Synopsis
A growing literature shows the pervasiveness and importance of comorbidity in youth with bipolar disorder (BPD). For instance, up to 90% of youth with BPD have been described to manifest comorbidity with attention deficit hyperactivity disorder. Multiple anxiety, substance use, and disruptive behavior disorders are the other most commonly reported comorbidities with BPD. Moreover, important recent data highlights the importance of obsessive compulsive and pervasive developmental illness in the context of BPD. Data suggests that not only special developmental relationships are operant in context to comorbidity, but also that the presence of comorbid disorders with BPD results in a more severe clinical condition. Moreover, the presence of comorbidity has therapeutic implications for the treatment response for both BPD and the associated comorbid disorder. Future longitudinal studies to address the relationship and the impact of comorbid disorders on course and therapeutic response over time are required in youth with BPD.
Keywords: bipolar disorder, comorbidity, treatment
Pediatric onset bipolar disorder (BPD) seldom occurs in the absence of comorbid conditions. The co-occurrence of additional disorders complicates both the accurate diagnosis of BPD and its treatment. BPD is one of the most debilitating psychiatric disorders, estimated to cost Americans 45 billion dollars per year. It is not so infrequent as previously reported, with rates of bipolar spectrum disorder reaching an estimated 4%. Youth with BPD are amongst the most impaired population and the presence of comorbidity compounds disability, complicates treatment, and appears to worsen the prognosis in this population. Comorbid disorders may have a significant impact on various indices of BPD correlates. Knowledge of their comorbid presence with BPD could be informative in determining course, prognosis, and functional and therapeutic outcomes. Early identification and appropriate management may lead to improved functioning, prevention of impending emergence of comorbid disorders (Oppositional defiant disorder/Conduct disorder [ODD/CD], Substance use disorders [SUD]), and attenuation of the untreated course of BPD27,163. On the other hand, if comorbidity is not appropriately acknowledged then misattribution of impairing symptoms could lead to inappropriate therapeutic interventions, unnecessary exposure to neuroleptics, worsening of symptoms, delayed diagnosis, and misuse of mental health resources.
Recognition of comorbidity is important as it has therapeutic implications such as 1) increased risk of mood destabilization that is inherent to the therapeutic options for the comorbidity, as is the case with anti-anxiety, antidepressant, or anti-ADHD medications that have manicogenic potential, 2) atypical response (efficacy and tolerability) to psychotropics associated with certain disorders like Pervasive developmental disorders (PDD), or 3) less than expected anti-manic response to thymoleptic agents in the presence of certain comorbid disorders (for instance Attention deficit hyperactivity disorder [ADHD], Obsessive – Compulsive disorder [OCD]).
Comorbid disorders may be challenging to diagnose due to overlapping symptoms and developmentally sensitive complicated patterns of symptom development. Several methods have been applied to scientifically understand comorbidity. Structured diagnostic interviews (for instance, Schedule for Affective Disorders and Schizophrenia-Epidemiological Fifth Version [K-SADS-E]6 are helpful in clinically parsing out the comorbid conditions as they comprehensively assesses the spectrum of psychopathologies described in DSM including past and present severity of symptoms. Diagnoses are considered positive only if the diagnostic criteria are met to a degree that would be considered clinically meaningful. “Clinically meaningful” means that the data collected from the structured interview indicated that the diagnosis should be a clinical concern due to the nature of the symptoms, the associated impairment, and the coherence of the clinical picture. For a given disorder the overlapping non-specific symptoms are considered for the diagnosis if the respective cardinal symptoms are present and the disorder is cause for significant impairment. Furthermore, although DSM criteria do not permit comorbid presence of certain disorders and assigns diagnoses based on hierarchy, in order to fully characterize the clinical picture a non-hierarchical diagnostic approach is taken to assess for comorbid disorders. Thus the approach taken by structured interview objectively and comprehensively documents symptom presentation and minimizes diagnostic biases.
Perhaps the most compelling scientific method to examine comorbidity is familial risk analysis which addresses uncertainties regarding complex phenotypes in probands by examining the transmission of comorbid disorders in families55,56. Therapeutic response has also provided evidence of the existence of separate conditions. For instance, in a review of clinical records in manic children, Biederman et al. (1998) reported that whereas mood stabilizers significantly improved mania-like symptoms, antidepressants and stimulants did not and conversely, tricyclic antidepressants and not mood stabilizers were associated with improvement of ADHD symptoms14. Finally, attributes of comorbidity can also be addressed by applying neurobiological probes to seek the existence of underlying changes commensurate with each comorbid disorder, either disorder, or neither disorder indicating a unique subtype with distinct neurobiological attributes. The emerging proton magnetic resonance spectroscopic (1HMRS) imaging intervention research in youth with BPD is suggesting a profile of cerebral metabolites in a specific region of the brain which may facilitate the understanding of neurochemical correlates of BPD in the context of comorbidity. For instance, the 1HMRS profile of cerebral metabolites in the anterior cingulate cortex region of the brain in children with ADHD appears to have a significantly higher ratio of glutamate plus glutamine to myo-inositol-containing compounds than does the profile of children with comorbid BPD and ADHD129.
Present studies addressing comorbidity generally rely either on cross-sectional observations or on recall of disorders over the whole life course. Both of these approaches pose limitations. Longitudinal studies are required that offer the best possibility for observing the developmental progression of the emergence of comorbid conditions.
Treatment guidelines for pediatric BPD indicate that the treatment plan must include treatment for each comorbid disorder, which may become a complex process of trial and error to find the most effective combination of medications105. These guidelines further recommend that in the absence of treatment trials specifically studying a population of children with BPD and specific comorbid disorders, clinicians should use psychopharmacological and psychosocial treatments which are generally recommended for each comorbid disorder when that disorder occurs as the primary problem. Though certain comorbid disorders associated with BPD respond to anti-manic agents (disruptive behavior disorders [DBD], PDD), there are frequently co-occurring disorders (ADHD, anxiety disorders, depression) with typical onset prior to the emergence of mania that require treatment with agents that have manicogenic potential. Available empirical evidence and clinical acumen dictates that treatment of comorbid conditions can be addressed only after the symptoms of BPD are stabilized181. Decision to treat the comorbid disorders following stabilization of mania should be guided by clinically determining the level of impairment associated with the disorder. As a rule, medications with lower manicogenic potential are preferred in this population.
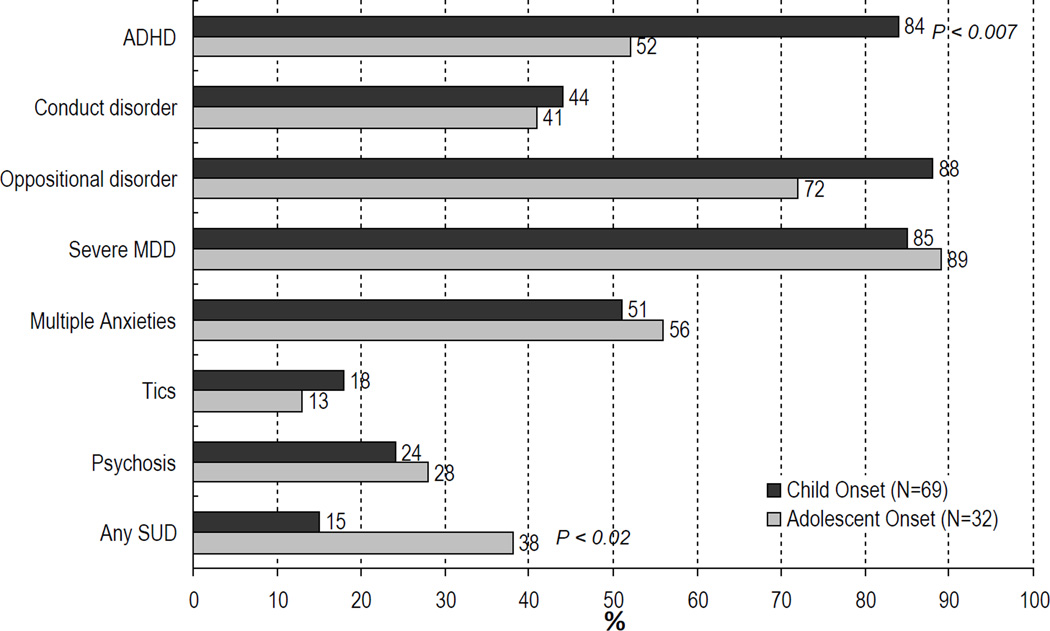
Comorbid conditions frequently associated with pediatric onset BPD include ADHD, disruptive behavior disorders, substance use disorders, anxiety disorders (including panic disorder, posttraumatic stress disorder, obsessive-compulsive disorder), and pervasive developmental disorders (Figure-1). In the following sections we will address the characteristics and management of frequently occurring comorbid disorders in children and adolescents with BPD.
Figure-1.
Rates of Psychiatric Comorbidity in Bipolar Youth Stratified by Age at Onset of BPD
ATTENTION DEFICIT HYPERACTIVITY DISORDER
Systematic studies of pediatric populations with BPD show that the rates of comorbid ADHD range from 60 to 90%71,168,171. While a high prevalence of ADHD is reported in youth with BPD, a modest rate (22%) of comorbid BPD is reported in pediatric populations with ADHD12. Although the rates of ADHD in youth with BPD are universally high, the age at onset modifies the risk for comorbid ADHD. ADHD comorbidity is more often associated with early onset BPD (< 18 years)34,147. Rates of ADHD in adolescents with BPD are reported to be greater in childhood onset BPD (• 12 years) than in adolescent onset BPD (> 13 years)18,54. Sachs et al. (2000) reported that, among adults with BPD, a history of comorbid ADHD was only evident in those subjects with onset of BPD before the age 19 years.
Although the adult literature on the subject has been less extensive, similar findings have been documented. The National Comorbidity Survey Replication epidemiological study documented significantly higher rates of BPD in the adult population with ADHD compared with adults without ADHD (19.4% vs. 3.1%)96. Consistent with the documented association of comorbid ADHD almost exclusively with early-onset BPD, a relatively low lifetime prevalence (9.5%) of comorbid ADHD is reported in an adult research population with BPD133. Similarly, Winokur et al. (1993) reported childhood hyperactivity in 21.3% of their adult BPD population (N=189) and in 19% of their first-degree adult relatives with BPD. ADHD comorbidity with BPD is over-represented in males and in its presence, BPD adults had the onset of their mood disorder approximately 5 years earlier, had shorter periods of euthymia, were more frequently depressed, and had a greater burden of additional comorbid psychiatric disorders, especially anxiety disorders and SUD133.
In an analysis of prepubertal children with BPD and ADHD, Wozniak et al. (1995) demonstrated correlates of both conditions. Affected children had high rates of major depression, psychosis, multiple anxiety disorders, impaired psychosocial function, and hospitalization, consistent with BPD. Similarly, phenotypic features of ADHD and associated neuropsychiatric correlates (that is, high rates of learning disabilities and need for educational services) have striking homology to the presentation of ADHD in the context of comorbidity with BPD, suggesting that ADHD may be a bona fide disorder when comorbid with BPD14. As ADHD and mania diagnostically share nonspecific symptoms (distractibility, motoric hyperactivity, and talkativeness), there is a risk of unintentional over-diagnosis. Several studies have addressed this issue128. Biederman et al. (1996) showed that the majority of children with the combined condition continued to meet criteria of both mania and ADHD after removing overlapping symptoms suggesting that BPD and ADHD comorbidity is not a methodologic artifact due to shared diagnostic criteria. Although limited information is available regarding the potential for different rates of comorbidity with BPD among the DSM-IV subtypes of ADHD, the rates of BPD are reported to be highest among youth with combined-type ADHD (26.5%), but also elevated among hyperactive-impulsive (14.3%) and inattentive (8.7%) youth51. Wilens and his colleagues (2003) examined a research population of adults with ADHD where nearly half the population also met the criteria for BPD (47%); the vast majority were BPD-II (88%). Although ADHD adults with comorbid BPD shared the prototypic characteristics of both the disorders, they had higher rates of combined type ADHD, with a greater number of DSM-IV ADHD symptoms (especially attentional symptoms), a higher prevalence of anxiety disorders, and poorer global functioning171.
While the mechanisms that mediate the association between BPD and ADHD are not entirely clear, other data suggest that subforms of these disorders share genes in common50. Children of bipolar parents have an elevated risk for ADHD and relatives of ADHD children have an increased risk for BPD52. The transmission of these disorders has been studied in families ascertained through pediatric BPD patients, ADHD boys52 and ADHD girls53. In each of these reports, the pattern of transmission supported the hypothesis that early onset BPD may be a developmental subtype of BPD.
In BPD youth with ADHD whose mood is well stabilized, ADHD symptoms often become the second most severe presenting complaint181. If the symptoms of inattentiveness, distractibility, talkativeness, and impulsivity are not recognized as comorbid ADHD then they may be inappropriately treated as residual symptoms of mania. Conversely, identification of mania in youth with ADHD has therapeutic implications as failure to recognize comorbidity could lead to administration of anti-ADHD medications that could exacerbate the mania.
