studies in the literature have demonstrated the efficacy of different types of periodontal therapy alone and in combination in improving, on average, clinical periodontal parameters for different lengths of time post-therapy (Apatzidou & Kinane 2010, Berglundh et al. 1998, Caton et al. 2000, Drisko 2001, Guerrero et al. 2005, Haffajee et al. 1995, 2003, Heitz-Mayfield et al. 2002, Herrera et al. 2002, Huynh-Ba et al. 2009, Lang et al. 2008, Palmer et al. 1999, Pavicic et al. 1994, Pihlstrom et al. 1983, Ramberg et al. 2001, van Winkelhoff et al. 1992, Winkel et al. 1999, 2001, Xajigeorgiou et al. 2006). Fewer studies have evaluated the microbiological response to periodontal therapies. However, those that did examine the changes in the subgingival microbiota post-therapy demonstrated a decrease in the levels, proportions or prevalence of periodontal pathogens that accompanied the clinical improvements (Berglundh et al. 1998, Haffajee et al. 1995, 2006, Pavicic et al. 1994, Teles et al. 2006, van Winkelhoff et al. 1992, Winkel et al. 1999, 2001, Xajigeorgiou et al. 2006, Oteo et al. 2010, Aimetti et al. 2011, Sampaio et al. 2011, Silva et al. 2011).
Recently, Goodson et al (2012), described the clinical effects achieved by different periodontal therapies including SRP alone or combined with one or more of periodontal surgery (S), systemically administered amoxicillin and metronidazole (AM) and locally delivered tetracycline (LDD) in a multi-center clinical study of 187 subjects with moderate to advanced chronic periodontitis. It was shown that, on average, therapy improved clinical periodontal parameters and that subjects receiving AM as part of their therapy demonstrated a better clinical outcome 24 months post-therapy. However, since all treatments led to an improvement in the mean clinical parameters, it was of interest to determine what microbiological changes accompanied this improvement and whether there were differences in the nature and rate of change of the individual species in the subgingival ecosystem.
Clearly, something happens to the composition of the subgingival microbiota as a result of periodontal therapy that leads to an initially rapid clinical improvement often followed by a sustained continued reduction in mean pocket depth and “gain’ in clinical attachment. The purpose of the present investigation was to evaluate the effect of the different periodontal therapies over a 2 year post-therapy monitoring period on the counts of 40 bacterial species, to examine the kinetics of change for these species and to determine how species change over time impacted the subgingival ecosystem. The overall microbiological changes will be described in this manuscript and the effects of different treatments on the subgingival microbiota in a companion paper (Haffajee et al. 2012).
Material and Methods
Experimental Design
The data in this manuscript came from a multi-center, single-blind, randomized, prospective clinical trial that used a 2 × 2 × 2 factorial design to evaluate the adjunctive effects to scaling and root planing (SRP) of eight possible combinations of; S, AM and LDD, on clinical and microbiological outcomes at 3, 6, 12, 18 and 24 months post-therapy. The treatment groups and the number of subjects within each group are presented in Table 1. Details of the design and clinical outcomes have been described by Goodson et al. (2012).
Table 1.
Adjunctive therapies for subjects in treatment groups 1 – 8. All subjects received SRP.
| Group | N | Surgery | Systemic amoxicillin + metronidazole | Locally delivered tetracycline |
|---|---|---|---|---|
| 1 | 22 | + | + | + |
| 2 | 17 | + | + | − |
| 3 | 21 | + | − | + |
| 4 | 21 | + | − | − |
| 5 | 27 | − | + | + |
| 6 | 25 | − | + | − |
| 7 | 24 | − | − | + |
| 8 | 21 | − | − | − |
Subject population
The subject population for the microbiological analysis consisted of 178 subjects who had baseline and at least 3 post-therapy microbiological sets of samples. The 30 subjects with one or two missing microbiological visits had data carried forward as described previously (Goodson et al. 2012). The study protocol was approved by the Institutional Review Boards at The Forsyth Institute, Boston University School of Dental Medicine and by the Ethics Committee at University of Gothenburg. The nature of the study was described thoroughly to all subjects prior to obtaining informed consent. Inclusion and exclusion criteria, treatment of subjects as well as clinical monitoring were described earlier (Goodson et al. 2012). The subjects were randomly assigned to 8 treatment groups receiving 8 different combinations of therapy (Goodson et al. 2012).
Microbiological sampling and enumeration of species
Subgingival biofilm samples were taken using individual sterile Gracey curettes from the mesial surface of each tooth (excluding third molars) and placed into separate Eppendorf tubes containing 0.15 ml Tris EDTA buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.6). 0.10 ml of freshly prepared 0.5 M NaOH was added immediately to each sample. At this stage, the samples were stable and could be shipped to Forsyth from the University of Gothenburg and Boston University for processing. Each sample consisted of a single stroke of a Gracey curette on the tooth surface to ensure standardization of sample taking. Samples were individually analyzed for their content of 40 bacterial species using checkerboard DNA-DNA hybridization (Socransky et al. 1994, 2004). A total of 27,710 subgingival biofilm samples were evaluated (an average of 25.4 samples per subject per visit).
Data analysis
The counts of 40 test species were available at up to 28 subgingival sites in each of 178 subjects prior to and from at least 3 follow-up visits. Counts of individual species were averaged within a subject and then averaged across subjects for each time point separately. Significance of differences over time were sought using the Wilcoxon signed ranks test when baseline and 24 month data were compared, and the Friedman test when data from all time points were compared. The Dunn’s multiple comparison post-hoc test was used to determine significance of differences between median baseline counts and median counts at 3, 6, 12, 18 and 24 months post-therapy. All analyses were adjusted for 40 comparisons (Socransky et al. 1991).
