Abstract
Background & Aims
5-HT3 receptor (5-HT3R) antagonists are effective in treating patients with irritable bowel syndrome (IBS) and have anxiolytic effects. Their therapeutic effects are related, in part, to reducing amygdala engagement during expected visceral pain. A single nucleotide polymorphism (SNP) in HTR3A, c.-42C>T;(C178T; rs1062613), is associated with altered reactivity of the amygdala during emotional face processing in healthy subjects (controls). We evaluated the influence of this SNP on amygdala reactivity to emotional faces and non-emotional stimuli in female patients with IBS and controls.
Methods
We measured brain responses during an affect-matching paradigm in 54 women (26 with IBS, 29 controls) using functional magnetic resonance imaging. We examined associations between HTR3A c.-42C>T genotype (C/C vs. T carrier) and responses in amygdala and other regions of brain that expressed high levels of 5-HT3R.
Results
The C/C genotype was associated with greater anxiety symptoms in patients with IBS and controls and increased activation of the amygdala under emotional and non-emotional conditions. Among patients with IBS, the C/C genotype was associated with greater symptom ratings; a subset of IBS patients with the C/C genotype had increased amygdala responses to non-emotional stimuli, compared to other subjects with C/C genotype.
Conclusions
Regardless of diagnosis, the C/C genotype of the c.-42C>T polymorphism in HTR3A, compared to T carrier status, is associated with increased anxiety and amygdala responsiveness during emotional and non-emotional tasks. This polymorphism was associated with severity of IBS symptoms. Although this genotype is not sufficient for diagnosis of IBS, it is associated with severity of symptoms.
Keywords: serotonin, genetics, emotion, fMRI, digestive disorders
INTRODUCTION
Preclinical and clinical evidence supports an important role of the serotonin (5-HT) signaling system in the modulation of the brain gut axis, and alterations in this signaling system may play a role in functional gastrointestinal disorders such as IBS 1, psychiatric disorders (anxiety, depression, eating disorders 2) and pregnancy-related nausea 3. 5-HT3R antagonists are one of the most effective treatments for patients with diarrhea predominant IBS (IBS-D) 4–6 and have also shown effectiveness in comorbid chronic pain disorders, including functional dyspepsia and fibromyalgia 7. Several clinical studies reported beneficial effects of 5-HT3R antagonists in the treatment of anxiety 8, which is highly comorbid with IBS, eating disorders and fibromyalgia 9. Animal studies support the concept that 5-HT3R antagonists have anxiolytic effects by attenuating the response of emotional arousal circuits in the brain10–12. Even though the precise mechanism(s) underlying the effectiveness of the most widely tested 5-HT3R antagonists (Alosetron, Cilansetron) in treating IBS-D symptoms remain incompletely understood, evidence supports both peripheral and central mechanisms of action 4. For example, symptom improvement during Alosetron treatment was associated with a reduction in amygdala activity during anticipation of abdominal pain 13, consistent with a possible role of increased activity of amygdala-related emotional arousal circuits in the pathophysiology of IBS 14.
5-HT3Rs are widely distributed in the central (CNS) and peripheral nervous system, including the enteric nervous system 15, 16. Within the CNS, 5-HT3Rs are found in amygdala, hippocampus, cingulate cortex, striatum, entorhinal frontal cortex as well as brainstem and the superficial layers of the spinal cord 17–19. 5-HT3Rs are unique, representing the only ligand gated cation channel amongst 5-HT receptors, which are primarily expressed presynaptically 20, 21. The presynaptic expression of 5-HT3Rs on nerve endings is consistent with their physiological role in the release of neurotransmitters such as dopamine, cholecystokinin, glutamate, acetylcholine and GABA 22. Consequently, both excitatory and inhibitory effects of 5-HT3R activation have been reported, which may be mediated by activation of excitatory and inhibitory interneurons respectively 23. For example, 5-HT3R activation on GABAergic interneurons innervating the amygdala, exert an inhibitory influence on amygdala activity, while those on excitatory glutaminergic interneurons have the opposite effect 23 (see Figure 1).
Figure 1.

5-HT3R activation on GABAergic interneurons innervating the amygdala exerts an inhibitory influence on amygdala activity, while those on excitatory glutaminergic interneurons will exert an excitatory influence.
There is considerable molecular diversity within the 5-HT3R family resulting from variable composition of 5 different subunits (5-HT3A-E), with differential expression of subunits in different regions of the nervous system 23. Several polymorphisms of the 5-HT3AR, 5-HT3BR, 5-HT3CR and 5-HT3ER encoding genes (HTR3A, B, C and E) have been reported to be associated with gastrointestinal disorders (including IBS) 24, pregnancy-related nausea 3, and several psychiatric conditions (including anxiety, depression and eating disorders) 2, 25, 26. For example, the novel HTR3E 3′-UTR variant c.*76G>A has been shown to be associated with a diagnosis of IBS-D in female patients in two independent samples, suggesting a possible role of this variant in intestinal fluid handling 24.
The relatively common variant c.-42C>T (minor allele frequency 0.15–0.23) within the 5′ untranslated region of the HTR3A gene is associated with increased 5-HT3A subunit expression in vitro 24, 26. The less common T allele, versus the C allele is associated with reduced amygdala and prefrontal cortical activation during a face recognition task and may be associated with the personality trait of lower harm avoidance in women 27, 28. These findings are consistent with T allele-related increased expression of 5-HT3Rs on inhibitory GABAergic interneurons, resulting in greater inhibition of the amygdala.