The response to lithium has been reported to be less robust in the presence of ADHD comorbidity in youth with BPD154,161 suggesting that this subgroup of BPD may constitute a unique genetic subform with a differential treatment response. Chart review evaluating treatment outcome of youth with BPD treated over a 4-year period showed that mood stabilizers selectively improved manic symptoms, whereas stimulants had no effect10. Furthermore, in youngsters with BPD comorbid ADHD could be addressed selectively with the anti-ADHD armamentarium, but only after mood stabilization.
The stimulants have been reported to be efficacious in treating comorbid ADHD without precipitating (hypo)mania in mood stabilized BPD youth in two controlled trials. A controlled trial of stimulants as an adjunctive therapy for ADHD in BPD youth with manic symptoms stabilized on divalproex found mixed amphetamine salts to be safe and efficacious for the treatment of ADHD in the context of BPD149. More recently, Findling et al. (2007) reported that in youth stabilized with a stable dose of at least one mood stabilizer, concomitant treatment with methylphenidate improved ADHD in a dose dependent manner without destabilization of mood. Furthermore, in an open trial of the non-stimulant anti-ADHD agent bupropion in adults with predominately mood stabilized bipolar II disorder and ADHD, we previously reported a significant improvement in ADHD without activation of mania174. These aggregate data suggest that treatment for BPD needs to precede ADHD treatment; and that in general, stimulants and non-stimulants may be cautiously introduced.
OPPOSITIONAL DEFIANT DISORDER
High rates with bidirectional overlap of comorbid ODD and BPD are reported by various studies. Rates of ODD in the BPD population range from 47% – 88%61,72 and conversely, 20% of children with ODD are reported to have comorbid BPD76. A recent meta-analysis reported ODD as the second most common comorbidity after ADHD, with a weighted rate of 53% among samples of children and adolescents with BPD105.
The diagnosis of ODD in the context of BPD is challenging as nosologically ODD shares overlapping symptoms with mania without any symptom specific to ODD that could diagnostically differentiate it from mania. Because ODD is so frequently comorbid with pediatric BPD, the understanding of the relationship of ODD with BPD ranges from ODD being a secondary disorder as a consequence of bipolar illness or being a prodrome or early manifestation of BPD, to ODD representing a “true independent” comorbid psychopathological phenomenon. However, many children with disruptive behavior disorders do not go on to develop BPD12, suggesting that different forms of disruptive behavior disorders may exist, one that could be prodromal to BPD and another form that is not. More work is needed to further evaluate this issue.
A clinical inquiry summarized 8 reviews on ODD treatments of children and found improved behavior with a 20– 30% decrease in disruptive or aggressive behaviors with parenting interventions and behavioral therapy including cognitive-behavioral therapy (CBT), social problem-solving skills training, and parent management training involving the child and / or parent for 12–25 sessions57.
Though treatment of ODD is primarily behavioral in nature, when comorbid with other medication-responsive psychiatric conditions (BPD, ADHD), pharmacological treatment of the comorbid disorder often reduces overall symptoms of the ODD. While there is currently no data available on the treatment of ODD in the context of BPD comorbidity, an emerging body of literature points to the role of thymoleptic agents in the treatment of DBD (CD, ODD, and DBD-NOS) including ODD in youth with significant aggression. Evidence suggests that pharmacotherapy may be effective in youth with DBD, especially those experiencing problematic aggression, but response of the DBD per se to these thymoleptics is understudied. Multiple studies have examined the safety and efficacy of atypical antipsychotics (risperidone, olanzapine, quetiapine, and aripiprazole) in treating aggression in children with DBD. Atypical antipsychotics have generally been significantly more efficacious than placebo in treating aggression in DBD youth.
To date, risperidone is the most extensively studied atypical antipsychotic for DBD. Several trials indicate that risperidone can be useful for DBD, especially the aggressive features, in both short term and long term use5,40,60,167. Short- and long-term efficacy and tolerability of risperidone as pharmacotherapy for DBDs in children with sub-average intelligence has been demonstrated in over 1,300 children and adolescents in the literature5,40,60,143,152,167. In two short-term (6-week) controlled trials Aman and colleagues (2005) studied the role of low dose risperidone (mean dose 1.16 and 0.98 mg/day) in borderline intellectual functioning ((IQ of 36–84) DBD youth (N=223; ages 5–12) and reported an acceptable tolerability profile with significant improvement in aggression and behaviors associated with DBD5,152. As the five long-term (1–3 year) follow-up trials suggest, a low dose of risperidone (mean dose ranged from 1.38–1.92 mg/day) was equally well tolerated and effective in controlling the DBD and aggressive behaviors in this population40,60,143,144,167. Low dose risperidone (0.02 mg/kg/day) is also reported to be well tolerated and efficacious in treating DBD behaviors in youth with normal intelligence as suggested by short and long-termtrials79,143.
However, a post-hoc analysis of the data from the controlled trial of risperidone in DBD conducted by Aman and colleagues5 examined 24 candidate affective symptoms extracted from the 64 item Nisonger Child Behavior Rating Form15. These symptoms reflected the bipolar symptoms of explosive irritability, agitation, expansiveness, grandiosity, and depression. Risperidone was also effective in treating these putative symptoms of mania. This analysis raises the question of whether studies which examine the effects of anti-manic agents on DBD may include subjects with comorbid bipolar spectrum illness, and, further, whether the improvement in DBD is a function in part of the improvement in BPD.
Quetiapine is the other most studied atypical antipsychotic for DBD in youth. In youth with DBD and ADHD who fail to respond to OROS methylphenidate monotherapy (at 54 mg/day dose), the addition of quetiapine (at a mean dose 329 mg/day) has been shown to be effective in controlling symptoms of ODD and aggression107. Open-label and placebo-controlled studies suggest that divalproex is efficacious for the treatment of mood lability and explosive temper in children and adolescents with DBD46,47. Further prospective studies addressing the course and treatment of ODD when comorbid with BPD are warranted.
CONDUCT DISORDER
In a comprehensive literature review, Geller70 concluded that: “Available data strongly suggest that prepubertal-onset BPD is a non-episodic, chronic, rapid-cycling, mixed manic state that may be comorbid with attention-deficit hyperactivity disorder (ADHD) and conduct disorder (CD) or have features of ADHD and/or CD as initial manifestations.” This observation is supported by a body of research documenting a bi-directional overlap between CD and BPD in children. As both CD and BPD are highly impairing conditions, their co-occurrence heralds a particularly severe clinical picture and raises important clinical questions. From a diagnostic standpoint, the question remains as to whether antisocial behaviors in a child with BPD such as stealing, lying or vandalizing, should be attributed to the disinhibition of mania with its attendant impulsivity, irritability and grandiosity or to comorbid CD. From a treatment standpoint in such a child, the question further remains as to whether the symptoms of CD will diminish when the symptoms of BPD are adequately treated.
The association between CD and mania is consistent with the well documented comorbidity between CD and major depression7 and the frequently bipolar nature of juvenile depression69,160. Moreover, pediatric onset BPD is frequently mixed (dysphoric) and commonly associated with “affective storms,” with prolonged and aggressive temper outbursts30,41. These irritable outbursts often include threatening or attacking behavior towards family members, children, adults, and teachers, behaviors that overlap with CD. For example, McGlashan et al.126 reported that juvenile onset BPD may be particularly explosive and disorganized and that children with mania tended to have more trouble with the law and more “psychotic assaultiveness” than adults with BPD. Kovacs104 reported that some youngsters with mania showed serious acting out behaviors including burglary, stealing, vandalism and a history of school suspensions. Although these aberrant behaviors are consistent with the diagnosis of CD, they may be due to the behavioral disinhibition that characterizes BPD. Thus, it is not surprising that youth with BPD frequently meet diagnostic criteria for CD. High rates of CD (69%) are reported in youth with BPD104 and the comorbid presence of BPD and CD in youth heralds a more complicated course with high rates of hospitalization (42%)108. Furthermore, CD is reported to be severe in the presence of comorbid BPD130.
Epidemiologic studies report high rates of comorbidity between BPD and DBD111,146. There seems to be an increase in the risk for BPD with a higher number of CD symptoms146 and a nearly 7-fold increase in the risk for BPD in individuals with antisocial personality disorder22. The risk of CD is three fold higher in younger bipolar individuals (< 30 years) with comorbid SUD than those without SUD (52% vs. 14.8%)29.
Comorbid CD in BPD youth might confuse the clinical presentation of childhood BPD and possibly account for some of the documented failure to detect bipolarity in children. Isaac86 examined a group of adolescents found to be the most problematic, crisis prone, and treatment resistant in a special educational day school and treatment program. These authors found that two-thirds of these youngsters satisfied DSM-III-R criteria for BPD, which had often been misdiagnosed as ADHD and CD. Most of the remaining youngsters showed significant bipolar features but did not fully satisfy DSM-III-R criteria for BPD. Considering the heterogeneity of BPD and that of CD, these findings may have important implications in helping to identify a subtype of BPD with early onset characterized by high levels of comorbid CD104 and a subtype of CD with high levels of dysphoria and explosiveness.
In a large, well characterized, prospective sample of children referred with ADHD9, ADHD children with comorbid BPD and CD reported higher familial and personal risk for mood disorders than youth with ADHD and CD alone, who were found to have higher personal risk for antisocial personality disorder. This suggests that the presence of BPD in some CD children could be clinically meaningful, at least in the context of ADHD. Further analysis of structured interview-derived data from a large sample of consecutive, clinic-referred children and adolescents, showed again a large and symmetrical overlap between BPD and CD10. Examination of the clinical features, patterns of psychiatric comorbidity and functioning in multiple domains showed that children with CD and BPD had similar features of each disorder irrespective of comorbidity with the other disorder. These findings further supported the hypothesis that children satisfying diagnostic criteria for BPD and CD suffer from both disorders, rather than one being misdiagnosed as the other, even outside the context of comorbid ADHD. These authors also documented that psychiatric hospitalizations among CD probands were almost entirely accounted for by those with comorbid BPD. This finding is consistent with the notion that CD plus BPD probands, along with other symptoms of CD, engage in a disorganized type of aggression associated with BPD. Since many children in psychiatric hospitals with the diagnosis of CD commonly have a profile of severe aggressiveness, it is likely that these children required psychiatric hospitalizations because of the manic picture and not necessarily due to the CD.
Further evidence that a subtype of CD linked to BPD could be identified derives from pilot familial risk analyses13,183. These results suggest that relatives of BPD probands were at an increased risk for BPD but not CD. On the other hand, relatives of CD probands had an increased risk for CD but not for BPD, while relatives of CD plus BPD probands had an elevated risk for both disorders183. Among relatives in this latter group, BPD and antisocial disorders showed significant cosegregation, i.e., relatives with one disorder were highly likely to have the other. As a result of this cosegregation, CD plus BPD was significantly elevated among relatives of CD plus BPD probands but was rare among the relatives of the other proband groups. Probands with the combined condition of CD and BPD also had high rates of non-BPD conduct/antisocial disorders among the relatives, suggesting a genetic loading with two sub-types of CD: with and without BPD. These results provide compelling evidence that subtypes of CD and of BPD can be identified based on patterns of comorbidity with the other disorder suggesting that, their cooccurrence may correspond to a distinct familial syndrome13,51–53,183.
The delineation of a subgroup of manic CD children would have important clinical implications. It could lead to improvement in our efforts to ameliorate the guarded outcome of some CD youth. Since BPD may respond to specific pharmacological treatments, correctly identifying those CD children with BPD may afford the opportunity to introduce these medications in the treatment of antisocial and aggressive youth.
There is limited evidence from various trials of atypical antipsychotics in youth with BPD on the possible role of thymoleptics in managing CD when comorbid with BPD. In our open-label short-term (8-week) trials of risperidone (N=30) and ziprasidone (N=21) in children and adolescents aged 6–17 years with BPD, these atypical antipsychotics were associated not only with significant improvement in symptoms of pediatric BPD, but also with improvement in the severity of comorbid CD (a CGI rating of much or very much improved) in the subset of BPD youth with comorbid CD16,17. Likewise, a similar response of comorbid CD to open-label short-term trial (8-week) of risperidone (N=16) and olanzapine (N=15) is recorded in a younger population of preschool-age children (4–6 years) with BPD17. The promising role of atypical antipsychotics in treating comorbid CD in youth with BPD as suggested by these open trials requires further validation by conducting controlled studies that apply specific measures to assess the response of CD.
As discussed earlier under the role of thymoleptic agents in the DBD population, potential pharmacotherapy for CD with marked aggression includes mood stabilizers, typical and atypical anti-psychotics - the very medications most commonly recommended for the treatment of BPD. The anti-manic agent lithium has been found to be an effective anti-aggressive agent in youth with CD as reported by various studies conducted in ambulatory and hospitalized youth with CD27,151. A small literature suggests that typical antipsychotic medications such as haloperidol and molindone are helpful in decreasing aggression in youth with CD26,28,77. However, typical antipsychotic treatment was associated with range of problematic adverse effects. This led to the use of the atypical antipsychotic agents in treating CD with aggressive features.