In order to estimate whether the rate of change was different for various bacterial species, we performed regression models for each possible pair of species. We fit generalized poisson models with bacterial counts as the dependent variable, subject as a random effect, time (continuous), and squared time as a fixed effect. The curvature of change in levels of each species over time was captured by the quadratic term of time. We accounted for multiple comparisons by applying a stepdown false discovery rate procedure (Benjamini and Yekutieli 2001). Since bacterial species live symbiotically within the biofilm, the assumption of independence between statistics may not be realistic. Because the dependence structure is unknown, a procedure which is conservative for such a situation, like Benjamini and Yekutieli method, works better. In the procedure, the p-values from each test are sorted increasingly, and then each p-value is compared with a corresponding cut-off value to see if it is significant. The cut-off values are calculated based on the total number of tests, the order of the test, and a penalty to correct the dependence between tests. Hierarchical cluster analysis of the coefficient estimates of the linear and quadratic terms of time from the regression models was used to group subgingival species with similar kinetics of change after treatment. The Gower similarity coefficient and an averaged unweighted linkage sort were used in the cluster analysis (Sneath & Sokal, 1973).
Results
The baseline and 2 year post-therapy clinical parameters of the 178 subjects are presented in Table 2. The mean age (± SD) of the study population was 49 ± 10 years; 49% of the 178 subjects were Swedes, 52% males, and 41 % current smokers.
Table 2.
Mean (± SD) clinical features of the subjects at baseline and 24 months after therapy.
| N = 178 | Baseline | 24 months | ||
|---|---|---|---|---|
|
| ||||
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | |
|
| ||||
| % of sites with: | ||||
| Plaque | 50 ± 31 | 37, 61 | 34 ± 32 | 13, 34 |
| BOP | 54 ± 24 | 45, 59 | 26 ± 19 | 18, 25 |
| Mean pocket depth (mm) | 4.2 ± 0.7 | 4.1, 4.3 | 3.2 ± 0.5 | 3.1, 3.2 |
| Mean attachment level (mm) | 4.2 ± 1.2 | 3.8, 4.4 | 3.9 ± 1.1 | 3.6, 4.1 |
| Number of missing teeth | 3.7 ± 3.2 | 3.0, 4.0 | 3.7 ± 3.2 | 3.0, 4.0 |
SD – standard deviation
CI – confidence interval
Overall effect of therapy
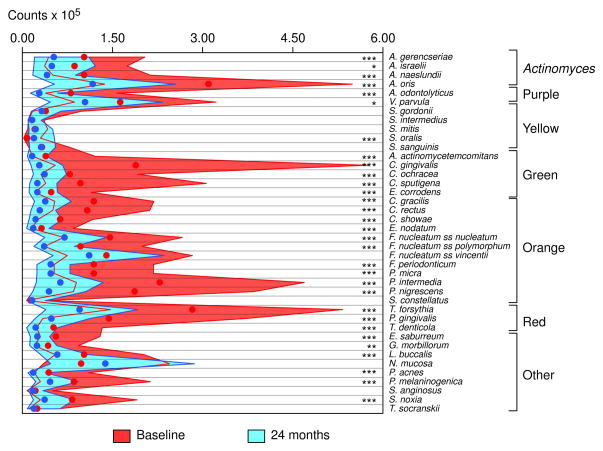
The change in median counts of the 40 test species in the subgingival biofilm samples from the mesial sites of all teeth, excluding third molars, at baseline and 24 months post-therapy are presented in Fig. 1. After adjusting for multiple comparisons, the mean counts of 30 of 40 species were significantly reduced post-therapy and one species, Streptococcus oralis, was significantly increased 24 months after treatment. The shape of the microbial profiles was markedly altered as a result of therapy. In particular the high numbers of the periodontal pathogens, Tannerella forsythia and Porphorymonas gingivalis as well as Prevotella nigrescens and Prevotella intermedia, the three Capnocytophaga species were dramatically reduced by therapy. The least changed were the Streptococcus species, Veillonella parvula, Neisseria mucosa and Treponema socranskii.
Fig. 1.
Median counts × 105 of 40 subgingival species in subgingival plaque samples taken pre-therapy and 24 months post-therapy. Counts of each species were averaged within a subject and then the medians across subjects for the baseline visit and the 24 month visit were computed separately. Significance of differences was determined using the Wilcoxon signed ranks test and adjusted for 40 comparisons (Socransky et al. 1991) * p< 0.05, ** p< 0.01, *** p< 0.001. The circles represent the medians and the interquartile ranges are represented by the colored bands. The species were ordered according to the complexes described by Socransky et al. (1998).
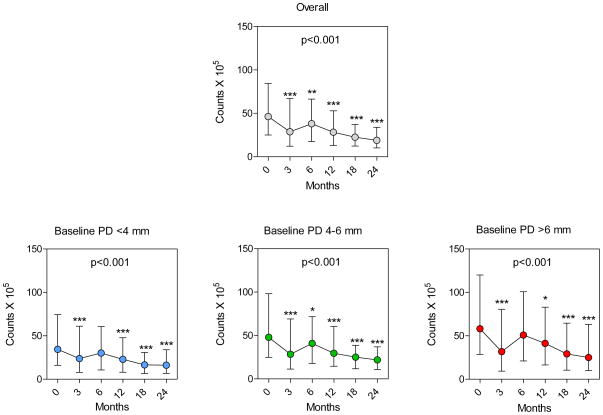
The overall reduction in counts of microorganisms, as reflected by the total DNA probe count, is presented in Fig. 2, top panel. Median total DNA probe counts (interquartile range [IQR]) declined from 46.3 (25.0 – 84.5) × 105 to 18.9 (10.2 – 33.9) × 105 at 24 months. There was a rapid reduction in numbers immediately after therapy to 3 months. Median total counts rebounded at 6 months and then began to slowly decline from 6 to 24 months. It may be observed that the kinetics of change in total DNA probe count after treatment was similar for all categories of baseline pocket depth (<4 mm, 4–6 mm, >6 mm [Fig. 2, lower panels]). The median counts (IQR, ×105) at baseline and 24 months were for BPD< 4 mm, 34.4 (16.1 – 74.4) and 16.1 (6.7 – 33.9); for BPD 4–6 mm, 47.9 (24.8 – 98.1) and 22.0 (10.8 – 37.0); for BPD >6 mm, 58.2 (28.6 – 120.0) and 25.1 (10.0 – 63.2).