We aimed to examine, in female IBS patients and HCs, the impact of HTR3A c.- 42C>T on responses in 5-HT3R-rich brain regions during an affect matching paradigm known to reliably activate the amygdala29. Based on the evidence summarized above, and the concept of increased engagement of amygdala-related emotional arousal circuits in IBS pathophysiology 14, our main hypothesis was that subjects carrying the C/C genotype of the HTR3A single nucleotide polymorphism c.-42C>T would show greater responses of the amygdala, and possibly greater IBS severity.
METHODS
Subjects
A sample of 26 female IBS patients and 29 female healthy controls (demographics are presented in Table 1) participated in fMRI studies of emotional reactivity and provided saliva samples for DNA analyses. Subjects were recruited through the UCLA Digestive Disease Clinic and from community advertisements. The diagnosis of IBS was confirmed using Rome II criteria during a clinical examination by a gastroenterologist or nurse practitioner experienced in functional GI disorders 30. 19% of IBS patients rated their usual symptoms as moderate (cannot be ignored but does not affect your lifestyle), 62 % as severe (affects your lifestyle), and 19 % as very severe (markedly affects your lifestyle). Control subjects received medical exams to confirm absence of functional pain disorders. All subjects were free of current or past psychiatric illness, substance abuse disorder and major medical or neurological conditions. No subjects took medications for 30 days prior to scanning. The UCLA institutional review board approved all studies; informed consent was obtained from all subjects.
Table 1.
| C/C | T carrier | ||
|---|---|---|---|
|
| |||
| N: | Control | 15 | 14 |
| IBS | 16 | 10 | |
|
| |||
| Age: | Control | 27.2(3.1) | 39.93(3.7) |
| IBS | 32.06(2.5) | 31.00(3.6) | |
|
| |||
| Anxiety: | Control | 4.5(0.5) | 2.6(0.8) |
| IBS | 5.8(0.9) | 4.5(0.9) | |
|
| |||
| Bowel Habit: | IBS | 6 IBS-C, 8 IBS-D, 2 IBS-A | 2 IBS-C, 3 IBS-D, 5 IBS-A |
|
| |||
| Overall Symptom Severity: | IBS | 13.3(1.2) | 9.6(1.0) |
|
| |||
| Bloating Severity: | IBS | 13.1(1.1) | 7.7(1.9) |
|
| |||
| Abdominal Pain Severity: | IBS | 11.3(1.5) | 9.0(1.2) |
IBS-C = constipation predominate; IBS-D = diarrhea predominate; IBS-A = alternating bowel habit
Genotyping
DNA was extracted at the UCLA Biological Samples Processing Core (BSPC). Samples for DNA isolation were collected using the Oragene™ DNA Self-Collection Kit (DNA Genotek Inc., Ottawa, Canada). DNA obtained using this kit is comparable in quality and quantity to DNA extracted from blood31. SNP genotyping was performed with the KASPar® assay system (KBiosciences Ltd, Hoddesdon, UK) as recommended by the manufacturer. The used primers for HTR3A c.-42C>T were: rs1062613_ALC: 5′-GAAGGTGACCAAGTTCATGCTGCCTCCGAGTGCTCAGGG-3′; rs1062613_ALT: 5′-GAAGGTCGGAGTCAACGGATTGTGCCTCCGAGTGCTCAGGA-3′; and rs1062613_C1: 5′-AGGTTGGCAGAGGGCAGGCAA-3′. An initial 15 min cycle at 94 °C was followed by 20 cycles consisting of 10 sec at 94 °C, 5 sec at 57 °C and 10 sec at 72 °C; 23 cycles consisting of 10 sec at 94 °C, 20 sec at 57 °C and 40 sec at 72 °C; and a cooldown at 10 °C. After the thermal cycling, results were analyzed using the fluorescence plate reader Taqman 7500 system (Applied Biosystems, Foster City, CA).
Study Design
Prior to scanning, IBS patients rated: (1) overall severity of their gastrointestinal symptoms, (2) severity of abdominal pain and (3) severity of bloating on a numerical rating scale of 0 (no symptoms) to 20 (the most intense symptoms imaginable). All subjects completed the Hospital Anxiety and Depression scale, a measure of current anxiety symptoms validated for non-psychiatric samples 32.
Subjects completed two runs of an emotional reactivity task (adapted from 29) consisting of two conditions: Match Emotion (ME) and Match Forms (MF). During the ME condition, subjects viewed a target face33 depicting an angry or fearful expression and were asked to select one of two other faces that expressed the same emotion. In the ME task, subjects tend to Match the Emotional faces based on perceptual characteristics, such as a furrowed brow 29. The MF condition is a neutral control task in which subjects perform a similar perceptual decision (Match Forms), but no emotional stimuli are presented. During the MF condition, subjects viewed a circular shaped target (approximately the same size as a human face) and were asked to select one of two other shapes that best matched the target. Traditionally, the difference in activity during the emotional condition compared to neutral condition (ME-MF) has been considered a measure of emotional reactivity 29.
Stimuli were presented in randomized sequences, counter-balanced across runs. Each condition was presented as a block of 6 images, with each image presented for 3 sec, for a total block length of 18 sec. In each run, 4 blocks of ME and 4 blocks of MFs were randomly presented. Subjects completed two runs, thus a total of 48 images were presented in the ME condition and 48 in the MF condition. An instruction cue was presented for 3 sec prior to each block and a rest period of 6 sec followed each block. Subjects were presented with additional facial stimuli as part of an emotional modulation task; however, the current study involves the results of the emotional reactivity task only.
fMRI Procedures
fMRI was performed using a 3.0T MRI scanner (Siemens Trio; Siemens, Erlangen, Germany). A high resolution structural image was acquired from each subject with a magnetization-prepared rapid acquisition gradient-echo (MP-RAGE) sequence, repetition time (TR) = 2300 ms, echo time (TE) = 2.85 ms, 256 slices, 160*240 matrix, 13 mm voxel size. Functional blood oxygen-level dependent (BOLD) images were acquired (TR = 3000 ms, TE = 28 ms, flip angle = 90°, 38 slices, slice thickness = 3 mm) while subjects completed two runs of the emotional reactivity task. Stimuli were presented via MRI-compatible goggles using Superlab 4.0 software (Cedrus Corp, San Pedro, CA). Subjects responded using an MRI-compatible button box by pressing one of two buttons with the right hand.