In an open-label short-term (8-week; N=17) followed by long-term (18-week; N=9) trial of quetiapine (at median dose 150 mg/day) in children aged 6–12 years with the primary diagnosis of CD, quetiapine was found to be effective in treating aggression and conduct problems by week 8; the benefit was sustained during long-term treatment with quetiapine in children who responded to an acute therapeutic trial of quetiapine63,64. In this trial, no subject developed extra-pyramidal symptoms or discontinued the trial due to adverse events, suggesting that short and long-term treatment with quetiapine was safe and well tolerated. More recently, in a controlled trial, quetiapine 294 (±78) mg/day was reported to be superior to placebo in treating adolescents with CD (N=9/10) on clinician-assessed measures of global severity but failed to separate from placebo on parent-assessed specific measures of aggression and conduct behaviors; this discrepancy could be attributed to small sample size, leading to diminished statistical power to detect differences38.
There is preliminary evidence on the role of olanzapine in treating aggression with CD from a retrospective chart review of adolescents with CD (N=23) who were treated with olanzapine (mean dose of 8±3.2 mg/day) for an extended period of time (6–12 months)120. Treatment with olanzapine resulted in improvement of aggression but was also associated with a modest weight gain (4.6±3 Kg). These aforementioned empirical evidence from clinical trials exclusively conducted in CD populations in addition to the previous discussion of trials conducted in DBD populations strongly suggest the role of atypical antipsychotics in managing behaviors related to CD and aggression.
SUBSTANCE USE DISORDERS
In recent years, a focus on mood disorders in SUD youth has emerged as a major clinical and public health concern, particularly given the implications for reduction of SUD, delinquency, and mood symptoms with treatment145. In epidemiological and clinically based studies, SUD is one of the most common comorbidities found in BPD in adolescents and adults125,159,179. McElroy et al. reported that drug and alcohol use disorders were found in 39% and 32% of BPD adults, respectively. In terms of linking SUD to early-onset adult BPD, Dunner and Feiman48 showed that BPD onset prior to adulthood was strongly and specifically related to SUD development in young adults. Similarly, McElroy and colleagues showed a retrospective association between early onset BPD, mixed symptoms and comorbidity, and SUD.
A smaller but growing literature suggests that juvenile-onset BPD is a risk factor for SUD177. An excess of SUD has been reported in the literature in adolescents with BPD or prominent mood lability and dyscontrol and BPD is over-represented in youth with SUD19,169,170. A high prevalence of SUD (40%) is reported in an inpatient adolescent population with BPD169. In a five-year follow-up study of 54 inpatients adolescent with BPD, Strober et al. reported an increase in the rates of SUD from 10% at baseline (mean age 16 yrs) to 22% at follow-up and described a mixed presentation with highly relapsing course in the presence of comorbidity with SUD162.
We previously documented that adolescents with SUD referred to a psychiatric ambulatory care clinic were more likely than those without SUD to have comorbid BPD170. Preliminary prospective findings from our longitudinal study of ADHD youth signaled that youth with early-onset BPD, independent of ADHD, were reported to be at risk for SUD19 and were also found to be at higher risk for early initiation and higher rates of cigarette smoking172. Similarly, clinically referred adolescents with BPD are at heightened risk for the development of SUD, independent of the status of CD, as compared to non-BPD psychiatric adolescents176.
More recently, we reported the baseline findings of an ongoing, controlled longitudinal family-based study of SUD in BPD adolescents175 where we evaluated specifically the relationship between SUD and the age at onset of BPD (child vs. adolescent onset) and the presence of comorbid CD. Participating youth (N=105 BPD and 98 non-mood disordered controls; mean age of 13.6 +/− 2.5 years) did not differ by group (BPD vs. controls) for any clinical characteristics or demographics. Nicotine dependence was found in 23% of BPD and 4% of controls whereas full SUD was found in 34% of BPD youth compared to 4% of controls. BPD youth with SUD had higher rates of additional comorbidities and poorer overall functioning than youth with BPD without SUD and controls. Comorbidity with ADHD, conduct, or anxiety disorders did not account for the high risk of SUD in BPD175.
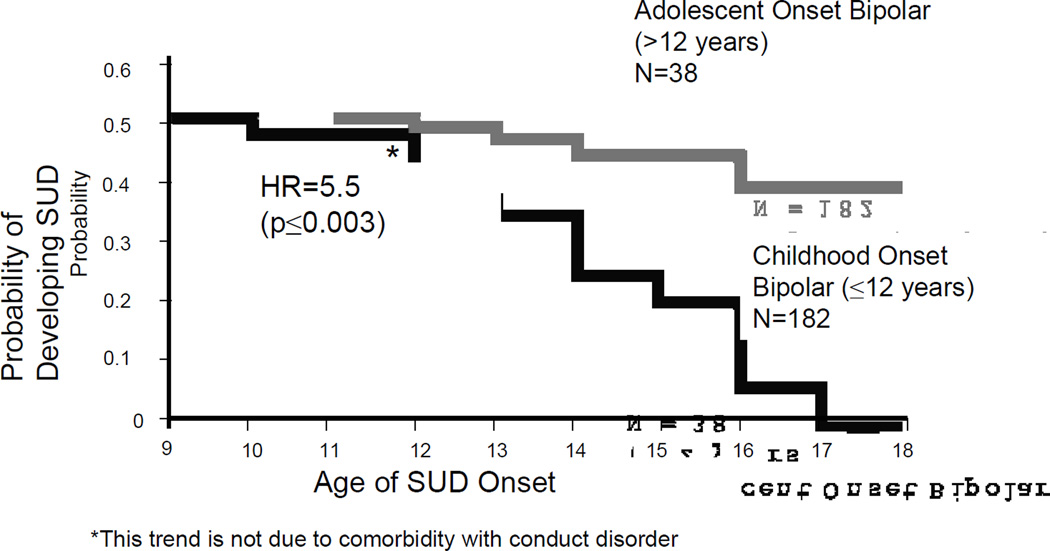
We previously reported that the onset of BPD in adolescence was more pernicious in terms of SUD onset than if the BPD began prepubertally176 (Figure-3). We further evaluated the relationship between the developmental effect of BPD onset and the onset of SUD. Interestingly, we replicated our previous findings in that youth with the onset of their BPD in adolescence were at higher risk for cigarette smoking and SUD compared to those with the onset prepubertally175.
Figure-3.
Development of Substance Use Disorders in Child & Adolescent-Onset BPD Adolescent Onset Bipolar (>12 years) N=38
Because CD is an important comorbidity of both BPD and predictor of SUD, we very recently examined the contribution of CD on later development of SUD. We found that while CD increases the risk for SUD in BPD, the risk of SUD in BPD was not substantially changed with or without CD173. Thus we can conclude that juvenile BPD is a risk factor for SUD irrespective of the status of CD, ADHD, or other comorbid psychopathology. Youth with the onset of their BPD during adolescence were at particularly high risk for SUD and the severity and course of both the disorders is worse when comorbid.
Mechanism of SUD Risk in BPD
The reasons that juvenile BPD is a risk factor for SUD in particular, and that adolescent- vs. child-onset BPD confer a differential risk for SUD remain unclear. Given the prominent genetic influences in both BPD and ADHD (as individual disorders and perhaps co-segregating), it remains unclear if SUD in BPD youth represents a subtype of BPD and/or SUD, or if a vulnerability to SUD development exists in these youth. For instance, we previously speculated that the development of BPD may be particularly predictive of the development of SUD during adolescence, considering that adolescence is a time of vulnerability for the development of SUD33,176.
Among disturbances reported in BPD youth, severe affective and self-regulation problems may predispose them to seek drugs of abuse120. By nature of their intrapsychic distress and behavioral disinhibition, these youth may try to modulate their irritable and labile mood with substances of abuse; such has been described in adults99. We recently examined this issue and found evidence that youth with BPD tended to initiate substances of abuse to attenuate mood relative to non-mood disordered adolescents who reported using substance more often to get high113.
Genes and Adolescent SUD
Child- and adolescent-onset BPD may be etiologically distinct with a variable course and outcome including the risk for SUD. It may also be that adolescent-onset BPD and adolescent-onset SUD may represent variable expressivity of a shared risk factor37,49. In order to better understand these competing influences, family studies are necessary. To this end, we examined the parents of our adolescents with and without BPD (and/or SUD) as part of our NIH funded longitudinal study. We found the parents of proband youth with BPD (without SUD) and BPD & SUD were more likely to develop BPD than the parents of controls (omnibus test 2=10.18, p=0.006); we also found no differences between the two bipolar groups. Parents of proband youth with BPD and with BPD & SUD were more likely than relatives of controls to develop SUD (omnibus test 2=14.69, p<0.001); however, we found no differences between the parents of the two proband bipolar groups. Within the parents of proband youth with BPD & SUD, we found higher risk of SUD in parents with BPD than in those without BPD ( 2=8.39, p=0.004) leading us to speculate that BPD and SUD are prevalent in the first-degree relatives of adolescents with BPD, adults with BPD were more likely to manifest SUD, and that BPD and SUD cosegregated. Interestingly, recent work from our group with a candidate gene for BPD – namely the dopamine transporter protein127 - has also been found to be associated with the development of early –onset SUD.
Diagnostic and Treatment Considerations
The first consideration that clinicians should have in mind in the establishment of a specific treatment plan for adolescents with co-occurring mental and substance-related disorders is the determination of the level of care needed. When treating dually diagnosed disorders such as BPD and SUD, clinicians should consider a simultaneous approach. Given limited, albeit important, data on the effects of medication treatment reducing SUD in BPD, both psychosocial and medication strategies should be considered simultaneously in these comorbid adolescents. There is evidence that pharmacological interventions are effective for youth with SUD and BPD. Two studies, including one randomized controlled study, have reported that mood stabilizers, specifically lithium and valproic acid, significantly reduced substance use in bipolar youth45,68. A controlled, 6-week study of treatment with lithium in youth with affective dysregulation and substance dependence Geller et al.68 reported a clinically significant decrease in the number of positive urines as well as a significant increase in overall global functioning.
In a 5-week open trial of valporic acid in adolescent outpatients with marijuana abuse/dependence and “explosive mood disorder” (mood symptoms were not classified using the DSM IV) Donovan et al.45 reported significant improvement in their marijuana use and their affective symptoms. The use of atypical neuroleptics as mood-stabilizing agents in comorbid SUD-BPD remains understudied, albeit compelling, and further work in this area is needed.
ANXIETY DISORDERS
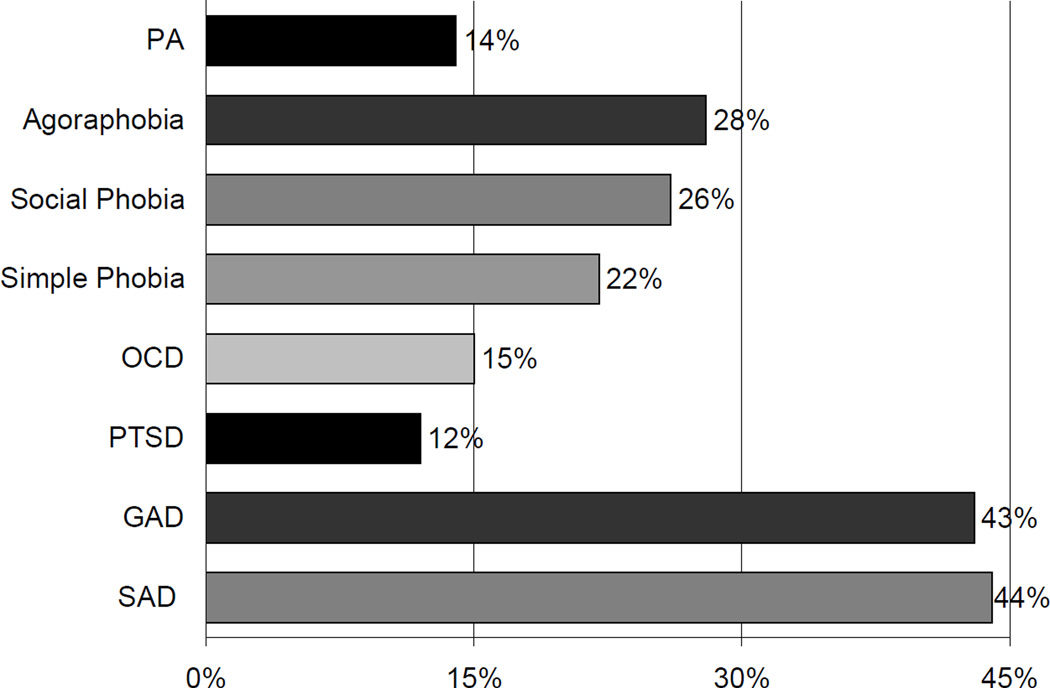
The presence of anxiety disorders in individuals who suffer from BPD has been under-recognized and understudied. One reason for this lack of recognition could be the notion that it is counterintuitive to suggest that BPD, which is characterized by high levels of disinhibition, could coexist with anxiety, which is characterized by fear and inhibition. However, in the first paper to demonstrate the high frequency of BPD in an outpatient pediatric psychopharmacology clinic (16%), 56% of the children with BPD suffered from 2 or more lifetime anxiety disorders (multiple anxiety disorders) comorbidly. Furthermore, a recent detailed analysis of the comorbidity between pediatric BPD and anxiety disorders in a clinically referred population revealed that 76% of youth with BPD have one or more anxiety disorders comorbid with their BPD81. In a community sample Lewinsohn et al.111 reported that a third of non-referred BPD adolescents had comorbid anxiety disorders, a significantly higher rate than that found in those without a history of BPD. Because the anxiety disorders are heterogeneous (eight are included in the DSM-IV1) and as most studies lump them, uncertainties remain as to which anxiety disorders are associated with BPD. Various clinical and epidemiological studies in adult and pediatric populations have identified a wide range of anxiety disorders associated with BPD with rates ranging between 12.5% and 76%11,52,54,58,81,88,111,121–123,137,164,175,184 (Figure-4).