Fig. 2.
Plots of median total DNA probe counts (× 105, interquartile ranges) (top panel) at baseline (pre-therapy), and at 3, 6, 12, 18 and 24 months post-therapy for all sites and sites subset according to baseline pocket depth categories of <4, 4–6, >6 mm (lower panels). Total counts were averaged within a subject and then the medians across subjects for each visit and pocket depth category were computed separately. Significance of differences over time was determined using the Friedman test (p values presented in each graph). Dunn’s multiple comparison post-hoc test was used to determine significance of differences between baseline median counts and median counts at 3, 6, 12, 18 and 24 months post-therapy* p< 0.05, ** p< 0.01, *** p< 0.001.
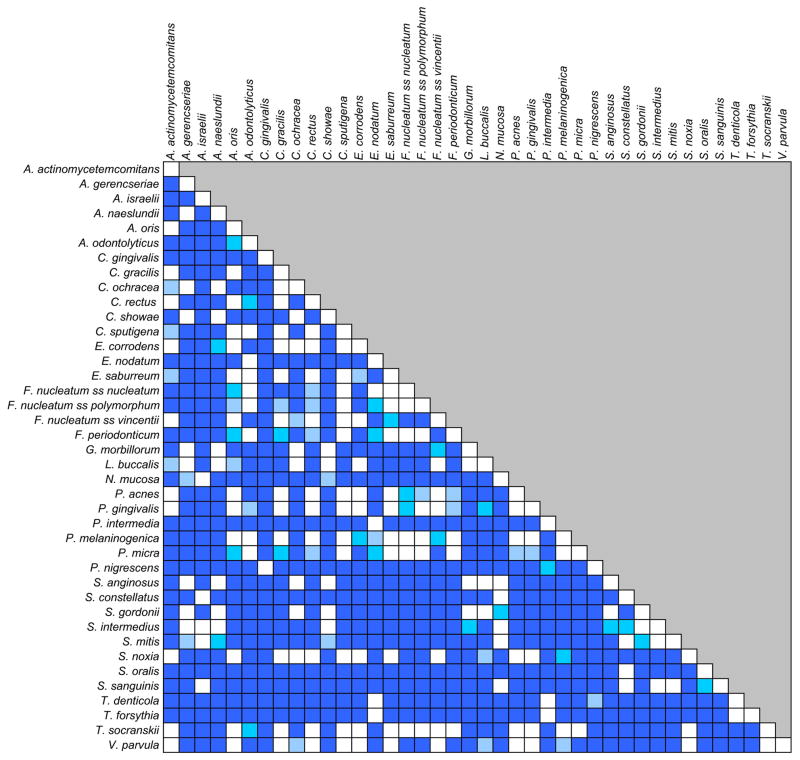
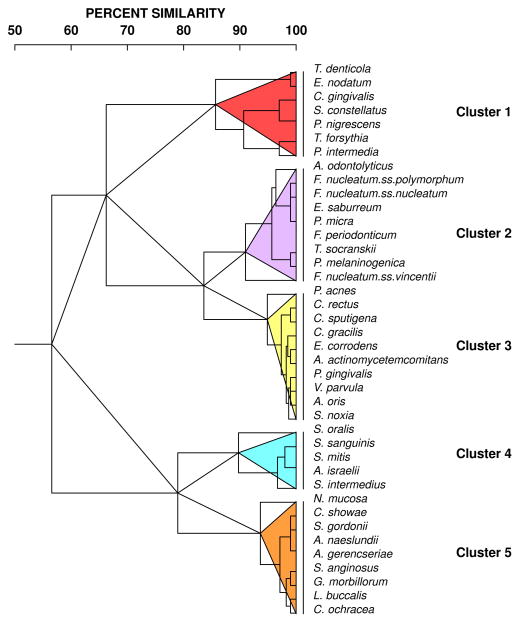
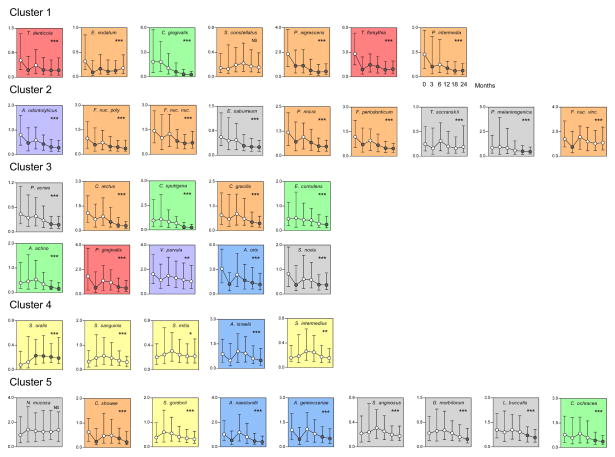
The next question was whether the kinetics of the reduction was the same for all species. Fig. 3 is a grip-plot of the p values obtained with the regression models testing if the rate of change was different for various bacterial species after adjusting for multiple comparisons. It can be observed that for the vast majority of the pairwise comparisons were statistically significantly different. Fig. 4 presents the dendrogram of the cluster analysis of the slope estimates for each bacterial species obtained with the regression models. The colored triangles lines within the dendrograms indicate the 5 clusters of species that formed at >84% similarity. Fig. 5 presents the mean changes over time of the 40 test taxa from pre-therapy to 24 months post-therapy. The panels were ordered according to the clusters illustrated in Fig. 4.
Fig. 3.