Data Analysis
The first two volumes were discarded to allow for stabilization of the magnetic field. The remaining functional images were slice-time and motion corrected, spatially normalized to the MNI template, and spatially smoothed with a 8 mm3 Gaussian kernel using SPM5 (Welcome Department of Cognitive Neurology, London, UK). While the amygdala was the primary region of interest (ROI), additional ROIs were chosen a priori and consisted of brain areas previously associated with 5-HT3 receptor distribution 17–19. ROIs for the amygdala, caudate, hippocampus, insula, anterior cingulate (ACC) and medial prefrontal cortex (mPFC) were created using the Wake Forest University PickAtlas toolbox in SPM5 34–37. Functional data were analyzed using the general linear model within SPM5. For each individual subject, fixed effect analyses were performed comparing (1) ME versus an implicit baseline; (2) MF versus an implicit baseline; and (3) ME versus MF (ME-MF). Parameter estimates for ME-MF were considered a measure of emotional reactivity 29. We included analyses of brain responses during ME and MF conditions (considered individually) to provide additional information on potential group differences in the specificity of brain responsiveness. Random effect analyses were performed to examine genotype and diagnosis effects with age of the subject entered as a covariate. Small volume correction (SVC) for ROIs was applied using SPM5 familywise error (FWE) algorithm and a volume-corrected probability value of <0.05 was considered significant. A priori power analyses conducted in G*Power 3.1.2 (www.psycho.uni-duesseldorf.de/aap/projects/gpower/) indicated that 10 subjects per group were required to provide adequate power (1-Beta=.80) to detect an effect size difference (d=1.20, z=2.4) in brain activity commonly seen in our research studies, using a one-sample t-test.
The association of genotype with diagnosis was analyzed by chi square and risk assessment tests; genetic impact on anxiety and symptom ratings was analyzed by analysis of variance (ANOVA) using PASW 17 (Chicago, IL).
RESULTS
Clinical characteristics
HTR3A polymorphism
Fifteen of 29 HCs and 16 of 26 IBS patients were homozygous for the C allele, nine HCs and seven patients carried the heterozygous C/T genotype, and five HCs and three patients carried the homozygous T/T genotype. The c.-42C>T SNP had been previously found to be associated with IBS-D in a UK sample applying a minor alllele dominant model 24. Thus, subjects carrying a T allele (C/T or T/T) were combined into a single group (“T carriers”) for analyses.
A HTR3A genotype (C/C, T carrier) × Diagnosis (HC, IBS) ANOVA revealed a significant interaction of diagnosis and T carrier status on anxiety symptoms, with IBS patients reporting higher anxiety ratings, and T carriers (IBS and HCs combined) reporting lower anxiety ratings (F(1,51)=4.20, p=.046; F(1,51)=4.37, p=.042, respectively). Among IBS patients, T carriers had significantly lower overall symptom severity and abdominal discomfort (bloating) ratings (t(24)=2.102, p=.046, t(24)=2.654, p=.014, respectively). However, T carriers with IBS did not differ in abdominal pain ratings (p>.05). Means and standard errors are displayed in Table 1. Chi-square tests and risk estimates indicated no significant association between T carrier status and IBS diagnosis (p’s>.05).
Emotional reactivity (ME-MF) at the whole brain level
Activity during ME relative to MF (ME-MF; with age entered as a covariate) was first examined at the whole brain level to determine if regions differentially activated in response to emotional faces compared to neutral visual stimuli were similar to previous studies of emotional reactivity. Areas that were significantly activated (p<.05, FWE, extent ≥ 30 voxels) across all subjects included right amygdala, bilateral visual cortex (BA 17/18/19), bilateral fusiform gyrus (BA 37), bilateral thalamus, bilateral dorsolateral and ventrolateral PFC (BA 8/9/45/46/47), bilateral BA 7, and right BA 22/41 (Table 2). Areas that were significantly deactivated (p<.05, FWE, extent ≥ 30 voxels) during ME relative to MF included bilateral mPFC (BA 24/32), bilateral insula, bilateral posterior cingulate cortex (BA 23/31), and left BA39 (Table 2). These results are consistent with previous studies using a similar emotional reactivity paradigm 29, 38.
Table 2.