Figure-4.
Anxiety Disorders in Clinically Referred Youth with Bipolar Disorder
Among various anxiety disorders, a specific association of certain anxiety disorders more than others has been suggested in youth with BPD20,164. A preponderance of investigators have suggested that a particular link exists between panic disorder and BPD in adults35,75 and children20. Data from adult studies report a lifetime prevalence of panic disorder in 21%–33% of individuals with BPD35,75,98 and conversely, lifetime BPD in 6%–23% of individuals with panic disorder21,137. MacKinnon et al.115,116 utilize family genetic methodology in 57 families to argue that panic disorder with BPD is a genetic subtype of BPD. Savino et al.148 systematically explored the intra-episodic and longitudinal comorbidity of 140 adults with panic disorder and reported comorbidity with BPD in 13.5% of the patients with panic disorder. They also note that an additional 34.3% met features of “hyperthymic temperament”, a possible bipolar spectrum condition. Biederman et al. reported high rates of panic disorder (52%) among youth with BPD11 consistent with the observation by Birmaher et al.20 suggesting that the association between BPD and panic disorder in children and adolescents might be unique and specific. However, recent emerging literature indicates high prevalence of various anxiety disorders - including but not limited to panic disorder - in pediatric81,88,122 and adult31,65,123 populations with BPD, that challenges the notion of specific link between BPD and panic disorder. Thus, more information is needed as to whether the association between BPD and anxiety disorders in youth is limited to a single anxiety disorder or is more extensive and includes other anxiety disorders as well.
In the presence of high levels of anxiety, adults with BPD experience greater symptom severity, increased risk for suicide and alcohol abuse, higher frequency of a polypharmacy regimen, more severe adverse effects, poor treatment response, and higher rates of non-remission with poor course and functioning58,67,136,185. Olvera et al.134 found that children with BPD and multiple anxiety disorders displayed manic symptoms at an earlier age and were more likely to have been hospitalized for their illness.
Improving the understanding of the relationship between anxiety disorders and BPD in youth has important treatment implications. Masi et al.122 reported high rates of pharmacologic hypo/mania in youth with anxiety disorders with mean age of onset of for anxiety disorders preceding that for BPD. This finding suggests caution when considering antidepressant pharmacotherapy in a pediatric population with multiple anxiety disorders. Considering that treatments for BPD with traditional mood stabilizers do not generally treat anxiety disorders, and that treatment of anxiety disorders with SSRIs can aggravate the BPD, the pharmacological approach to bipolar children with comorbid anxiety disorders needs to be defined. As BPD and anxiety disorders respond to different treatments, identification of the comorbid state is essential for proper treatment and for achieving optimal functioning.
No systematic data is available which examines treatment of anxiety disorders in the context of bipolar comorbidity. Trials of pediatric anxiety disorders exclude children with BPD by protocol design and similarly, children with a BPD diagnosis are typically excluded from the trials of treatment for both depression and anxiety.
To-date, only one open-label trial has assessed response of co-occurring panic attacks and generalized anxiety disorder in adults with BPD, reporting significant decrease in or remission of anxiety symptoms with divalproex therapy25. Corroborative evidence for anti-anxiety effect of mood stabilizers comes from various open-label and controlled trials in adult population with anxiety disorders that suggest valproate to be effective in treating panic disorder and Posttraumatic stress disorder (PTSD)8,36,59,114,124,135,140. Anti-anxiety response of certain mood stabilizers could be specific to certain anxiety disorder(s). For instance, carbamazepine, though effective in treating certain symptoms of PTSD, it is found to be ineffective in treating other anxiety disorders in adults namely panic disorder and OCD87,112,157,165,180.
Obsessive – Compulsive Disorder
Descriptions of OCD symptoms in bipolar patients date back to the 19th century131. Most data on comorbid OCD and BPD are not based on systematic studies74,142, but are documentation from naturalistic studies. In adults, evidence of a higher-than-expected overlap between OCD and BPD first came from the ECA study, where 23% of those with BPD also met criteria for OCD146. Subsequent studies have consistently found the overlap between OCD and BPD at rates as high as 15–35%35,138,175. When comorbid with BPD, OCD in adults has a more episodic course, often featuring higher rates of sexual and religious obsessions, lower rates of checking rituals, and greater frequency of concurrent major depressive episodes and panic disorder. They also exhibit increased rates of suicidality, more frequent hospitalizations and more complex pharmacological interventions than those without BPD32,35,138,139. A recent survey conducted among the French Association of OCD patients provides corroborating evidence for this comorbidity in subjects giving retrospective childhood reports. While reporting a high prevalence of comorbid lifetime bipolarity, they also noted that many of these subjects had a juvenile onset of their OCD80,102.
While the available literature suggests substantial impact on clinical presentation, global functioning, and treatment decisions when BPD and OCD co-occur in young patients121 the nature of this relationship is not clearly delineated. For example, the agitation, racing thoughts and feelings of distress which can be associated with severe OCD could mimic a bipolar picture; conversely, the manic symptom of increase in goal directed activity (“mission mode” behavior) or repetitive, unwanted hypersexual thoughts in a child or adolescent with BPD could mimic an OCD presentation. In one of the two studies that addresses this comorbid presentation Masi et al.121 reported that in comparison to OCD, comorbid OCD and BPD youth were significantly more impaired, had earlier age of onset of OCD and had more frequent existential, philosophical, odd and/or superstitious obsessions, indicating that comorbidity with BPD may have a clinically relevant influence on the symptom expression of the OCD. Half of the comorbid population in this study had type II BPD and one-third experienced pharmacologic hypomania. This high risk of (hypo) manic switches reported with antidepressant treatment in pediatric OCD is suggestive of a bipolar diathesis44,100.
In other study of youth ascertained for family genetic study of BPD and OCD we previously92 documented a significant and symmetrical bidirectional overlap between BPD and OCD (21% of the BPD cohort and 15% of the OCD cohort satisfied criteria for both BPD and OCD). In the presence of comorbid BPD, youth with OCD more often presented with the symptom of hoarding/saving, experienced a higher prevalence of other comorbid disorders especially ODD, major depressive disorder (MDD), and psychosis, suffered from poorer psychosocial functioning, and required hospitalization at a greater frequency. Higher prevalence of comorbidity with multiple anxiety disorders, especially General anxiety disorder (GAD) and social phobia, was observed in youth with comorbid OCD and BPD than when either disorder occurred in youth without reciprocal comorbidity. Limited family genetic data suggests a genetic linkage between OCD and BPD. Coryell39 reported an equal incidence (2.3%) of BPD in families of probands with OCD and in families with BPD. Similarly, an increased incidence of obsessional traits has been reported in the offspring of bipolar probands101.
Though no systematic data to date are available that address the therapeutic response of BPD in the presence of comorbid anxiety disorder, Joshi et al.90 conducted a secondary data analysis to examine the antimanic response of BPD youth to olanzapine in the context of comorbidity status with OCD and GAD and concluded that in children and adolescents with BPD the comorbid presence of lifetime OCD, but not GAD, is associated with poor antimanic response (Figure-5). This suggests that certain anxiety disorders when comorbid with BPD may have a larger mediating effect on BPD treatment outcome than others.
Figure-5.
Endpoint YMRS Score Mean Change BPD+OCD Vs. BPD+GAD
Conversely, the presence of BPD with anxiety disorders may have a negative effect on the treatment outcome of the anxiety disorder. For instance, compared to OCD youth without comorbid BPD, children and adolescents with comorbid OCD and BPD have been reported to show poor response to psychotropic medications and are more frequently on a combined pharmacy regimen119. Non-pharmacological treatments such as CBT have been found to be useful and should be instituted when possible, along with any of the pharmacological alternatives to SSRIs.
Posttraumatic Stress Disorder
Individuals who work with trauma victims make the clinical observation that mood swings are common in this group. Although considerable literature implicates psychosocial stresses in the onset and recurrence of BPD24,84,106, there is paucity of research on the association of PTSD with BPD. While Breslau et al. reported that 40–76% of children had been exposed to a traumatic event by age 1723,73, the estimated lifetime prevalence of the full syndromatic PTSD in the general population is between 1 and 14%, and its prevalence in children is around 6%73. By contrast, reported rates of PTSD comorbidity in patients with BPD have varied widely from 7% to 50%.
Rates of SUD are substantially higher in youths who had a lifetime diagnosis of PTSD before age 18 compared to youths who had never experienced a trauma108. Given the increasing recognition of SUD in BPD and PTSD, we further examined the relationship of BPD, SUD, and PTSD in an older group of adolescents with BPD. We found significantly more PTSD in adolescents with BPD compared to non-mood disordered controls. Sixteen percent of youth with BPD had broad PTSD compared to 4% of controls. Moreover, a higher risk of SUD was found in BPD adolescents with PTSD. While exploratory in nature, interesting temporal patterns emerged suggesting a pattern of onset of BPD first, followed by substance use, trauma, PTSD and then the onset of full SUD. These data highlight the high risk of PTSD in BPD adolescents that appears to be related to SUD in these youth155.
Although many studies have looked at possible links between early traumatic events and development of psychopathology over the life span78,94,97, few studies have examined the potential role of early traumatic life stresses on the development of BPD and PTSD. Emerging evidence suggests that trauma significantly compromises the course of BPD. Leverich et al.110 evaluated 631 outpatients with BPD and reported that nearly half of the females (49%) and one-third of the males (36%) reported early sexual and physical abuse. Those who endorsed a history of child or adolescent physical or sexual abuse, compared with those who did not, had significantly higher rates of comorbid PTSD, a history of an earlier onset of bipolar illness, and a higher rate of suicide attempts. On the other hand Geller et al.72 reported high rates (43%) of the symptom of hypersexuality (higher in pubertal versus prepubertal BPD population) and low rates (< 1%) of history of sexual abuse in their prepubertal and early adolescent BPD cohort, suggesting that the symptom of hypersexuality in pediatric BPD is etiologically unrelated to sexual abuse and more reflective of mania and puberty. Similarly, Garno et al.66 studied 100 adult patients with BPD, and half (51%) reported a history of abuse while a quarter suffered from with comorbid PTSD (24%).
A report by Wozniak et al. raises the question as to whether a diagnosis of BPD may pose a risk factor for trauma. Using data from a large longitudinal sample of well-characterized boys with and without ADHD, these authors failed to find meaningful associations between ADHD, trauma, and PTSD. Instead, they identified early BPD as an important antecedent for later trauma. When traumatized children present with severe irritability and mood lability there may be a tendency to associate these symptoms to the trauma. On the contrary, these longitudinal results suggest that rather than a consequence, BPD may be an antecedent risk factor for later trauma (possibly because of the attendant reckless, disinhibited state). If confirmed, these results could help dispel the commonly held notion that mania-like symptoms in youths represent a reaction to trauma and would further suggest that children with BPD should be adequately treated and monitored closely to avoid trauma.
Though no empirical evidence is available for the treatment of comorbid BPD and PTSD, corroborative evidence comes from trials of mood stabilizers in adult population suggesting valproate to be effective in treating certain combat related (but not non-combat-related) PTSD symptoms8,36,59,135,140. Certain mood stabilizers have shown promise in treating specific symptoms of PTSD. Several open trials report that carbamazepine may be useful for treating PTSD symptoms of flashbacks, nightmares, and intrusive thoughts112,157,180. In a preliminary controlled trial lamotrigine exhibited potential efficacy in the treatment of PTSD symptoms of re-experiencing, avoidance, and numbing in adults82.
PERVASIVE DEVELOPMENTAL DISORDERS
Recently, there has been immense interest in the overlap of BPD and autistic spectrum disorders. A limited literature exists on the diagnosis and treatment of comorbid BPD and PDD in children and adolescents. In the absence of systematic research on comorbid BPD and PDD, indirect evidence suggestive of comorbid BPD in pediatric populations with PDD comes from high rates of aggressive behaviors documented in children with PDD, a high incidence of BPD in family members of children with PDD, and from a small literature documenting the presence of BPD comorbidity in PDD populations.
High rates of aggressive behaviors and severe mood disturbances are documented in children with PDD95,103,153,156. There is considerable evidence suggesting that a subset of PDD youth with extreme disturbance of mood suffer from a symptom cluster that is phenomenologically consistent with the syndrome of BPD. In a study of a group of patients with Asperger’s disorder who were followed into adolescence, Wing et al.178 found that nearly one-half of the patients developed affective disorders. Conversely, high rates of PDD or PDD traits are reported in children and adolescents with BPD. Presence of significant PDD traits are reported to be as high as 62% in pediatric mood and anxiety disorder research populations166.