Grid-plot of the p values obtained with regression models to estimate whether the rate of change was different for various bacterial species. The color code indicates the level of the p value after adjusting for multiple comparisons using a stepdown false discovery rate procedure (Benjamini and Yekutieli 2001): light blue p< 0.05, medium blue p< 0.01, dark blue p< 0.001.
Fig. 4.
Dendrogram of hierarchical cluster analysis of the coefficient estimates of the linear and quadratic terms of time from the regression models. The cluster analysis used the Gower similarity coefficient and an averaged unweighted linkage sort. The colored triangles within the dendrograms indicate the 5 clusters of species that formed at > 84% similarity.
Fig. 5.
Plots of median counts (× 105, IQR) for subgingival species at baseline (pre-therapy), and at 3, 6, 12, 18 and 24 months post-therapy. Counts of each species were averaged within a subject and then the medians across subjects for each visit were computed separately. Significance of differences over time was determined using the Friedman test and adjusted for 40 comparisons (Socransky et al. 1991) * p< 0.05, ** p< 0.01, *** p< 0.001. Dunn’s multiple comparison post-hoc test was used to determine significance of differences between baseline median counts and median counts at 3, 6, 12, 18 and 24 months post-therapy. Circles indicate medians and whiskers the 25th and 75th quartiles. Black circles indicate statistically significant differences from baseline (p< 0.001) according to the Dunn’s test. The y-axes values represent median counts × 105. After adjusting for multiple comparisons, all taxa differed significantly (p< 0.05) over time except S. constellatus and N. mucosa (NS – not significant). Graphs were grouped according to the clusters described in Fig. 4. The background shading represents the complexes described by Socransky et al. (1998).
Cluster 1 included species showing rapid post-therapy reduction at 3 months to levels that remained stable for up to 24 months such as Treponema denticola, Eubacterium nodatum, and T. forsythia. Other members of this cluster such as P. intermedia and P. nigrescens also had reductions in their levels at 3 months and continued to have their median counts reduced for up to 24 months. Interestingly, this cluster included several periodontal pathogens of the orange and red complexes. The other two members of the complex, Capnocytophaga gingivalis and Streptococcus constellatus presented different kinetics of change, characterized by significant decreases after 12 months and no changes over time, respectively.
Cluster 2 was formed mainly by taxa that showed a rapid reduction to 3 months post-therapy followed by a rise at 6 months and then a lowering of counts to 24 months, including Actinomyces odontolyticus, Fusobacterium nucleatum ss polymorphum, Fusobacterium nucleatum ss nucleatum, Parvimonas micra, and Fusobacterium periodonticum. Other members of this cluster either did not show significant changes over time (T. socranskii and Fusobacterium nucleatum ss vincentii) or only presented significant reductions at later times (Eubacterium saburreum and Prevotella melaninogenica).
Cluster 3 encompassed 10 taxa; some species exhibited a slow but consistent rate of reduction in median numbers over the 24 months of the study, including Propionibacterium acnes, Campylobacter rectus, Capnocytophaga sputigena, Campylobacter gracilis, Eikenella corrodens, Aggregatibacter actinomycetemcomitans, and V. parvula. Other members of this cluster such as P. gingivalis, Actinomyces oris and Selenomonas noxia had a kinetics characterized by a decrease in median levels at 3 months followed by a rebound at 6 months and a slow decrease thereafter.
Cluster 4 included species showing mild increases in their median levels at 6 and 12 months after treatment followed by a modest decline to levels similar to baseline values such as Streptococcus sanguinis, Streptococcus mitis, Actinomyces israelii, and Streptococcus intermedius. S. oralis was the only species that showed a marked increase in mean counts by 3 months post-therapy which was maintained to 24 months.
Cluster 5 was composed of species with 3 types of kinetics of changes after therapy: 1) species that were not significantly affected by periodontal therapy such as Neisseria mucosa, Streptococcus gordonii and Streptococcus anginosus; 2) species that had decreases to 3 months followed by a rebound at 6 months and slow reductions to 24 months, including Campylobacter showae, Actinomyces naeslundii and Actinomyces gerencseriae; and 3) species that only had reductions to median levels lower than baseline values at later time points, including Gemella morbillorum, Leptotrichia buccalis and Capnocytophaga ochracea.
Changes in species counts at sites with different baseline pocket depths
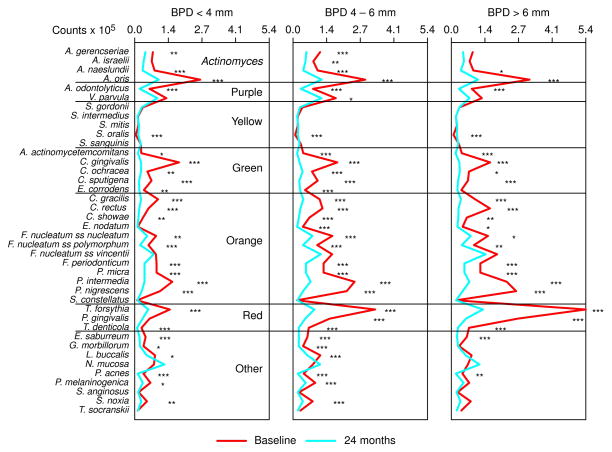
The change in median counts from baseline to 24 months at sites with BPD< 4, 4–6 and >6 mm is presented in Fig. 6. There were significant reductions in the majority of species for all pocket depth categories. In particular, species of the red, orange and green complexes were significantly reduced at the 24 month visit. S. oralis was the only species to significantly increase at 24 months in the 3 pocket depth ranges. These microbial changes were accompanied by reductions in the mean PD for the sampled sites. The mean PD (± SD, mm) at baseline and 24 months were for BPD< 4 mm, 2.9 ± 0.3 and 2.6 ± 0.4; for BPD 4–6 mm, 4.9 ± 0.3 and 3.5 ± 0.6; for BPD >6 mm, 7.5 ± 0.7 and 4.5 ± 1.2.