| Hemisphere | Region | Cluster Size | p | T | X(mm) | Y(mm) | Z(mm) | |
|---|---|---|---|---|---|---|---|---|
| Whole Brain | ||||||||
| ME-MF: | ||||||||
| All subjects | bilateral | BA 17/18/19/37 | 11576 | <.001 | 18.87 | 22 | −92 | −10 |
| <.001 | 16.49 | −18 | −92 | −6 | ||||
| bilateral | thalamus | 919 | <.001 | 10.62 | 20 | −30 | −2 | |
| <.001 | 10.39 | −20 | −30 | −2 | ||||
| right | amyg | 59 | <.001 | 7.90 | 20 | −6 | −14 | |
| right | BA 45/46/47 | 2747 | <.001 | 10.45 | 50 | 20 | 22 | |
| left | BA 9/45/47 | 2020 | <.001 | 10.40 | −40 | 8 | 28 | |
| left | BA 7 | 240 | <.001 | 7.35 | −26 | −58 | 44 | |
| right | BA 22/41 | 351 | <.001 | 7.23 | 54 | −42 | 6 | |
| right | BA 7 | 171 | <.001 | 7.16 | 34 | −58 | 42 | |
| bilateral | BA 8 | 182 | .003 | 6.27 | −4 | 16 | 50 | |
| .006 | 5.99 | 4 | 18 | 50 | ||||
| MF-ME: | ||||||||
| All subjects | bilateral | BA 24/32 | 2145 | <.001 | 11.79 | −6 | 34 | −4 |
| <.001 | 9.54 | 6 | 34 | −4 | ||||
| right | insula | 1171 | <.001 | 7.38 | 42 | −18 | 18 | |
| left | insula | 742 | <.001 | 7.58 | −44 | −22 | 6 | |
| left | BA 39 | 175 | <.001 | 7.57 | −42 | −72 | 36 | |
| left | BA 23 | 136 | <.001 | 7.54 | −8 | −56 | 14 | |
| right | BA 31 | 369 | <.001 | 7.35 | 4 | −28 | 44 | |
| ROI Analyses | ||||||||
| MF - baseline: | ||||||||
| IBS>HC | left | amyg | 104 | 0.043 | 2.90 | −22 | −10 | −12 |
| left | caud | 65 | 0.023 | 3.74 | −26 | −40 | 4 | |
| left | hippo | 156 | 0.005 | 3.85 | −26 | −40 | 2 | |
| C/C>T carrier | left | amyg | 81 | 0.032 | 3.03 | −26 | −4 | −26 |
| right | amyg | 72 | 0.012 | 3.46 | 30 | −6 | −22 | |
| right | hippo | 67 | 0.031 | 3.13 | 30 | −8 | −22 | |
| ME - baseline: | ||||||||
| IBS>HC | left | caud | 59 | 0.015 | 3.94 | −26 | −40 | 4 |
| left | hippo | 69 | 0.005 | 3.95 | −26 | −38 | 2 | |
| C/C>T carrier | right | amyg | 59 | 0.03 | 3.12 | 30 | −6 | −20 |
| ME-MF: | ||||||||
| HC>IBS | right | insula | 1257 | .015 | 4.28 | 42 | −4 | 16 |
| T carrier>C/C | left | caud | 142 | .022 | 3.83 | −8 | 2 | 18 |
| left | hippo | 61 | .046 | 3.05 | −26 | −24 | −12 | |
| right | hippo | 161 | .014 | 3.52 | 32 | −22 | −14 | |
| C/C Genotype Median Split | ||||||||
| MF - baseline: | ||||||||
| IBS L > IBS H | right | amyg | 135 | 0.006 | 4.04 | 26 | −2 | −20 |
| IBS L > HC | right | amyg | 138 | 0.013 | 3.69 | 26 | −2 | −20 |
HTR3A effects on brain responses in specific regions
An ROI analysis was conducted to examine diagnosis and HTR3A genotype effects on activity of the amygdala during a neutral visual control task (MF) and during an emotional task (ME) as well as effects on emotional reactivity (ME – MF). We hypothesized greater amygdala responses in C/C genotype subjects. In addition, we performed exploratory analyses of potential diagnosis and HTR3A genotype effects on activity of other 5-HT3R-rich brain regions.
MF
During MF, IBS subjects relative to HCs had significantly greater activity in the left amygdala while C/C genotype subjects (IBS and HCs combined) had greater activity in bilateral amygdala (Figure 2) relative to T carriers (Table 2; p<.05, SVC). No significant interactions in amygdala responses were found. Exploratory analyses demonstrated significantly greater activity in the left caudate and hippocampus in IBS subjects relative to HCs and greater activity in the right hippocampus in C/C genotype subjects (IBS and HCs combined) relative to T carriers (Table 2; p<.05, SVC); however, only the left hippocampus remained formally significant with Bonferroni correction for multiple comparisons.
Figure 2.
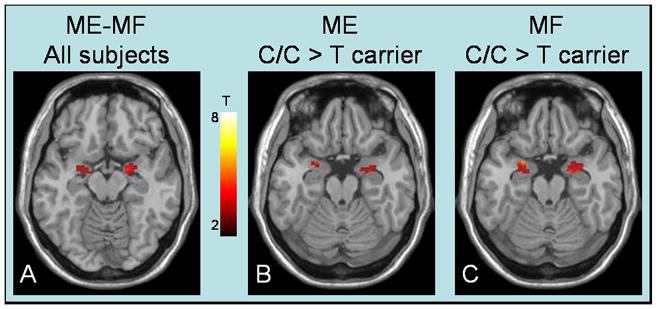
The emotional reactivity paradigm activated the amygdala (A). C/C genotype subjects, regardless of diagnosis, displayed increased amygdala responsiveness during the Match Emotions condition (B) and during the Match Forms condition (C).
ME
During ME, C/C genotype subjects (IBS and HCs combined) had greater activity in the right amygdala (Figure 2; Table 2; p<.05, SVC). No significant diagnosis effects or interactions in amygdala responses were found. Exploratory analyses demonstrated significantly greater activity in the left caudate and hippocampus of IBS subjects relative to HCs (Figure 2; Table 2; p<.05, SVC), however only the left hippocampus remained formally significant with Bonferroni correction for multiple comparisons.
ME – MF
Although group differences existed in amygdala responses during ME and MF considered individually (see above), no significant group differences or interactions in amygdala emotional reactivity were found. Exploratory analyses demonstrated that HCs relative to IBS patients had greater differential activity in the right insula during ME relative to MF condition (Table 2; p<.05, SVC). Also, T carriers relative to C/C genotype subjects (IBS and HCs combined) had greater differential activity in the left caudate and right hippocampus (Table 2; p<.05, SVC). However, none of these results remained formally significant with Bonferroni correction for multiple comparisons.