In the first study using accepted operationalized criteria, our group assessed clinically referred children and adolescents (N=727) using a comprehensive diagnostic battery including structured diagnostic interview. We reported a bidirectional overlap: BPD occurred in 21% of PDD and PDD occurred in 11% of BPD youth182. There was striking homology in the clinical characteristics of PDD and BPD when clinical features were compared based on the presence or absence of reciprocal comorbidity. BPD and PDD irrespective of the reciprocal comorbidity were similar in phenotypic features including symptom profile, pattern of comorbidity, and measures of functioning. This work suggests that BPD and PDD are bona fide disorders when they co-occur in youth.
Recently we replicated our earlier findings and reported that one third of our clinically referred population of children and adolescents with PDD also received the diagnosis of BPD on structured diagnostic interviews91. Similarly we found consistently high rates of comorbid PDD (15%) in our research populations of children and adolescents with BPD irrespective of the aims for ascertainment - family genetic study or treatment trials of BPD89,93. In the presence of comorbid PDD, youth with BPD experience an earlier age at onset and increased severity of BPD with a poorer level of functioning89,91,93. Furthermore, PDD youth with a family history of BPD are more often high functioning and their mood disturbance is characterized by a severe cycling pattern, agitation, and aggression along with neurovegetative disturbances43. There is an accumulating body of literature from family genetic studies suggesting higher than expected incidence of BPD in first degree relatives of about one-third of the population with PDD42,43,83.
Treatment response to psychotropics in youth with PDD is noted to be less robust with higher rates of adverse effects to both medication and placebo2,4,141. Thus, due to an atypical response and higher susceptibility to adverse effects, it is advisable to initiate and titrate psychotropics at a lower dose and titrate upward in smaller increments in this population.
Though there is substantial evidence documenting the role of pharmacotherapy for the management of extreme mood difficulties, there is minimal published literature and no systematic data on the treatment of comorbid BPD in this population. Limited literature on the treatment of comorbid BPD in children with PDD suggests that first-generation antipsychotics (haloperidol, chlorpromazine, thioridazine) and traditional mood stabilizers (lithium, carbamazepine) are minimally effective for the treatment of mania109. On the contrary, in a recent secondary analysis of acute atypical antipsychotic monotherapy trials in BPD youth we reported acceptable tolerability and robust antimanic response to atypical antipsychotics (risperidone, olanzapine, quetiapine, ziprasidone, or aripiprazole) in the presence of PDD comorbidity89. No difference was observed in the rate of anti-manic response and tolerability with the exception that PDD youth were more susceptible to the adverse effect of slurred speech and teary eyes. Furthermore, compared to other atypical antipsychotics, risperidone had a superior anti-manic response in BPD youth with comorbid PDD. However this study is limited by the retrospective nature of post-hoc analysis and lack of direct measures of PDD symptomatology.
There is evidence from the treatment trials of risperidone, aripiprazole, and ziprasidone that second-generation neuroleptics are well-tolerated and efficacious in treating symptoms of irritability and aggression in youth with PDD, a spectrum of symptoms suggestive of BPD. Controlled trials of risperidone consistently report favorable safety, tolerability, and efficacy profile for treating symptoms of irritability and aggression in youth with PDD3,132,150. Although risperidone is the only atypical antipsychotic that is FDA approved for the treatment of irritability and aggression in autistic children, weight gain associated with risperidone is a significant adverse effect that often limits continuation of treatment in this population. In contrast, results from recent short-term open trials with newer atypical antipsychotics - aripiprazole and ziprasidone-- show promise as a treatment for irritability in children with PDD, and are associated with negligible weigh gain117,158. Contrary to the encouraging response observed with the afore-mentioned atypical antipsychotics, the atypical antipsychotics quetiapine and olanzapine are noted to be ineffective in treating symptoms of irritability and aggression in this population62,85,118.
Thus in choosing thymoleptic agent for the treatment of BPD in youth with comorbid PDD, consideration should be given to those anti-manic agents that are also shown to be efficacious in treating associated and core features of PDD. Furthermore, in this population due to higher susceptibility to adverse effects, it is advisable to initiate and titrate psychotropics at a lower dose. As afore-mentioned empirical evidence suggests, risperidone appears to be efficacious in treating both core and associated features of PDD and may be superior to other atypical antipsychotics as an anti-manic agent in youth with PDD. Youth should be closely monitored for adverse effects especially weight gain, as it remains a concern in short and long term therapy with risperidone.
Summary
The diagnosis and treatment of BPD needs to address psychiatric comorbidity given its ubiquitous nature. ADHD comorbidity is particularly associated with very early onset BPD, whereas the risk of SUD comorbidity is much higher for adolescent-onset versus child-onset BPD. Onset of pediatric BPD is generally either prior or simultaneous to the onset of SUD and severity of both the disorders is worse in the comorbid state. A higher than expected prevalence of anxiety disorders is documented in individuals with BPD. In the presence of an anxiety disorder, individuals with BPD experience greater symptom severity, poorer treatment response, and poorer course and functioning. Hypersexuality in pediatric BPD is etiologically unrelated to sexual abuse and more reflective of dysregulation related to mania and puberty. BPD may be an antecedent risk factor for later trauma and subsequent PTSD and not merely represent a reaction to the trauma. There is an accumulating body of literature that suggests that a subset of PDD youth with extreme disturbance of mood suffer from a symptom cluster that is phenomenologically consistent with the syndrome of BPD and this is equally substantiated by family genetic studies that document a higher than expected incidence of BPD in first degree relatives of youth with PDD.
In general, the presence of comorbid disorders with BPD results in a more severe clinical condition. Earlier onset BPD seems to be related to additional comorbidity and more severe episodes and cycle acceleration. Identifying and treating these co-occurring psychiatric conditions may help alleviate the severity of impairment and duration of mood episodes in BPD.
Knowledge of the impact of comorbid disorders on the therapeutic response in youth with BPD is growing rapidly. BPD response to lithium is less robust in the presence of ADHD comorbidity. Stimulants are safe and efficacious for the treatment of comorbid ADHD once mania is stabilized in youth with BPD. Treatment of BPD with lithium or valporic acid results in attenuation of active SUD. In youth with BPD, the comorbid presence of lifetime OCD, but not GAD, is associated with poor antimanic response. Response to psychotropics in PDD youth with mood dysregulation is noted to be less robust with higher susceptibility to adverse effects.
The scientific interface between BPD and comorbidities remains unclear. Comorbidity may represent an important genetic and clinical subtype with distinct psychopathology, familiality, and cognitive, neural, and genetic underpinnings. Future longitudinal studies addressing the impact of comorbidity on the clinical presentation, course, and response to treatment along with studies examining the cognitive correlates, genetic-candidate genes, and neurobiological overlap would assist in further clarifying the relationship of BPD with its comorbidities.
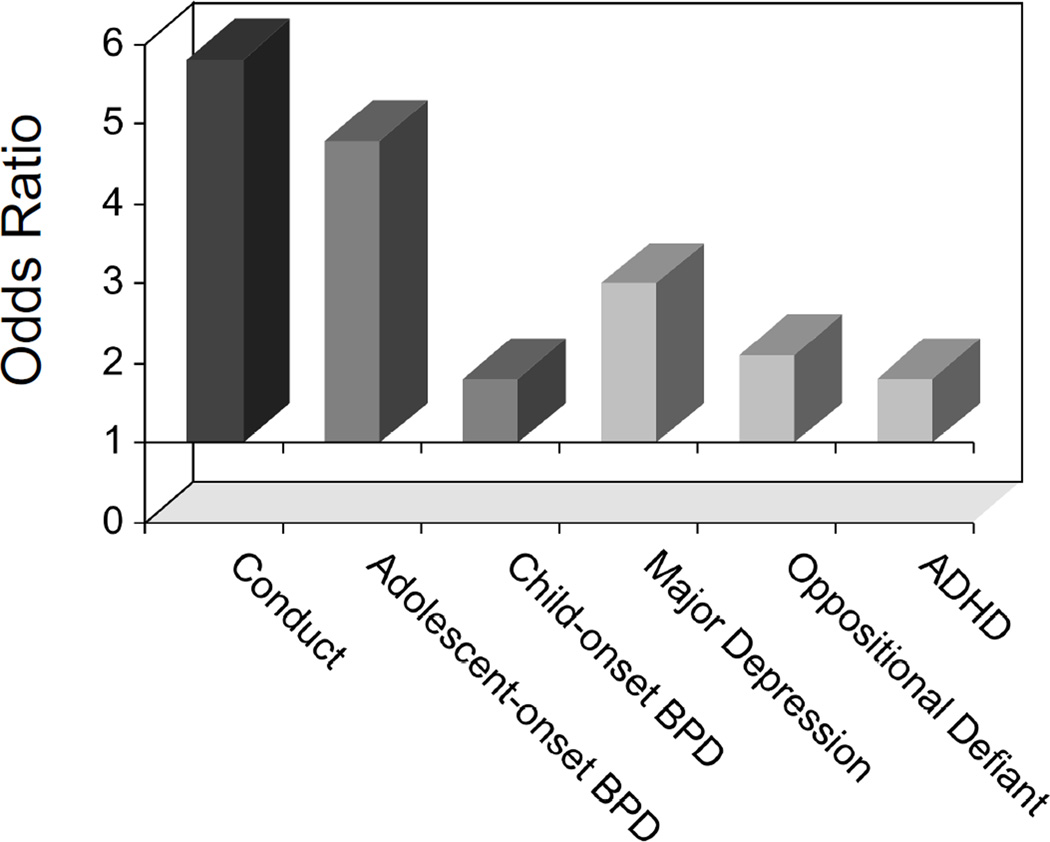
Figure-2.