Fig. 6.
Median counts (×105) of 40 subgingival species in subgingival plaque samples taken pre-therapy and 24 months post-therapy at sites with baseline pocket depth of <4 (left panel), 4–6 (middle panel) and >6 mm (right panel). Counts of each species were averaged within a subject for each pocket depth category separately and then the medians across subjects for the baseline and the 24 month visits were computed separately. Significance of differences in species counts between visits was determined using the Wilcoxon signed ranks test and adjusted for 40 comparisons (Socransky et al. 1991) * p< 0.05, ** p< 0.01, *** p< 0.001. The species were ordered according to the complexes described by Socransky et al. (1998).
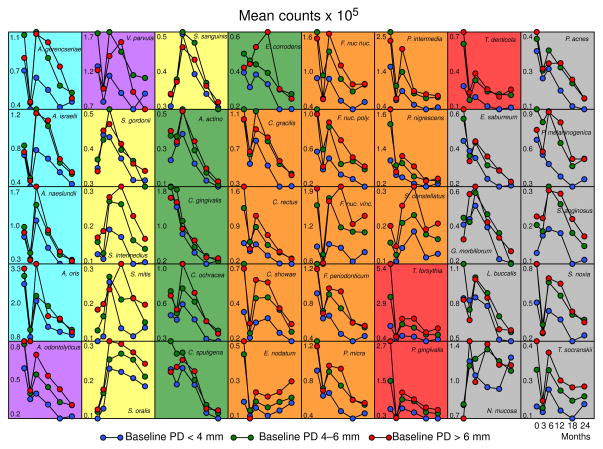
Fig. 7 presents the change in median counts for each species from baseline to 24 months at sites subset according to BPD category. Certain species showed similar patterns of change irrespective of initial pocket depth including; C. gingivalis, S. gordonii, P. acnes and many of the Actinomyces species. The red complex species, T. forsythia, P. gingivalis and T. denticola, showed dramatic decreases in mean counts at 3 months to virtually identical mean levels for each pocket depth category. However, from 6 to 24 months mean counts were higher at the sites with initially deeper pockets and least at the sites with initially shallow pockets. At sites with BPD >6 mm, species including C. showae, S. constellatus, V. parvula and F. nucleatum ss polymorphum showed different patterns of recolonization post-therapy than the same species at sites with shallow pocket depths.
Fig. 7.
Plots of median counts (×105) for subgingival species at baseline (pre-therapy), and at 3, 6, 12, 18 and 24 months post-therapy at sample sites with baseline pocket depths of <4, 4–6 and >6 mm. Counts of each species were averaged within a subject for each pocket depth category separately and then the medians across subjects for each visit were computed separately. Significance of differences over time was determined using the Friedman test and adjusted for 40 comparisons (Socransky et al. 1991). The y-axes values represent median counts × 105. Species counts that did not differ significantly over time were F. nucleatum ss vincentii (PD <4 mm), L. buccalis (PD >6 mm), N. mucosa (PD <4, 4–6 mm), S. anginosus (PD >6 mm), S. constellatus (PD <4, 4–6, >6 mm), S. gordonii (PD >6 mm), S. intermedius (PD <4, 4–6, >6 mm), S. mitis (PD <4, 4–6, >6 mm), S sanguinis (PD >6 mm), T. socranskii (PD >6 mm), V. parvula (PD 4–6, >6 mm). The background shading represents the complexes described by Socransky et al. (1998).
Discussion
This and other studies have demonstrated that therapies that include SRP alone or along with adjunctive agents or procedures such as AM, S or LDD have a beneficial effect on pocket depth reduction and attachment level change. The question posed in this manuscript was what changes in the subgingival microbiota caused and also resulted from the improved clinical status of the treated subjects. It was observed that there was an overall decrease in the total number of bacteria from pre-therapy to 24 months post-therapy and that the reductions were associated with a wide range of test species including those thought to be periodontal pathogens (Fig. 1). The suppression of periodontal pathogens by various forms of periodontal therapy has been observed in many other studies (Berglundh et al. 1998, Haffajee et al. 1995, 2006, Pavicic et al. 1994, Teles et al. 2006, van Winkelhoff et al. 1992, Winkel et al. 1999, 2001, Xajigeorgiou et al. 2006 Oteo et al. 2010, Aimetti et al. 2011, Sampaio et al. 2011, Silva et al. 2011).
This study provides unique data by following subgingival microbiological counts before and at multiple time points up to 2 years post-therapy. The kinetics of population shifts in the subgingival microbial ecosystem may be thought of in different time frames. There is an immediate shift during and immediately post-periodontal therapy that may occur in minutes (e.g. as a result of scaling) or days. This perturbation would be followed by a re-organization of the subgingival microbiota which might take weeks to months. Finally, a longer term shift in the ecosystem would lead to a new climax community that might be stable for years to decades. Rapid shifts in the ecosystem as a result of therapy have been observed in a number of studies (Lindhe et al. 1983, Loesche et al. 1984, 1991, Feres et al. 2001). Lindhe et al. (1983) demonstrated that the percentage of spirochetes and motile rods was reduced at sites that received scaling with or without metronidazole by 2 weeks and this reduction persisted to 50 weeks. Non-scaled sites in subjects receiving metronidazole showed a similar reduction. Loesche and co-workers (1984, 1991) examined changes in subgingival taxa following metronidazole administration and found that spirochetes and other motile forms were decreased in numbers and proportions by 0–2 weeks and this reduction lasted for at least 15–30 weeks (Loesche et al. 1984). In a second study, 1–3 days after metronidazole administration, the percents of P. gingivalis and spirochetes were reduced significantly and the reduction in spirochetes was maintained at least to 3 to 6 months (Loesche et al. 1991). Feres et al. (2001) found that dramatic decreases occurred 3 days into antibiotic therapy in the counts of several species including P. gingivalis, T. forsythia, T. denticola, C. rectus, F. nucleatum ss vincentii and P. intermedia. These reductions lasted for at least 1 year.