Parameter estimates for emotional reactivity (ME-MF) of the right amygdala were extracted for each subject using the Marsbar toolbox 39. As shown in Figure 3, right amygdala reactivity estimates varied greatly among IBS patients with C/C genotype. Bartlett’s test for homogeneity of variances demonstrated significant differences in variance (χ2(3) = 9.62, p=.022) such that IBS patients with C/C genotype had significantly more variance than IBS T carriers and HCs with C/C genotype (χ2(1) = 5.22, p=.022; χ2(1) = 6.58, p=.01, respectively). The greater variance appeared to be due to a subgroup of IBS patients with C/C genotype with unusually low differential activity during ME relative to MF. Therefore, additional analyses were performed to determine if this subgroup had higher amygdala responses during MF and/or lower amygdala responses during ME compared to other subjects with C/C genotype.
Figure 3.
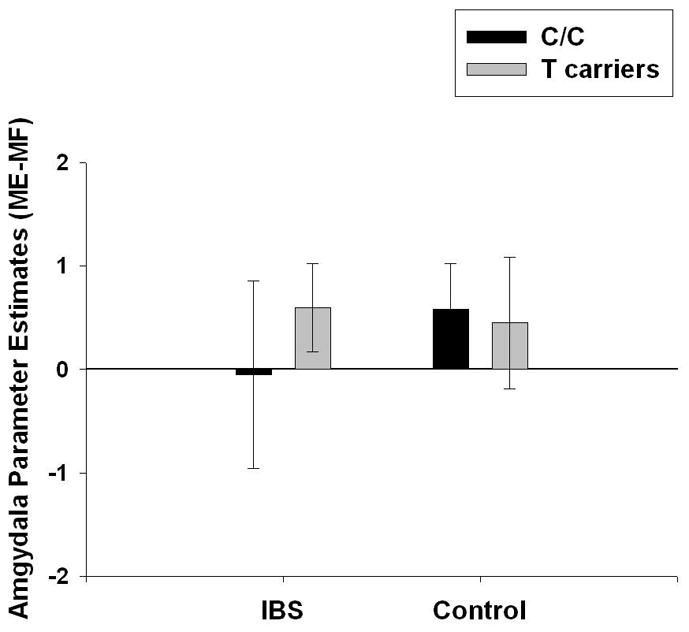
Mean and standard deviation for right amygdala emotional reactivity (defined as BetaME-MF) is plotted for healthy controls and IBS patients by HTR3A c.-42C>T genotype (C/C vs. T carrier). A subset of IBS subjects with C/C genotype had an unusual pattern of amygdala response.
C/C genotype median split
An ROI analysis using a median split was performed, comparing IBS patients with C/C genotype and below median reactivity (IBS L; n=8) with: (1) IBS patients with C/C genotype and above median reactivity (IBS H; n=8) and (2) HC with C/C genotype (n=15). Among subjects with C/C genotype, IBS subjects with low differential activity had significantly greater activity in the right amygdala during MF compared to both IBS and HCs with above median reactivity (Table 2). No significant differences were found during ME. Also, no significant differences between IBS patients with C/C genotype and above median reactivity and HC with C/C genotype were found.
DISCUSSION
We used a validated emotional reactivity paradigm to identify differences between IBS patients and HCs in emotional arousal-related brain responses. When contrasting the brain’s response to the negative emotional faces with the response to neutral geometric forms (ME-MF), consistent activations in amygdala, thalamus, lateral prefrontal and parietal cortices, and deactivations in mPFC, insula and posterior cingulate cortex were seen. C/C genotype, regardless of diagnosis, was associated with significantly greater anxiety and greater amygdala responses to emotional facial as well as neutral visual stimuli when brain responses during each condition were examined individually (ME-baseline, and MF-baseline). In patients, the C/C genotype was associated with higher IBS symptom ratings, and a subset of IBS patients with C/C genotype had even greater amygdala responses during the neutral stimulus (e.g. subjects with usually low differential activity during ME relative to MF), compared to other subjects with C/C genotype (e.g. IBS/HC with a normal pattern of responses). Thus, even though the CC genotype conferred altered amygdala responsiveness and associated anxiety ratings across both IBS and HCs, there was also an interaction of this genotype with IBS diagnosis.
C/C genotype across groups showed greater amygdala responses to both emotional and non-emotional stimuli, when compared to T carriers. This indiscriminate pattern of response suggests a generalized hyper-responsiveness of the amygdala in C/C genotype subjects. Indiscriminate or generalized increased amygdala responsiveness to emotional facial stimuli following acute experimental stress has been recently reported 40. The authors suggested that a shift of amygdala function occurred toward heightened sensitivity with lower levels of specificity, consistent with a state of hypervigilance under stress. Our findings suggest that the C/C genotype predisposes individuals to such indiscriminate hypervigilance. Consistent with this interpretation, Iidaka et al 28 showed increased amygdala and PFC responses to pictures of neutral faces (relative to pictures of houses) in HCs with C/C genotype compared to C/T genotype subjects. Despite a difference in paradigms, the current results are similar in that subjects with C/C genotype appear to be hyper-responsive to facial stimuli compared to T carriers. However, in the current study, subjects with C/C genotype were not specifically hyper-responsive to facial stimuli, since they showed heightened amygdala responses when processing both emotional faces and non-emotional geometric forms. In other words, they showed a generalized amygdala hyper-responsiveness regardless of the emotional content of the images. Both HCs and IBS with the C/C genotype may have increased sustained or tonic activation of the amygdala, consistent with their greater anxiety symptoms relative to T carrier subjects.