Risk of SUD in Psychiatrically Referred Adolescent Outpatient Population
Acknowledgments
This work was supported by the NIH RO1 DA129452, K24 DA0162642, the Dupont Warren Fellowship Award1, the Norma Fine Pediatric Psychopharmacology Fellowship Fund1, and the Pediatric Psychopharmacology Council Fund1,2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, D.C: American Psychiatric Association; [Google Scholar]
- 2.Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry. 2005;62:1266. doi: 10.1001/archpsyc.62.11.1266. [DOI] [PubMed] [Google Scholar]
- 3.Research Units on Pediatric Psychopharmacology Autism Network: Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- 4.Aman MG, Arnold MDL, McDougle CJ, et al. Acute and long-term safety and tolerability of risperidone in children with autism. J Child Adolesc Psychopharmacol. 2005;15:869. doi: 10.1089/cap.2005.15.869. [DOI] [PubMed] [Google Scholar]
- 5.Aman MG, De Smedt G, Derivan A, et al. Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. Am J Psychiatry. 2002;159:1337. doi: 10.1176/appi.ajp.159.8.1337. [DOI] [PubMed] [Google Scholar]
- 6.Ambrosini PJ. Historical development and present status of the schedule for affective disorders and schizophrenia for school-age children (K-SADS) Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:49. doi: 10.1097/00004583-200001000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Angold A, Costello EJ. Depressive comorbidity in children and adolescents: Empirical, theoretical and methodological issues. American Journal of Psychiatry. 1993;150:1779. doi: 10.1176/ajp.150.12.1779. [DOI] [PubMed] [Google Scholar]
- 8.Baetz M, Bowen RC. Efficacy of divalproex sodium in patients with panic disorder and mood instability who have not responded to conventional therapy. Can J Psychiatry. 1998;43:73. doi: 10.1177/070674379804300109. [DOI] [PubMed] [Google Scholar]
- 9.Biederman J, Faraone S, Hatch M, et al. Conduct disorder with and without mania in a referred sample of ADHD children. Journal of Affective Disorders. 1997;44:177. doi: 10.1016/s0165-0327(97)00043-8. [DOI] [PubMed] [Google Scholar]
- 10.Biederman J, Faraone SV, Chu MP, et al. Further evidence of a bidirectional overlap between juvenile mania and conduct disorder in children. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:468. doi: 10.1097/00004583-199904000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Biederman J, Faraone SV, Marrs A, et al. Panic disorder and agoraphobia in consecutively referred children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:214. doi: 10.1097/00004583-199702000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Biederman J, Faraone SV, Mick E, et al. Attention deficit hyperactivity disorder and juvenile mania: An overlooked comorbidity? Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:997. doi: 10.1097/00004583-199608000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Biederman J, Faraone SV, Wozniak J, et al. Parsing the association between bipolar, conduct, and substance use disorders: a familial risk analysis. Biological Psychiatry. 2000;48:1037. doi: 10.1016/s0006-3223(00)00906-9. [DOI] [PubMed] [Google Scholar]
- 14.Biederman J, Mick E, Bostic J, et al. The naturalistic course of pharmacologic treatment of children with manic-like symptoms: A systematic chart review. Journal of Clinical Psychiatry. 1998;59:628. doi: 10.4088/jcp.v59n1111. [DOI] [PubMed] [Google Scholar]
- 15.Biederman J, Mick E, Faraone SV, et al. Risperidone in the Treatment of Affective Symptoms: A Secondary Analysis of a Randomized Clinical Trial in Children with Disruptive Behavior Disorder. Clinical Therapeutics. 2006;28:794. doi: 10.1016/s0149-2918(06)00132-9. [DOI] [PubMed] [Google Scholar]
- 16.Biederman J, Mick E, Spencer TJ, et al. A prospective open-label treatment trial of ziprasidone monotheray in children and adolescents with bipolar disorder. Bipolar Disorders. doi: 10.1111/j.1399-5618.2007.00450.x. in press. [DOI] [PubMed] [Google Scholar]
- 17.Biederman J, Mick E, Wozniak J, et al. An Open-Label Trial of Risperidone in Children and Adolescents with Bipolar Disorder. Journal of Child and Adolescent Psychopharmacology. 2005;15:311. doi: 10.1089/cap.2005.15.311. [DOI] [PubMed] [Google Scholar]
- 18.Biederman J, Petty C, Faraone SV, et al. Moderating effects of major depression on patterns of comorbidity in referred adults with panic disorder: A controlled study. Psychiatry Research. 2004;126:143. doi: 10.1016/j.psychres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Biederman J, Wilens T, Mick E, et al. Is ADHD a risk for psychoactive substance use disorder? Findings from a four year follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:21. doi: 10.1097/00004583-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Birmaher B, Kennah A, Brent D, et al. Is bipolar disorder specifically associated with panic disorder in youths? J Clin Psychiatry. 2002;63:414. doi: 10.4088/jcp.v63n0507. [DOI] [PubMed] [Google Scholar]
- 21.Bowen R, South M, Hawkes J. Mood swings in patients with panic disorder. Canadian Journal of Psychiatry. 1994 Mar;39:91. doi: 10.1177/070674379403900205. [DOI] [PubMed] [Google Scholar]
- 22.Boyd JH, Burke JD, Gruenberg E, et al. Exclusion criteria of DSM-III: A study of co-occurrence of hierarchy-free syndromes. Archives of General Psychiatry. 1984;41:983. doi: 10.1001/archpsyc.1984.01790210065008. [DOI] [PubMed] [Google Scholar]
- 23.Breslau N, Lucia VC, Alvarado GF. Intelligence and other predisposing factors in exposure to trauma and posttraumatic stress disorder: a follow-up study at age 17 years. Arch Gen Psychiatry. 2006;63:1238. doi: 10.1001/archpsyc.63.11.1238. [DOI] [PubMed] [Google Scholar]
- 24.Brown GW, Harris T. Disease, distress and depression. A comment. J Affect Disord. 1982;4:1. doi: 10.1016/0165-0327(82)90012-x. [DOI] [PubMed] [Google Scholar]
- 25.Calabrese J, Delucchi G. Spectrum of efficacy of valproate in 55 patients with rapid-cycling bipolar disorder. American Journal of Psychiatry. 1990;147:431. doi: 10.1176/ajp.147.4.431. [DOI] [PubMed] [Google Scholar]
- 26.Campbell M, Anderson LT, Green WH. Behavior-disordered and aggressive children: New advances in pharmacotherapy. Journal Of Developmental And Behavioral Pediatrics. 1983;4:265. [PubMed] [Google Scholar]
- 27.Campbell M, Cueva JE. Psychopharmacology in child and adolescent psychiatry: A review of the past seven years. part II. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:1262. doi: 10.1097/00004583-199510000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Campbell M, Small AM, Green WH, et al. Behavioral efficacy of haloperidol and lithium carbonate: A comparison in hospitalized aggressive children with conduct disorder. Archives of General Psychiatry. 1984;41:650. doi: 10.1001/archpsyc.1984.01790180020002. [DOI] [PubMed] [Google Scholar]
- 29.Carlson G, Kelly K. Manic symptoms in psychiatrically hospitalized children- what do they mean? Journal of Affective Disorders. 1998;51:123. doi: 10.1016/s0165-0327(98)00211-0. [DOI] [PubMed] [Google Scholar]
- 30.Carlson GA. Classification issues of bipolar disorders in childhood. Psychiatric Developments. 1984;2:273. [PubMed] [Google Scholar]
- 31.Cassano GB, Pini S, Saettoni M, et al. Multiple anxiety disorder comorbidity in patients with mood spectrum disorders with psychotic features. Am J Psychiatry. 1999;156:474. doi: 10.1176/ajp.156.3.474. [DOI] [PubMed] [Google Scholar]
- 32.Centorrino F, Hennen J, Mallya G, et al. Clinical outcome in patients with bipolar I disorder, obsessive compulsive disorder or both. Hum Psychopharmacol. 2006;21:189. doi: 10.1002/hup.760. [DOI] [PubMed] [Google Scholar]
- 33.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. Am J Psych. 2003;160:1041. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang KD, Steiner H, Ketter TA. Psychiatric phenomenology of child and adolescent bipolar offspring. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:453. doi: 10.1097/00004583-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Dilsaver S. Comorbidity of panic disorder in bipolar illness: Evidence from the epidemiologic catchment area survey. American Journal of Psychiatry. 1995;152:280. doi: 10.1176/ajp.152.2.280. [DOI] [PubMed] [Google Scholar]
- 36.Clark RD, Canive JM, Calais LA, et al. Divalproex in posttraumatic stress disorder an open-label clinical trial. J Trauma Stress. 1999;12:395. doi: 10.1023/A:1024797014210. [DOI] [PubMed] [Google Scholar]
- 37.Comings D, Comings B, Muhleman D, et al. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. Journal of the American Medical Association. 1991;266:1793. [PubMed] [Google Scholar]
- 38.Connor DF, McLaughlin TJ, Jeffers-Terry M. Randomized controlled pilot study of quetiapine in the treatment of adolescent conduct disorder. J Child Adolesc Psychopharmacol. 2008;18:140. doi: 10.1089/cap.2006.0007. [DOI] [PubMed] [Google Scholar]
- 39.Coryell W. Obsessive-compulsive disorder and primary unipolar depression. Comparisons of background, family history, course, and mortality. J Nerv Ment Dis. 1981;169:220. doi: 10.1097/00005053-198104000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Croonenberghs J, Fegert JM, Findling RL, et al. Risperidone in children with disruptive behavior disorders and subaverage intelligence: a 1-year, open-label study of 504 patients. J Am Acad Child Adolesc Psychiatry. 2005;44:64. doi: 10.1097/01.chi.0000145805.24274.09. [DOI] [PubMed] [Google Scholar]
- 41.Davis RE. Manic-depressive variant syndrome of childhood: A preliminary report. American Journal of Psychiatry. 1979;136:702. doi: 10.1176/ajp.136.5.702. [DOI] [PubMed] [Google Scholar]
- 42.DeLong GR, Dwyer JT. Correlation of family history with specific autistic subgroups: Asperger’s syndrome and bipolar affective disease. J Autism Dev Disord. 1988;18:593. doi: 10.1007/BF02211877. [DOI] [PubMed] [Google Scholar]
- 43.DeLong GR, Nohria C. Psychiatric family history and neurological disease in autistic spectrum disorders. Developmental Medicine and Child Neurology. 1994;36:441. [PubMed] [Google Scholar]
- 44.Diler RS, Avci A. SSRI-induced mania in obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 1999;38:6. doi: 10.1097/00004583-199901000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Donovan S, Nunes E. Treatment of comorbid affective and substance use disorders Therapeutic potential of anticonvulsants. American Journal of Addiction. 1998;7:210. [PubMed] [Google Scholar]
- 46.Donovan S, Susser E, Nunes E, et al. Divalproex treatment of disruptive adolescents A report of 1 cases. Journal of Clinical Psychiatry. 1997;58:12. doi: 10.4088/jcp.v58n0102. [DOI] [PubMed] [Google Scholar]
- 47.Donovan SJ, Stewart JW, Nunes EV, et al. Divalproex Treatment for Youth With Explosive Temper and Mood Lability: A Double-Blind, Placebo-Controlled Crossover Design. American Journal of Psychiatry. 2000;157:818. doi: 10.1176/appi.ajp.157.5.818. [DOI] [PubMed] [Google Scholar]
- 48.Dunner DL, Feinman J. 34th Annual Meeting of the American College of Neuropsychopharmacology. San Juan: Puerto Rico; The effect of substance abuse on the course of bipolar disorder; p. 171. [Google Scholar]
- 49.Ebstein R, Novick O, Umansky r, et al. Dopamine D4 receptor exon III polymorphism associated with the human personality trait of novelty seeking. Nature Genetics. 1996;12:78. doi: 10.1038/ng0196-78. [DOI] [PubMed] [Google Scholar]
- 50.Faraone S, Glatt S, Tsuang M. The genetics of pediatric onset bipolar disorder. Biological Psychiatry. 2003;53:970. doi: 10.1016/s0006-3223(02)01893-0. [DOI] [PubMed] [Google Scholar]
- 51.Faraone SV, Biederman J, Mennin D, et al. Bipolar and antisocial disorders among relatives of ADHD children: Parsing familial subtypes of illness. Am J Med Genet. 1998;81:108. [PubMed] [Google Scholar]
- 52.Faraone SV, Biederman J, Mennin D, et al. Attention-deficit hyperactivity disorder with bipolar disorder: a familial subtype? Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1378. doi: 10.1097/00004583-199710000-00020. [DOI] [PubMed] [Google Scholar]
- 53.Faraone SV, Biederman J, Monuteaux MC. Attention deficit hyperactivity disorder with bipolar disorder in girls: further evidence for a familial subtype? Journal of Affective Disorders. 2001;64:19. doi: 10.1016/s0165-0327(00)00213-5. [DOI] [PubMed] [Google Scholar]
- 54.Faraone SV, Biederman J, Wozniak J, et al. Is comorbidity with ADHD a marker for juvenile onset mania? Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1046. doi: 10.1097/00004583-199708000-00012. [DOI] [PubMed] [Google Scholar]
- 55.Faraone SV, Tsuang MT. Methods in Psychiatric Genetics. In: Tohen M, Tsuang MT, Zahner GEP, editors. Textbook in Psychiatric Epidemiology. New York, NY: John Wiley; 1995. p. 81. [Google Scholar]
- 56.Faraone SV, Tsuang MT, Tsuang D. Genetics and Mental Disorders: A Guide for Students, Clinicians, and Researchers. New York, NY: The Guilford Press; 1999. [Google Scholar]
- 57.Farley SE, Adams JS, Lutton ME, et al. Clinical inquiries. What are effective treatments for oppositional and defiant behaviors in preadolescents? J Fam Pract. 2005;54:162. [PubMed] [Google Scholar]