The present investigation focused on the changes that take place over a longer time frame. Examination of Fig. 5 indicates that pathogenic species including T. forsythia, T. denticola and E. nodatum were markedly reduced at the 3 month post-therapy monitoring visit. It may be surmised from the above studies that the reduction in these species took place much earlier than 3 months and it was shown in this study that counts of these taxa continued at reduced levels out to 2 years. It may be speculated that the reduction in this group of species, and perhaps additional pathogens, initiated the change in the ecosystem that led to periodontal stability, on average, over the 2 years of the study. The ecosystem was altered directly by reduction in pathogen numbers and indirectly by altering the status of the local host tissues. In particular, reduction in local inflammation would lead to reduction in levels of gingival crevicular fluid, diminishing a prominent source of nutrients for the growth of subgingival taxa (Socransky et al. 2004, Teles et al. 2012). These alterations may have led to the slow but continued decline in a wide range of taxa including E. corrodens, V. parvula, C. rectus, C. gracilis, C. sputigena, P. melaninogenica, and P. acnes. Certain members of Clusters 2 and 5 such as A. odontolyticus, F. nucleatum ss. polymorphum, P. micra, F. periodonticum, C. showae, A. naeslundii, A. gerencseriae, L. buccalis, and C. ochracea were decreased in median counts at 3 months, rebound at 6 months and declined slowly thereafter. Other taxa, including Streptococci species, A. israelii, N. mucosa, and T. socranskii appeared to be less affected by therapy while one taxon, S. oralis, actually increased following periodontal therapy and remained at increased median levels. The result of periodontal therapy at 2 years (Fig. 1) was a markedly altered mean microbial profile. Dramatic reductions occurred in many taxa including members of the pathogenic red and orange complexes as well as members of the Actinomyces, Green complex, P. melaninogenica and S. noxia. This led to a more even distribution of taxa with species such as members of the genus Streptococcus, playing a more prominent post-therapy role. The overall change that was observed was likely due, in part, to reductions in pocket depth which led to the reduction of the deep periodontal pockets that foster colonization of red and orange complex species (Socransky et al. 1998, 2004) and reduction in inflammation that limits nutrient availability to colonizing species. Data presented in Fig. 6 demonstrate that the microbial shifts described above occurred irrespectively from the BPD of the site. The shift in the microbiota towards a more healthy-associated climax community in shallow sites might explain, in part, why clinical improvements were sustained for up to 2 years. For instance, it has been demonstrated that periodontal disease progression in periodontitis subjects during supportive periodontal therapy occurs primarily in shallow sites (Teles et al. 2008). Therefore, reduction of pathogens in these sites might contribute to the stability of the periodontal condition.
This study and others help to unravel the means by which periodontal therapies achieve their goal of controlling periodontal infections. The control of periodontal diseases, for the most part, does not involve the control of a single bacterial species, but rather the alteration of an ecosystem to one in which pathogens remain at levels lower than those needed to initiate or continue disease progression. As shown in the current manuscript, there is an additional effect on the ecosystem beyond suppression of the pathogens. The counts of other taxa are, over time, also reduced. This is interpreted as an effect of changing the ecosystem (by reducing certain members of the subgingival microbiota such as certain members of the red complex) and by altering the habitat; the adjacent periodontal tissues. The alteration of habitat inevitably leads to an altered ecosystem, in this case reduction of numbers of organisms of many other taxa. The ecosystem appears to return to one similar to, but not identical with, the ecosystem typical of periodontal health. Thus, the effect of therapy is to “set the clock back” to one that is more similar to the status prior to initiation of disease requiring the ecosystem to go through the time-consuming changes necessary to lead to disease re-initiation.
Clinical relevance.
Scientific rationale
The control of periodontal infections is brought about by the control of the organisms that cause them. This study examined the long-term changes that occur in the subgingival microbiota resulting from periodontal treatment in order to determine the sequence of changes that take place in the subgingival microbiota that lead to periodontal stability.
Principal findings
Overall, periodontal therapy reduced the mean counts of 30/40 test species, particularly species thought to play a major role in the initiation of periodontal infections such as T. forsythia, T. denticola and E. nodatum. These species were reduced rapidly by treatment and remained at lowered levels to 2 years post-therapy. Their reduction may have contributed to the decrease in other species in the ecosystem.
Practical implications
If the therapist is able to rapidly decrease and maintain at low levels the counts of periodontal pathogens, this will lead to a reduction in the counts of the entire ecosystem and improvement in clinical parameters.
Acknowledgments
Source of funding statement:
This research was supported by the National Institute of Dental and Craniofacial Research grants DE12861 and RR025771 and GCRC funding from RR00533 and RR01032.
The authors would like to express their gratitude to Dr. Jacqueline Starr (The Forsyth Institute), for her help with the statistical analysis during the revision of this manuscript.
Footnotes
Conflict of interest:
There are no conflicts of interest to report.