Further analyses revealed a subgroup of IBS patients with C/C genotype had unusually low differential amygdala activity during ME relative to MF (Figure 3). These subjects failed to discriminate between emotional and neutral stimuli due to heightened amygdala responses during the non-emotional condition. These findings suggest an interaction between IBS diagnosis and C/C genotype. It remains to be determined if the unusual response pattern of the amygdala to neutral and emotional stimuli represents an endophenotype 41 which is associated with the HTR3A SNP, or perhaps is related to increased stress perceived by some IBS patients during the experiment.
The T allele is related to an increase of HTR3A expression compared with the C allele 24, 26. Greater presynaptic expression of HTR3A on inhibitory GABAergic input to the amygdala is expected to result in greater GABAergic inhibition of the amygdala during serotonergic stimulation 42, 43. Increased inhibitory GABAergic function in the amygdala via benzodiazepines 44, micro-infusion of GABAA receptor agonists 45 or neuron-rich grafts 46 has been associated with decreased anxiety. These results suggest T carriers have enhanced amygdala 5-HT3A expression and GABAergic function associated with decreased anxiety. Both preclinical and clinical evidence supports a role for 5-HT3Rs in anxiety. For example, results from rodent studies have led to the consensus that 5-HT3R antagonists have anxiolytic effects by blocking limbic hyperactivity response 10–12. Clinical studies reported on beneficial effects of 5-HT3R antagonists in the treatment of anxiety 47. emotion-potentiated startle response 48. 5-HT3 receptor knockout mice have demonstrated sex differences in 5-HT3 regulation of depression- and anxiety-related behaviours 49. Future studies will be required to determine if sex differences exist in the association between C/C genotype and generalized amygdala responsiveness, anxiety, and IBS severity.
Limitations
IBS patient recruitment included all bowel habit types, and no relationship between bowel habit and genotype/amygdala response patterns was found in this relatively small sample (data not shown). Future studies with larger sample size for each bowel habit subtype, are needed to adequately examine the specificity of the relationship between HTR3A c.-42C>T polymorphism and amygdala responsiveness in terms of bowel habit.
Summary and Future Directions
Using a validated emotional reactivity paradigm, unrelated to the gastrointestinal system, we found significant correlations of the HTR3A c.-42C>T polymorphism with amygdala responsiveness, anxiety and IBS symptom severity in a relatively small sample. The study supports the important role of 5-HT3Rs in the modulation of emotional arousal circuits which have previously been shown to be related to 5-HT3R antagonist (Aolestron) mediated IBS symptom improvement 13. These findings suggest that while this gene polymorphism is not essential for a diagnosis of IBS, it influences symptom severity via greater engagement of amygdala-related emotional arousal circuits. This is consistent with previous reports showing an important role of anxiety in symptom severity in IBS 50 and in functional dyspepsia 51.
The differential responsiveness of the amygdala based on genotype could underlie differences in responsiveness of individual IBS patients to 5-HT3R antagonists, as well as other centrally directed therapies. For example, only about 40% of patients were identified as responders in clinical trials with the 5-HT3R antagonist alosetron 6. Alosetron’s effectiveness in reducing symptoms in non constipated IBS patients has been associated with reduced amygdala responsiveness during non-aversive and aversive conditions 13. The current study demonstrates an effect of HTR3A c.-42C>T polymorphism on amygdala reactivity to emotional and non-emotional stimuli. Additional research is needed to examine the relationship between emotional reactivity and visceral pain reactivity, and to directly examine the relationship between HTR3A c.-42C>T polymorphism, amygdala reactivity, and response to 5-HT3R antagonists. Patients with C/C genotype appear to have upregulated amygdala activity, thus they may be more resistant to treatment by 5-HT3R antagonists 52. In general, subtyping of IBS patients based on gene variants of central receptors modulating emotional arousal may improve the outcome of future clinical trials.