- 58.Feske U, Frank E, Mallinger AG, et al. Anxiety as a correlate of response to the acute treatment of bipolar I disorder. American Journal of Psychiatry. 2000;157:956. doi: 10.1176/appi.ajp.157.6.956. [DOI] [PubMed] [Google Scholar]
- 59.Fesler FA. Valproate in combat-related posttraumatic stress disorder. J Clin Psychiatry. 1991;52:361. [PubMed] [Google Scholar]
- 60.Findling RL, Aman MG, Eerdekens M, et al. Long-Term, Open-Label Study of Risperidone in Children With Severe Disruptive Behaviors and Below-Average IQ. Am J Psychiatry. 2004;161:677. doi: 10.1176/appi.ajp.161.4.677. [DOI] [PubMed] [Google Scholar]
- 61.Findling RL, Gracious BL, McNamara NK, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disorders. 2001;3:202. [PubMed] [Google Scholar]
- 62.Findling RL, McNamara NK, Gracious BL, et al. Quetiapine in nine youths with autistic disorder. J Child Adolesc Psychopharmacol. 2004;14:287. doi: 10.1089/1044546041649129. [DOI] [PubMed] [Google Scholar]
- 63.Findling RL, Reed MD, O’Riordan MA, et al. Effectiveness, safety, and pharmacokinetics of quetiapine in aggressive children with conduct disorder. J Am Acad Child Adolesc Psychiatry. 2006;45:792. doi: 10.1097/01.chi.0000219832.23849.31. [DOI] [PubMed] [Google Scholar]
- 64.Findling RL, Short EJ, McNamara NK, et al. Methylphenidate in the Treatment of Children and Adolescents With Bipolar Disorder and Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adoles Psychiatry. 2007;46:1445. doi: 10.1097/chi.0b013e31814b8d3b. [DOI] [PubMed] [Google Scholar]
- 65.Freeman MP. The comorbidity of bipolar and anxiety disorders: prevalence, psychobiology, and treatment issues. Journal Of Affective Disorders. 2002;68:1. doi: 10.1016/s0165-0327(00)00299-8. [DOI] [PubMed] [Google Scholar]
- 66.Garno JL, Goldberg JF, Ramirez PM, et al. Impact of childhood abuse on the clinical course of bipolar disorder. Br J Psychiatry. 2005;186:121. doi: 10.1192/bjp.186.2.121. [DOI] [PubMed] [Google Scholar]
- 67.Gaudiano BA, Miller IW. Anxiety disorder comobidity in Bipolar I Disorder: relationship to depression severity and treatment outcome. Depress Anxiety. 2005;21:71. doi: 10.1002/da.20053. [DOI] [PubMed] [Google Scholar]
- 68.Geller B, Cooper T, Sun K, et al. Double-blind and placebo controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:171. doi: 10.1097/00004583-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 69.Geller B, Fox L, Clark K. Rate and predictors of prepubertal bipolarity during follow-up of 6-to 12-year-old depressed children. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:461. doi: 10.1097/00004583-199405000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Geller B, Luby J. Child and adolescent bipolar disorder: A review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1168. doi: 10.1097/00004583-199709000-00008. [DOI] [PubMed] [Google Scholar]
- 71.Geller B, Sun K, Zimmerman B, et al. Complex and rapid-cycling in bipolar children and adolescents: A preliminary study. Journal of Affective Disorders. 1995;34:259. doi: 10.1016/0165-0327(95)00023-g. [DOI] [PubMed] [Google Scholar]
- 72.Geller B, Zimerman B, Williams M, et al. Diagnostic characteristics of 93 cases of a prepubertal and early adolescent bipolar disorder phenotype by gender, puberty and comorbid attention deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology. 2000;10:157. doi: 10.1089/10445460050167269. [DOI] [PubMed] [Google Scholar]
- 73.Giaconia RM, Reinherz HZ, Silverman AB, et al. Traumas and posttraumatic stress disorder in a community population of older adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:1369. doi: 10.1097/00004583-199510000-00023. [DOI] [PubMed] [Google Scholar]
- 74.Goodwin F, Jamison K. Manic-Depressive Illness. New York: Oxford University Press; 1990. [Google Scholar]
- 75.Goodwin RD, Hoven CW. Bipolar-panic comorbidity in the general population: prevalence and associated morbidity. J Affect Disord. 2002;70:27. doi: 10.1016/s0165-0327(01)00398-6. [DOI] [PubMed] [Google Scholar]
- 76.Greene RW, Doyle AE. Toward a transactional conceptualization of oppositional defiand disorder Implications for assessment and treatment. Clinical Child and Family Psychology Review. 1999;2:129. doi: 10.1023/a:1021850921476. [DOI] [PubMed] [Google Scholar]
- 77.Greenhill LL, Solomon M, Pleak R, et al. Molindone hydrochloride treatment of hospitalized children with conduct disorder. Journal of Clinical Psychiatry. 1985;46:20. [PubMed] [Google Scholar]
- 78.Grilo CM, Sanislow C, Fehon DC, et al. Psychological and behavioral functioning in adolescent psychiatric inpatients who report histories of childhood abuse. Am J Psychiatry. 1999;156:538. doi: 10.1176/ajp.156.4.538. [DOI] [PubMed] [Google Scholar]
- 79.Haas M, Karcher K, Pandina GJ. Treating disruptive behavior disorders with risperidone: a 1-year, open-label safety study in children and adolescents. J Child Adolesc Psychopharmacol. 2008;18:337. doi: 10.1089/cap.2007.0098. [DOI] [PubMed] [Google Scholar]
- 80.Hantouche EG, Demonfaucon C, Angst J, et al. [Cyclothymic obsessive-compulsive disorder. Clinical characteristics of a neglected and under-recognized entity] Presse Med. 2002;31:644. [PubMed] [Google Scholar]
- 81.Harpold T, Biederman J, Kwon A, et al. Examining the association between pediatric bipolar disorder and anxiety disorders in psychiatrically-referred children and adolescents. Atlanta, GA: 158th Annual meeting of the American Psyciatric Association; p. 187. [DOI] [PubMed] [Google Scholar]
- 82.Hertzberg MA, Butterfield MI, Feldman ME, et al. A preliminary study of lamotrigine for the treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;45:1226. doi: 10.1016/s0006-3223(99)00011-6. [DOI] [PubMed] [Google Scholar]
- 83.Herzberg B. The families of autistic children. In: Coleman M, editor. The autistic syndromes. North Holland: Amsterdam; 1976. p. 151. [Google Scholar]
- 84.Hlastala SA, Frank E, Kowalski J, et al. Stressful life events, bipolar disorder, and the “kindling model”. J Abnorm Psychol. 2000;109:777. doi: 10.1037//0021-843x.109.4.777. [DOI] [PubMed] [Google Scholar]
- 85.Hollander E, Wasserman S, Swanson EN, et al. A Double-Blind Placebo-Controlled Pilot Study of Olanzapine in Childhood/Adolescent Pervasive Developmental Disorder. J Child Adolesc Psychopharmacol. 2006;16:541. doi: 10.1089/cap.2006.16.541. [DOI] [PubMed] [Google Scholar]
- 86.Isaac G. Misdiagnosed bipolar disorder in adolescents in a special educational school and treatment program. Journal of Clinical Psychiatry. 1992;53:133. [PubMed] [Google Scholar]
- 87.Joffe RT, Swinson RP. Carbamazepine in obsessive-compulsive disorder. Biol Psychiatry. 1987;22:1169. doi: 10.1016/0006-3223(87)90061-8. [DOI] [PubMed] [Google Scholar]
- 88.Johnson JG, Cohen P, Brook JS. Associations between bipolar disorder and other psychiatric disorders during adolescence and early adulthood: A community-based longitudinal investigation. American Journal of Psychiatry. 2000;157:1679. doi: 10.1176/appi.ajp.157.10.1679. [DOI] [PubMed] [Google Scholar]
- 89.Joshi G, Biederman J, Wozniak J, et al. 161st American Psychiatric Association Annual Meeting (year?) Response to Second Generation Neuroleptics in Youth with Comorbid Bipolar Disorder and Pervasive Developmental Disorders. [Google Scholar]
- 90.Joshi G, Mick E, Wozniak J, et al. Impact of obsessive-compulsive disorder on antimanic response of pediatric bipolar disorder to second generation atypical antipsychotics. San Diego, CA: 53rd Annual Meeting of the American Academy of Child and Adolescent Psychiatry; (year?) [Google Scholar]
- 91.Joshi G, Morrow EM, Wozniak J, et al. 6th International Meeting for Autism Research. Prevalence and Clinical Correlates of Pervasive Developmental Disorders in Clinically Referred Population of Children and Adolescents. (year?) [Google Scholar]
- 92.Joshi G, Wozniak J, Geller D, et al. Clinical characteristics of comorbid obsessive-compulsive disorder and bipolar disorder in children and adolescents. Toronto, Ontario, Canada: The 52nd Annual Meeting of American Academy of Child and Adolescent Psychiatry; (year ?) [Google Scholar]
- 93.Joshi G, Wozniak J, Wilens T, et al. Clinical Characteristics of Youth with Comorbid Bipolar Disorder and Autism Spectrum Disorders. NIMH sponsored Annual Pediatric Bipolar Disorder Conference. (year?) [Google Scholar]
- 94.Kaplan SJ, Pelcovitz D, Salzinger S, et al. Adolescent physical abuse: risk for adolescent psychiatric disorders. Am J Psychiatry. 1998;155:954. doi: 10.1176/ajp.155.7.954. [DOI] [PubMed] [Google Scholar]
- 95.Kerbeshian J, Burd L. Case study Comorbidity among tourette’s syndrome, autistic disorder, and bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:681. doi: 10.1097/00004583-199605000-00024. [DOI] [PubMed] [Google Scholar]
- 96.Kessler RC, Adler L, Barkley R, et al. The prevalence correlates of adult ADHD in the United States Results from the national comorbidity survey replication. American Journal of Psychiatry. 2006;163:716. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol Med. 1997;27:1101. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- 98.Kessler RC, Stang PE, Wittchen HV, et al. Lifetime panic-depression comorbidity in the National Comorbidity Survey. Archives of General Psychiatry. 1998;55:801. doi: 10.1001/archpsyc.55.9.801. [DOI] [PubMed] [Google Scholar]
- 99.Khantzian EJ. The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review of Psychiatry. 1997;4:231. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- 100.King RA, Riddle MA, Chappel PB, Case study, et al. Emergence of self-destructive phenomena in children and adolescents during fluoxetine treatment. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:179. doi: 10.1097/00004583-199103000-00003. [DOI] [PubMed] [Google Scholar]
- 101.Klein D, Depue R, Slater J. Cyclothymia in the adolescent offspring of parents with bipolar affective disorder. Journal of Abnormal Psychology. 1985;94:115. doi: 10.1037//0021-843x.94.2.115. [DOI] [PubMed] [Google Scholar]
- 102.Kochman FJ, Hantouche EG, Millet B, et al. Trouble obsessionnel compulsif et bipolarite attenuee chez l’enfant et l’adolescent: re sultants de l’enquete ‘ABC-TOC’. Neuropsychiatr Enface Adolesc. 2002;50:1. [Google Scholar]
- 103.Komoto J, Seigo U, Hirata J. Infantile autism and affective disorder. Journal of Autism and Developmental Disorders. 1984;14:81. doi: 10.1007/BF02408557. [DOI] [PubMed] [Google Scholar]
- 104.Kovacs M, Pollock M. Bipolar disorder and comorbid conduct disorder in childhood and adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:715. doi: 10.1097/00004583-199506000-00011. [DOI] [PubMed] [Google Scholar]
- 105.Kowatch RA, Fristad M, Birmaher B, et al. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:213. doi: 10.1097/00004583-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 106.Kraepelin E. Manic-Depressive Insanity and Paranoia. Edinburgh: E. and S. Livingstone; 1921. [Google Scholar]
- 107.Kronenberger WG, Giauque AL, Lafata DE, et al. Quetiapine addition in methylphenidate treatment-resistant adolescents with comorbid ADHD, conduct/oppositional-defiant disorder, and aggression: a prospective, open-label study. J Child Adolesc Psychopharmacol. 2007;17:334. doi: 10.1089/cap.2006.0012. [DOI] [PubMed] [Google Scholar]
- 108.Kutcher SP, Marton P, Korenblum M. Relationship between psychiatric illness and conduct disorder in adolescents. Canadian Journal of Psychiatry. 1989;34:526. doi: 10.1177/070674378903400608. [DOI] [PubMed] [Google Scholar]
- 109.Lainhart JE, Folstein SE. Affective disorders in people with autism: a review of published cases. J Autism Dev Disord. 1994;24:587. doi: 10.1007/BF02172140. [DOI] [PubMed] [Google Scholar]
- 110.Leverich GS, Altshuler LL, Frye MA, et al. Risk of switch in mood polarity to hypomania or mania in patients with bipolar depression during acute and continuation trials of venlafaxine, sertraline, and bupropion as adjuncts to mood stabilizers. Am J Psychiatry. 2006;163:232. doi: 10.1176/appi.ajp.163.2.232. [DOI] [PubMed] [Google Scholar]
- 111.Lewinsohn P, Klein D, Seeley J. Bipolar disorders in a community sample of older adolescents: Prevalence, phenomenology, comorbidity, and course. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:454. [PubMed] [Google Scholar]
- 112.Looff D, Grimley P, Kuller F, et al. Carbamazepine for PTSD. J Am Acad Child Adolesc Psychiatry. 1995;34:703. doi: 10.1097/00004583-199506000-00008. [DOI] [PubMed] [Google Scholar]
- 113.Lorberg B, Martelon M, Parcell T, et al. AACAP. Chicago, IL: Self Medication in BPD Adolescents. (year and forum?) [Google Scholar]
- 114.Lum M, Fontaine R, Elie R, et al. Divalproex sodium’s anti-panic effect in panic disorder: A placebo-controlled study. Biological Psychiatry. 1990;27:164A. [Google Scholar]
- 115.MacKinnon D, Xu J, McMahon F, et al. Bipolar disorder panic disorder in families: An analysis of chromosome 18 data. American Journal of Psychiatry. 1998;155:829. doi: 10.1176/ajp.155.6.829. [DOI] [PubMed] [Google Scholar]