References
- Aimetti M, Romano F, Guzzi N, Carnevale G. One-stage full-mouth disinfection as a therapeutic approach for generalized aggressive periodontitis. Journal of Periodontology. 2011;82:845–853. doi: 10.1902/jop.2010.100468. [DOI] [PubMed] [Google Scholar]
- Apatzidou DA, Kinane DF. Nonsurgical mechanical treatment strategies for periodontal disease. Dental Clinics of North America. 2010;54:1–12. doi: 10.1016/j.cden.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Annals of Statistics. 2001;29:1165–1188. [Google Scholar]
- Berglundh T, Krok L, Liljenberg B, Westfelt E, Serino G, Lindhe J. The use of metronidazole and amoxicillin in the treatment of advanced periodontal disease. A prospective, controlled clinical trial. Journal of Clinical Periodontology. 1998;25:354–362. doi: 10.1111/j.1600-051x.1998.tb02455.x. [DOI] [PubMed] [Google Scholar]
- Caton JC, Ciancio SG, Blieden TM, Bradshaw M, Crout RJ, Hefti AF, Massaro JM, Polson AM, Thomas J, Walker C. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. Journal of Periodontology. 2000;71:521–532. doi: 10.1902/jop.2000.71.4.521. [DOI] [PubMed] [Google Scholar]
- Drisko CH. Nonsurgical periodontal therapy. Periodontology 2000. 2001;25:77–88. doi: 10.1034/j.1600-0757.2001.22250106.x. [DOI] [PubMed] [Google Scholar]
- Goodson JM, Haffajee AD, Socransky SS, Kent R, Teles R, Hasturk H, Bogren A, van Dyke T, Wennstrom J, Lindhe J. Control of Periodontal Infections: A randomized controlled trial I. The primary outcome attachment gain and pocket depth reduction at treated sites. Journal of Clinical Periodontology. 2012 doi: 10.1111/j.1600-051X.2012.01870.x. (in press) [DOI] [PubMed] [Google Scholar]
- Guerrero A, Griffiths GS, Nibali L, Suvan J, Moles DR, Laurell L, Tonetti MS. Adjunctive benefits of systemic amoxicillin and metronidazole in non-surgical treatment of generalized aggressive periodontitis: a randomized placebo-controlled clinical trial. Journal of Clinical Periodontology. 2005;32:1096–1107. doi: 10.1111/j.1600-051X.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- Feres M, Haffajee AD, Allard KA, Som S, Socransky SS. Change in subgingival microbial profiles in adult periodontitis subjects receiving either systemically administered amoxicillin or metronidazole. Journal of Clinical Periodontology. 2001;28:597–609. doi: 10.1034/j.1600-051x.2001.028007597.x. [DOI] [PubMed] [Google Scholar]
- Haffajee AD, Goodson JM, Teles R, Bogren A, Wennstrom J, Lindhe J, Hasturk H, van Dyke T, Socransky SS. Effect of periodontal therapy on the subgingival microbiota over a 2-year monitoring period. II. Main effects of periodontal surgery, local and systemic antibiotics therapy. Journal of Clinical Periodontology. 2013 doi: 10.1111/jcpe.12117. to be submitted shortly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffajee AD, Dibart S, Kent RL, Jr, Socransky SS. Clinical and microbiological changes associated with the use of 4 adjunctive systemically administered agents in the treatment of periodontal infections. Journal of Clinical Periodontology. 1995;22:618–627. doi: 10.1111/j.1600-051x.1995.tb00815.x. [DOI] [PubMed] [Google Scholar]
- Haffajee AD, Socransky SS, Gunsolley JC. Systemic anti-infective periodontal therapy. A systematic review. Annals of Periodontology. 2003;8:115–181. doi: 10.1902/annals.2003.8.1.115. [DOI] [PubMed] [Google Scholar]
- Haffajee AD, Teles RP, Socransky SS. The effect of periodontal therapy on the composition of the subgingival microbiota. Periodontology 2000. 2006;43:7–12. doi: 10.1111/j.1600-0757.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- Heitz-Mayfield LJ, Trombelli L, Heitz F, Needleman I, Moles D. A systematic review of the effect of surgical debridement vs non-surgical debridement for the treatment of chronic periodontitis. Journal of Clinical Periodontology. 2002;29(suppl 3):S92–S102. doi: 10.1034/j.1600-051x.29.s3.5.x. [DOI] [PubMed] [Google Scholar]
- Herrera D, Sanz M, Jepsen S, Needleman I, Roldan S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. Journal of Clinical Periodontology. 2002;29 (suppl 3):136–159. doi: 10.1034/j.1600-051x.29.s3.8.x. [DOI] [PubMed] [Google Scholar]
- Huynh-Ba G, Kuonen P, Hofer D, Schmid J, Lang NP, Salvi GE. The effect of periodontal therapy on the survival rate and incidence of complications of multirooted teeth with furcation involvement after an observation period of at least 5 years: a systematic review. Journal of Clinical Periodontology. 2009;36:164–176. doi: 10.1111/j.1600-051X.2008.01358.x. [DOI] [PubMed] [Google Scholar]
- Lang NP, Tan WC, Krähenmann MA, Zwahlen M. A systematic review of the effects of full-mouth debridement with and without antiseptics in patients with chronic periodontitis. Journal of Clinical Periodontology. 2008;35(suppl):S8–S21. doi: 10.1111/j.1600-051X.2008.01257.x. [DOI] [PubMed] [Google Scholar]
- Lindhe J, Liljenberg B, Adielson B, Borjesson I. Use of metronidazole as a probe in the study of human periodontal disease. Journal of Clinical Periodontology. 1983;10:100–112. doi: 10.1111/j.1600-051x.1983.tb01271.x. [DOI] [PubMed] [Google Scholar]
- Loesche WJ, Schmidt E, Smith BA, Morrison EC, Caffesse R, Hujoel PP. Effects of metronidazole on periodontal treatment needs. Journal of Periodontology. 1991;62:247–257. doi: 10.1902/jop.1991.62.4.247. [DOI] [PubMed] [Google Scholar]
- Loesche WJ, Syed SA, Morrison EC, Kerry GA, Higgine T, Stoll J. Metronidazole in periodontitis. I. Clinical and bacteriological results after 15 to 30 weeks. Journal of Periodontology. 1984;55:325–335. doi: 10.1902/jop.1984.55.6.325. [DOI] [PubMed] [Google Scholar]