Acknowledgments
Funding Sources: NIH grants DK 64531, DK 48351, AT 00268
Footnotes
Conflicts of Interest: No conflicts of interest exist
Author Involvement:
LA Kilpatrick – statistical analysis; drafting of manuscript
JS Labus – study concept and design; interpretation of data
K Coveleskie – analysis of fMRI data
C Hammer – analysis of genetic data; manuscript revision for content
G Rappold – analysis of genetic data
K Tillisch – study concept and design; interpretation of data
JA Bueller – study concept and design; analysis of fMRI data
B Suyenobu – study concept and design; acquisition of data; analysis of fMRI data
JM Jarcho – study concept and design; manuscript revision for content
JA McRoberts – interpretation of data; manuscript revision for content
B Niesler – technical support; interpretation of data; manuscript revision for content
EA Mayer – interpretation of data; manuscript revision for content
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bradesi S, Mayer EA. Novel therapeutic approaches in IBS. Curr Opin Pharmacol. 2007;7:598–604. doi: 10.1016/j.coph.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammer C, Kapeller J, Endele M, Fischer C, Hebebrand J, Hinney A, Friedel S, Gratacos M, Estivill X, Fichter M, Fernandez-Aranda F, Ehrlich S, Rappold G, Niesler B. Functional variants of the serotonin receptor type 3A and B gene are associated with eating disorders. Pharmacogenet Genomics. 2009;19:790–9. doi: 10.1097/FPC.0b013e32833132b3. [DOI] [PubMed] [Google Scholar]
- 3.Goecke TW, Ekici AB, Niesler B, Loehberg CR, Hammer C, Rappold G, Schanze D, Straub V, Altmann HH, Strissel P, Strick R, Beckmann MW, Fasching PA. Two naturally occurring variants of the serotonin receptor gene HTR3C are associated with nausea in pregnancy. Acta Obstet Gynecol Scand. 89:7–14. doi: 10.3109/00016340903322727. [DOI] [PubMed] [Google Scholar]
- 4.Mayer EA, Bradesi S. Alosetron and irritable bowel syndrome. Expert Opin Pharmacother. 2003;4:2089–98. doi: 10.1517/14656566.4.11.2089. [DOI] [PubMed] [Google Scholar]
- 5.Johanson JF. Options for patients with irritable bowel syndrome: contrasting traditional and novel serotonergic therapies. Neurogastroenterol Motil. 2004;16:701–11. doi: 10.1111/j.1365-2982.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- 6.Chang L, Ameen VZ, Dukes GE, McSorley DJ, Carter EG, Mayer EA. A dose-ranging, phase II study of the efficacy and safety of alosetron in men with diarrhea-predominant IBS. Am J Gastroenterol. 2005;100:115–23. doi: 10.1111/j.1572-0241.2005.40365.x. [DOI] [PubMed] [Google Scholar]
- 7.Faerber L, Drechsler S, Ladenburger S, Gschaidmeier H, Fischer W. The neuronal 5-HT3 receptor network after 20 years of research--evolving concepts in management of pain and inflammation. Eur J Pharmacol. 2007;560:1–8. doi: 10.1016/j.ejphar.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 8.Rajkumar R, Mahesh R. The auspicious role of the 5-HT3 receptor in depression: a probable neuronal target? J Psychopharmacol. 2010;24:455–69. doi: 10.1177/0269881109348161. [DOI] [PubMed] [Google Scholar]
- 9.Aina Y, Susman JL. Understanding comorbidity with depression and anxiety disorders. J Am Osteopath Assoc. 2006;106:S9–14. [PubMed] [Google Scholar]
- 10.Griebel G. 5-Hydroxytryptamine-interacting drugs in animal models of anxiety disorders: more than 30 years of research. Pharmacol Ther. 1995;65:319–95. doi: 10.1016/0163-7258(95)98597-j. [DOI] [PubMed] [Google Scholar]
- 11.Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 12.Lecrubier Y, Puech AJ, Azcona A, Bailey PE, Lataste X. A randomized double-blind placebo-controlled study of tropisetron in the treatment of outpatients with generalized anxiety disorder. Psychopharmacology (Berl) 1993;112:129–33. doi: 10.1007/BF02247373. [DOI] [PubMed] [Google Scholar]
- 13.Berman SM, Chang L, Suyenobu B, Derbyshire SW, Stains J, Fitzgerald L, Mandelkern M, Hamm L, Vogt B, Naliboff BD, Mayer EA. Condition-specific deactivation of brain regions by 5-HT3 receptor antagonist Alosetron. Gastroenterology. 2002;123:969–77. doi: 10.1053/gast.2002.35990. [DOI] [PubMed] [Google Scholar]
- 14.Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41:1032–43. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilpatrick GJ, Jones BJ, Tyers MB. Identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature. 1987;330:746–8. doi: 10.1038/330746a0. [DOI] [PubMed] [Google Scholar]
- 16.Fozard JR. Neuronal 5-HT receptors in the periphery. Neuropharmacology. 1984;23:1473–86. doi: 10.1016/0028-3908(84)90091-1. [DOI] [PubMed] [Google Scholar]
- 17.Laporte AM, Koscielniak T, Ponchant M, Verge D, Hamon M, Gozlan H. Quantitative autoradiographic mapping of 5-HT3 receptors in the rat CNS using [125I]iodo-zacopride and [3H]zacopride as radioligands. Synapse. 1992;10:271–81. doi: 10.1002/syn.890100402. [DOI] [PubMed] [Google Scholar]
- 18.Tecott LH, Maricq AV, Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci U S A. 1993;90:1430–4. doi: 10.1073/pnas.90.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morales M, Battenberg E, Bloom FE. Distribution of neurons expressing immunoreactivity for the 5HT3 receptor subtype in the rat brain and spinal cord. J Comp Neurol. 1998;402:385–401. [PubMed] [Google Scholar]
- 20.Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432–7. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- 21.Yakel JL, Jackson MB. 5-HT3 receptors mediate rapid responses in cultured hippocampus and a clonal cell line. Neuron. 1988;1:615–21. doi: 10.1016/0896-6273(88)90111-0. [DOI] [PubMed] [Google Scholar]
- 22.Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Barnes NM, Hales TG, Lummis SC, Peters JA. The 5-HT3 receptor--the relationship between structure and function. Neuropharmacology. 2009;56:273–84. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapeller J, Houghton LA, Monnikes H, Walstab J, Moller D, Bonisch H, Burwinkel B, Autschbach F, Funke B, Lasitschka F, Gassler N, Fischer C, Whorwell PJ, Atkinson W, Fell C, Buchner KJ, Schmidtmann M, van der Voort I, Wisser AS, Berg T, Rappold G, Niesler B. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 2008;17:2967–77. doi: 10.1093/hmg/ddn195. [DOI] [PubMed] [Google Scholar]
- 25.Walstab J, Rappold G, Niesler B. 5-HT(3) receptors: role in disease and target of drugs. Pharmacol Ther. 2010;128:146–69. doi: 10.1016/j.pharmthera.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Niesler B, Weiss B, Fischer C, Nothen MM, Propping P, Bondy B, Rietschel M, Maier W, Albus M, Franzek E, Rappold GA. Serotonin receptor gene HTR3A variants in schizophrenic and bipolar affective patients. Pharmacogenetics. 2001;11:21–7. doi: 10.1097/00008571-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Melke J, Westberg L, Nilsson S, Landen M, Soderstrom H, Baghaei F, Rosmond R, Holm G, Bjorntorp P, Nilsson LG, Adolfsson R, Eriksson E. A polymorphism in the serotonin receptor 3A (HTR3A) gene and its association with harm avoidance in women. Arch Gen Psychiatry. 2003;60:1017–23. doi: 10.1001/archpsyc.60.10.1017. [DOI] [PubMed] [Google Scholar]
- 28.Iidaka T, Ozaki N, Matsumoto A, Nogawa J, Kinoshita Y, Suzuki T, Iwata N, Yamamoto Y, Okada T, Sadato N. A variant C178T in the regulatory region of the serotonin receptor gene HTR3A modulates neural activation in the human amygdala. J Neurosci. 2005;25:6460–6. doi: 10.1523/JNEUROSCI.5261-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: Effects of a neocortical network on the limbic system. NeuroReport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- 30.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl 2):II43–7. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds JD, Do TT, Hongo DB, Kuramoto IK, Biggs WH, III, Frech CK. Comparison of high density genotyping results from saliva and blood samples on Affymetrix GeneChip® GenomeWide SNP 6.0 arrays. In annual meeting of the American Society of Human Genetics; San Diego, CA. 2007. [Google Scholar]
- 32.Zigmond As SRP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 33.Ekman P, Friesen WV. Constants across cultures in the face and emotion. J Pers Soc Psychol. 1971;17:124–9. doi: 10.1037/h0030377. [DOI] [PubMed] [Google Scholar]
- 34.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 35.Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–5. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 36.Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC. Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5:238–42. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Payer DE, Lieberman MD, Monterosso JR, Xu J, Fong TW, London ED. Differences in cortical activity between methamphetamine-dependent and healthy individuals performing a facial affect matching task. Drug Alcohol Depend. 2008;93:93–102. doi: 10.1016/j.drugalcdep.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Eighth International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- 40.van Marle HJ, Hermans EJ, Qin S, Fernandez G. From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry. 2009;66:649–55. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Labus JS, Tillisch K, Coveleskie K, Kilpatrick LA, Bueller JA, Smith S, Naliboff BD, Mayer EA. Functional neuroimaging paradigm identifies amygdala responsiveness as potential neurobiological endophenotype in IBS. Joint International Neurogastroenterology and Motility Meeting; Boston, Massachusetts. 2010. [Google Scholar]
- 42.Turner TJ, Mokler DJ, Luebke JI. Calcium influx through presynaptic 5-HT3 receptors facilitates GABA release in the hippocampus: in vitro slice and synaptosome studies. Neuroscience. 2004;129:703–18. doi: 10.1016/j.neuroscience.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 43.Koyama S, Matsumoto N, Kubo C, Akaike N. Presynaptic 5-HT3 receptor-mediated modulation of synaptic GABA release in the mechanically dissociated rat amygdala neurons. J Physiol. 2000;529(Pt 2):373–83. doi: 10.1111/j.1469-7793.2000.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kajimura N, Nishikawa M, Uchiyama M, Kato M, Watanabe T, Nakajima T, Hori T, Nakabayashi T, Sekimoto M, Ogawa K, Takano H, Imabayashi E, Hiroki M, Onishi T, Uema T, Takayama Y, Matsuda H, Okawa M, Takahashi K. Deactivation by benzodiazepine of the basal forebrain and amygdala in normal humans during sleep: a placebo-controlled [15O]H2O PET study. Am J Psychiatry. 2004;161:748–51. doi: 10.1176/appi.ajp.161.4.748. [DOI] [PubMed] [Google Scholar]
- 45.Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52:701–6. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- 46.Cunningham MG, Connor CM, Carlezon WA, Jr, Meloni E. Amygdalar GABAergic-rich neural grafts attenuate anxiety-like behavior in rats. Behav Brain Res. 2009;205:146–53. doi: 10.1016/j.bbr.2009.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dolnak DR. Treating patients for comorbid depression, anxiety disorders, and somatic illnesses. J Am Osteopath Assoc. 2006;106:S1–8. [PubMed] [Google Scholar]
- 48.Harmer CJ, Reid CB, Ray MK, Goodwin GM, Cowen PJ. 5HT(3) antagonism abolishes the emotion potentiated startle effect in humans. Psychopharmacology (Berl) 2006;186:18–24. doi: 10.1007/s00213-006-0337-z. [DOI] [PubMed] [Google Scholar]
- 49.Bhatnagar S, Nowak N, Babich L, Bok L. Deletion of the 5-HT3 receptor differentially affects behavior of males and females in the Porsolt forced swim and defensive withdrawal tests. Behav Brain Res. 2004;153:527–35. doi: 10.1016/j.bbr.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 50.Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, Naliboff BD. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 51.Van Oudenhove L, Vandenberghe J, Geeraerts B, Vos R, Persoons P, Demyttenaere K, Fischler B, Tack J. Relationship between anxiety and gastric sensorimotor function in functional dyspepsia. Psychosom Med. 2007;69:455–63. doi: 10.1097/PSY.0b013e3180600a4a. [DOI] [PubMed] [Google Scholar]
- 52.Jarcho JM, Chang L, Berman M, Suyenobu B, Naliboff BD, Lieberman MD, Ameen VZ, Mandelkern MA, Mayer EA. Neural and psychological predictors of treatment response in irritable bowel syndrome patients with a 5-HT3 receptor antagonist: a pilot study. Aliment Pharmacol Ther. 2008;28:344–52. doi: 10.1111/j.1365-2036.2008.03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