- Oteo A, Herrera D, Figuero E, O’Connor A, González I, Sanz M. Azithromycin as an adjunct to scaling and root planing in the treatment of Porphyromonas gingivalis-associated periodontitis: a pilot study. Journal of Clinical Periodontology. 2010;37:1005–1015. doi: 10.1111/j.1600-051X.2010.01607.x. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Matthews JP, Wilson RF. Non-surgical periodontal treatment with and without adjunctive metronidazole in smokers and non-smokers. Journal of Clinical Periodontology. 1999;26:158–163. doi: 10.1034/j.1600-051x.1999.260305.x. [DOI] [PubMed] [Google Scholar]
- Pavicic MJ, van Winkelhoff AJ, Douqué NH, Steures RW, de Graaff J. Microbiological and clinical effects of metronidazole and amoxicillin in Actinobacillus actinomycetemcomitans-associated periodontitis. A 2-year evaluation. Journal of Clinical Periodontology. 1994;21:107–112. doi: 10.1111/j.1600-051x.1994.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Pihlstrom BL, McHugh RB, Oliphant TH, Ortiz-Campos C. Comparison of surgical and nonsurgical treatment of periodontal disease. A review of current studies and additional results after 61/2 years. Journal of Clinical Periodontology. 1983;10:524–541. doi: 10.1111/j.1600-051x.1983.tb02182.x. [DOI] [PubMed] [Google Scholar]
- Ramberg P, Rosling B, Serino G, Hellstrom MK, Socransky SS, Lindhe J. The long-term effect of systemic tetracycline used as an adjunct to non-surgical treatment of advanced periodontitis. Journal of Clinical Periodontology. 2001;28:446–452. doi: 10.1034/j.1600-051x.2001.028005446.x. [DOI] [PubMed] [Google Scholar]
- Sampaio E, Rocha M, Figueiredo LC, Faveri M, Duarte PM, Gomes Lira EA, Feres M. Clinical and microbiological effects of azithromycin in the treatment of generalized chronic periodontitis: a randomized placebo-controlled clinical trial. Journal of Clinical Periodontology. 2011;38:838–846. doi: 10.1111/j.1600-051X.2011.01766.x. [DOI] [PubMed] [Google Scholar]
- Silva MP, Feres M, Sirotto TA, Soares GM, Mendes JA, Faveri M, Figueiredo LC. Clinical and microbiological benefits of metronidazole alone or with amoxicillin as adjuncts in the treatment of chronic periodontitis: a randomized placebo-controlled clinical trial. Journal of Clinical Periodontology. 2011;38:828–837. doi: 10.1111/j.1600-051X.2011.01763.x. [DOI] [PubMed] [Google Scholar]
- Sneath PHA, Sokal RR. The principles and practice of numerical classification. San Francisco: WH Freeman & Co; 1973. Numerical taxonomy. [Google Scholar]
- Socransky SS, Haffajee AD. Periodontal microbial ecology. In: Haffajee, A.D., & Socransky, S.S ed. Microbiology of periodontal diseases. Periodontology 2000. 2004;38:135–187. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. Journal of Clinical Periodontology. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Smith C, Dibart S. Relation of counts of microbial species to clinical status at the site. Journal of Clinical Periodontology. 1991;18:766–775. doi: 10.1111/j.1600-051x.1991.tb00070.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Smith C, Martin L, Haffajee JA, Uzel NG, Goodson JM. The use of checkerboard DNA-DNA hybridization to study complex microbial ecosystems. Oral Microbiology and Immunology. 2004;19:352–362. doi: 10.1111/j.1399-302x.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Smith C, Martin L, Paster BJ, Dewhirst FE, Levin AE. Checkerboard DNA-DNA hybridization. Biotechniques. 1994;17:788–792. [PubMed] [Google Scholar]
- Teles RP, Haffajee AD, Socransky SS. Microbiological goals of periodontal therapy. Periodontology 2000. 2006;43:180–218. doi: 10.1111/j.1600-0757.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- Teles RP, Socransky SS, Haffajee AD. Disease Progression in Periodontally Healthy and Maintenance Subjects. Journal of Periodontology. 2008;79:784–794. doi: 10.1902/jop.2008.070485. [DOI] [PubMed] [Google Scholar]
- Teles FR, Teles RP, Sachdeo A, Uzel NG, Song XQ, Torresyap G, Singh M, Papas A, Haffajee AD, Socransky SS. Comparison of microbial changes in early redeveloping biofilms on natural teeth and dentures. Journal of Periodontology. 2012 doi: 10.1902/jop.2012.110506. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Winkelhoff AJ, Tijhof CJ, de Graaff J. Microbiological and clinical results of metronidazole plus amoxicillin therapy in Actinobacillus actinomycetemcomitans-associated periodontitis. Journal of Periodontology. 1992;63:52–57. doi: 10.1902/jop.1992.63.1.52. [DOI] [PubMed] [Google Scholar]
- Winkel EG, Van Winkelhoff AJ, Timmerman MF, Van der Velden U, Van der Weijden GA. Amoxicillin plus metronidazole in the treatment of adult periodontitis patients. A double-blind placebo-controlled study. Journal of Clinical Periodontology. 2001;28:296–305. doi: 10.1034/j.1600-051x.2001.028004296.x. [DOI] [PubMed] [Google Scholar]
- Winkel EG, Van Winkelhoff AJ, Barendregt DS, Van der Weijden GA, Timmerman MF, Van der Velden U. Clinical and microbiological effects of initial periodontal therapy in conjunction with amoxicillin and clavulanic acid in patients with adult periodontitis. A randomised double-blind, placebo-controlled study. Journal of Clinical Periodontology. 1999;26:461–468. doi: 10.1034/j.1600-051x.1999.260708.x. [DOI] [PubMed] [Google Scholar]
- Xajigeorgiou C, Sakellari D, Slini T, Baka A, Konstantinidis A. Clinical and microbiological effects of different antimicrobials on generalized aggressive periodontitis. Journal of Clinical Periodontology. 2006;33:254–264. doi: 10.1111/j.1600-051X.2006.00905.x. [DOI] [PubMed] [Google Scholar]