- 116.MacKinnon DF, Zandi PP, Cooper J, et al. Comorbid bipolar disorder and panic disorder in families with a high prevalence of bipolar disorder. Am J Psychiatry. 2002;159:30. doi: 10.1176/appi.ajp.159.1.30. [DOI] [PubMed] [Google Scholar]
- 117.Malone RP, Delaney MA, Hyman SB, et al. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17:779. doi: 10.1089/cap.2006.0126. [DOI] [PubMed] [Google Scholar]
- 118.Martin A, Koenig K, Scahill L, et al. Open-label quetiapine in the treatment of children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol. 1999;9:99. doi: 10.1089/cap.1999.9.99. [DOI] [PubMed] [Google Scholar]
- 119.Masi G, Millepiedi S, Mucci M, et al. A naturalistic study of referred children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:673. doi: 10.1097/01.chi.0000161648.82775.ee. [DOI] [PubMed] [Google Scholar]
- 120.Masi G, Milone A, Canepa G, et al. Olanzapine treatment in adolescents with severe conduct disorder. Eur Psychiatry. 2006;21:51. doi: 10.1016/j.eurpsy.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 121.Masi G, Perugi G, Toni C, et al. Obsessive-compulsive bipolar comorbidity: Focus on children and adolescents. Journal of Affective Disorders. 2004;78:175. doi: 10.1016/S0165-0327(03)00107-1. [DOI] [PubMed] [Google Scholar]
- 122.Masi G, Toni C, Perugi G, et al. Anxiety disorders in children and adolescents with bipolar disorder: a neglected comorbidity. Can J Psychiatry. 2001;46:797. doi: 10.1177/070674370104600902. [DOI] [PubMed] [Google Scholar]
- 123.McElroy SL, Altshuler LL, Suppes T, et al. Axis I Psychiatric Comorbidity and Its Relationship to Historical Illness Variables in 288 Patients With Bipolar Disorder. American Journal of Psychiatry. 2001;158:420. doi: 10.1176/appi.ajp.158.3.420. [DOI] [PubMed] [Google Scholar]
- 124.McElroy SL, Keck PE, Jr, Lawrence JM. Treatment of panic disorder and benzodiazepine withdrawal with valproate. J Neuropsychiatry Clin Neurosci. 1991;3:232. doi: 10.1176/jnp.3.2.232-a. [DOI] [PubMed] [Google Scholar]
- 125.McElroy SL, Strakowski SM, Keck PE, et al. Differences and similarities in mixed and pure mania. Comprehensive Psychiatry. 1995;36:187. doi: 10.1016/0010-440x(95)90080-f. [DOI] [PubMed] [Google Scholar]
- 126.McGlashan T. Adolescent versus adult onset of mania. American Journal of Psychiatry. 1988;145:221. doi: 10.1176/ajp.145.2.221. [DOI] [PubMed] [Google Scholar]
- 127.Mick E, Kim JW, Biederman J, et al. Family based association study of pediatric bipolar disorder and the dopamine transporter gene (SLC6A3) Am J Med Genet B Neuropsychiatr Genet. 2008 doi: 10.1002/ajmg.b.30745. [DOI] [PubMed] [Google Scholar]
- 128.Milberger S, Biederman J, Faraone SV, et al. Attention Deficit Hyperactivity Disorder and comorbid disorders Issues of overlapping symptoms. American Journal of Psychiatry. 1995;152:1793. doi: 10.1176/ajp.152.12.1793. [DOI] [PubMed] [Google Scholar]
- 129.Moore CM, Biederman J, Wozniak J, et al. Differences in brain chemistry in children and adolescents with attention deficit hyperactivity disorder with and without comorbid bipolar disorder: a proton magnetic resonance spectroscopy study. Am J Psychiatry. 2006;163:316. doi: 10.1176/appi.ajp.163.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moore JM, Jr, Thompson-Pope SK, Whited RM. MMPI-A profiles of adolescent boys with a history of firesetting. J Pers Assess. 1996;67:116. doi: 10.1207/s15327752jpa6701_9. [DOI] [PubMed] [Google Scholar]
- 131.Morel BA. Traite de Maladies Metales. Paris: Libairie Victor Masson; 1860. [Google Scholar]
- 132.Nagaraj R, Singhi P, Malhi P. Risperidone in children with autism: randomized, placebo controlled, double-blind study. Journal of Child Neurology. 2006;21:450. doi: 10.1177/08830738060210060801. [DOI] [PubMed] [Google Scholar]
- 133.Nierenberg AA, Miyahara S, Spencer T, et al. Clinical and Diagnostic Implications of Lifetime Attention-Deficit/Hyperactivity Disorder Comorbidity in Adults with Bipolar Disorder: Data from the First 1000 STEP-BD Participants. Biol Psychiatry. 2005;57:1467. doi: 10.1016/j.biopsych.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 134.Olvera RL, Hunter K, Fonseca M, et al. Juvenile onset bipolar disorder and comorbid anxiety. Toronto, Ontario, Canada: The 52nd Annual Meeting of American Academy of Child and Adolescent Psychiatry; [Google Scholar]
- 135.Otte C, Wiedemann K, Yassouridis A, et al. Valproate monotherapy in the treatment of civilian patients with non-combat-related posttraumatic stress disorder: an open-label study. J Clin Psychopharmacol. 2004;24:106. doi: 10.1097/01.jcp.0000106234.36344.a4. [DOI] [PubMed] [Google Scholar]
- 136.Otto MW, Simon NM, Wisniewski SR, et al. Prospective 12-month course of bipolar disorder in outpatients with and without comorbid anxiety disorders. Br J Psychiatry. 2006;189:20. doi: 10.1192/bjp.bp.104.007773. [DOI] [PubMed] [Google Scholar]
- 137.Perugi G. Depressive comorbidity of panic, social phobic obsessive compulsive disorders re-examined: is there a bipolarII connection? Journal of Psychiatric Research. 1999;33:53. doi: 10.1016/s0022-3956(98)00044-2. [DOI] [PubMed] [Google Scholar]
- 138.Perugi G, Akiskal HS, Pfanner C, et al. The clinical impact of bipolar and unipolar affective comorbidity on obsessive-compulsive disorder. J Affect Disord. 1997;46:15. doi: 10.1016/s0165-0327(97)00075-x. [DOI] [PubMed] [Google Scholar]
- 139.Perugi G, Toni C, Frare F, Obsessive-compulsive-bipolar comorbidity, et al. a systematic exploration of clinical features and treatment outcome. J Clin Psychiatry. 2002;63:1129. [PubMed] [Google Scholar]
- 140.Petty F, Davis LL, Nugent AL, et al. Valproate therapy for chronic, combat-induced posttraumatic stress disorder. J Clin Psychopharmacol. 2002;22:100. doi: 10.1097/00004714-200202000-00021. [DOI] [PubMed] [Google Scholar]
- 141.Posey DJ, Wiegand RE, Wilkerson J, et al. Open-label atomoxetine for attention-deficit/ hyperactivity disorder symptoms associated with high-functioning pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16:599. doi: 10.1089/cap.2006.16.599. [DOI] [PubMed] [Google Scholar]
- 142.Rasmussen S, Eisen J. The epidemiology and differential diagnosis of obsessive compulsive disorder. Journal of Clinical Psychiatry. 1992;53:4. [PubMed] [Google Scholar]
- 143.Reyes M, Buitelaar J, Toren P, et al. A randomized, double-blind, placebo-controlled study of risperidone maintenance treatment in children and adolescents with disruptive behavior disorders. Am J Psychiatry. 2006;163:402. doi: 10.1176/appi.ajp.163.3.402. [DOI] [PubMed] [Google Scholar]
- 144.Reyes M, Croonenberghs J, Augustyns I, et al. Long-term use of risperidone in children with disruptive behavior disorders and subaverage intelligence: efficacy, safety, and tolerability. J Child Adolesc Psychopharmacol. 2006;16:260. doi: 10.1089/cap.2006.16.260. [DOI] [PubMed] [Google Scholar]
- 145.Riggs P, Mikulich S, Coffman L, et al. Fluoxetine in drug-dependent delinquents with major depression: An open trial. Journal of Child and Adolescent Psychopharmacology. 1997;7:87. doi: 10.1089/cap.1997.7.87. [DOI] [PubMed] [Google Scholar]
- 146.Robins L, Price R. Adult disorders predicted by childhood conduct problems: Results from the NIMH epidemiologic catchment area project. Psychiatry. 1991;54:116. doi: 10.1080/00332747.1991.11024540. [DOI] [PubMed] [Google Scholar]
- 147.Sachs GS, Baldassano CF, Truman CJ, et al. Comorbidity of attention deficit hyperactivity disorder with early- and late-onset bipolar disorder. American Journal of Psychiatry. 2000;157:466. doi: 10.1176/appi.ajp.157.3.466. [DOI] [PubMed] [Google Scholar]
- 148.Savino M, Perugi G, Simonini E, et al. Affective comorbidity in panic disorder: Is there a bipolar connection? Journal of Affective Disorders. 1993;28:155. doi: 10.1016/0165-0327(93)90101-o. [DOI] [PubMed] [Google Scholar]
- 149.Scheffer RE, Kowatch RA, Carmody T, et al. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry. 2005;162:58. doi: 10.1176/appi.ajp.162.1.58. [DOI] [PubMed] [Google Scholar]
- 150.Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114:e634. doi: 10.1542/peds.2003-0264-F. [DOI] [PubMed] [Google Scholar]
- 151.Sheard MH. Lithium in the treatment of aggression. J Nerv Ment Dis. 1975;160:108. doi: 10.1097/00005053-197502000-00005. [DOI] [PubMed] [Google Scholar]
- 152.Snyder SH. Fourty years of neurotransmitters: A personal account. Arch Gen, Psychiatry. 2002;59:983. doi: 10.1001/archpsyc.59.11.983. [DOI] [PubMed] [Google Scholar]
- 153.Sovner R, Hurley AD. Do the mentally retarded suffer from affective illness? Archives of General Psychiatry. 1983;40:61. doi: 10.1001/archpsyc.1983.01790010063008. [DOI] [PubMed] [Google Scholar]
- 154.State RC, Frye MA, Altshuler LL, et al. Chart review of the impact of attention-deficit/hyperactivity disorder comorbidity on response to lithium or divalproex sodium in adolescent mania. J Clin Psychiatry. 2004;65:1057. doi: 10.4088/jcp.v65n0805. [DOI] [PubMed] [Google Scholar]
- 155.Steinbuchel P, Wilens TE, Adamson J, et al. Posttraumatic Stress Disorder and Substance Use Disorder in Adolescent Bipolar Disorder. 2008 doi: 10.1111/j.1399-5618.2008.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Steingard R, Biederman J. Lithium responsive manic-like symptoms in two individuals with autism and mental retardation. Journal of American Academy of Child and Adolescent Psychiatry. 1987;26:932. doi: 10.1097/00004583-198726060-00021. [DOI] [PubMed] [Google Scholar]
- 157.Stewart JT, Bartucci RJ. Posttraumatic stress disorder and partial complex seizures. Am J Psychiatry. 1986;143:113. doi: 10.1176/ajp.143.1.113. [DOI] [PubMed] [Google Scholar]
- 158.Stigler KA, Diener JT, Kohn AE, et al. A prospective, open-label study of aripiprazole in youth with Asperger’s disorder and pervasive development disorder not otherwise specified. Neuropscyhopharmacology. 2006;31:S194. [Google Scholar]
- 159.Strakowski S, Sax K, McElroy S, et al. Course of psychiatric and substance abuse syndromes co-occurring with bipolar disorder after a first psychiatric hospitalization. Journal of Clinical Psychiatry. 1998;59:465. doi: 10.4088/jcp.v59n0905. [DOI] [PubMed] [Google Scholar]
- 160.Strober M, Carlson G. Bipolar illness in adolescents with major depressionClinical, genetic, and psychopharmacologic predictors in a three- to four-year prospective follow-up investigation. Archives of General Psychiatry. 1982;39:549. doi: 10.1001/archpsyc.1982.04290050029007. [DOI] [PubMed] [Google Scholar]
- 161.Strober M, DeAntonio M, Schmidt-Lackner S, et al. Early childhood attention deficit hyperactivity disorder predicts poorer response to acute lithium therapy in adolescent mania. Journal of Affective Disorders. 1998;51:145. doi: 10.1016/s0165-0327(98)00213-4. [DOI] [PubMed] [Google Scholar]
- 162.Strober M, Schmidt-Lackner S, Freeman R, et al. Recovery and relapse in adolescents with bipolar affective illness: A five-year naturalistic, prospective follow-up. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:724. doi: 10.1097/00004583-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 163.Tarter RE. Evaluation, treatment of adolescent substance abuseA decision tree method. American Journal of Drug & Alcohol Abuse. 1990;16:1. doi: 10.3109/00952999009001570. [DOI] [PubMed] [Google Scholar]
- 164.Tillman R, Geller B, Bolhofner K, et al. Ages of onset and rates of syndromal and subsyndromal comorbid DSM-IV diagnoses in a prepubertal and early adolescent bipolar disorder phenotype. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:1486. doi: 10.1097/00004583-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 165.Tondo L, Burrai C, Scamonatti L, et al. Carbamazepine in panic disorder. Am J Psychiatry. 1989;146:558. doi: 10.1176/ajp.146.4.558b. [DOI] [PubMed] [Google Scholar]
- 166.Towbin KE, Pradella A, Gorrindo T, et al. Autism spectrum traits in children with mood and anxiety disorders. J Child Adolesc Psychopharmacol. 2005;15:452. doi: 10.1089/cap.2005.15.452. [DOI] [PubMed] [Google Scholar]
- 167.Turgay A, Binder C, Snyder R, et al. Long-term safety and efficacy of risperidone for the treatment of disruptive behavior disorders in children with subaverage IQs. Pediatrics. 2002;110:e34. doi: 10.1542/peds.110.3.e34. [DOI] [PubMed] [Google Scholar]
- 168.West S, McElroy S, Strakowski S, et al. Attention deficit hyperactivity disorder in adolescent mania. American Journal of Psychiatry. 1995;152:271. doi: 10.1176/ajp.152.2.271. [DOI] [PubMed] [Google Scholar]
- 169.West SA, Strakowski SM, Sax KW, et al. Phenomenology and comorbidity of adolescents hospitalized for the treatment of acute mania. Biological Psychiatry. 1996;39:458. doi: 10.1016/0006-3223(95)00525-0. [DOI] [PubMed] [Google Scholar]
- 170.Wilens T, Biederman J, Abrantes A, et al. Clinical characteristics of psychiatrically referred adolescent outpatients with substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:941. doi: 10.1097/00004583-199707000-00016. [DOI] [PubMed] [Google Scholar]
- 171.Wilens T, Biederman J, Forkner P, et al. Patterns of comorbidity and dysfunction in clinically referred preschoolers with bipolar disorder. Journal of Child and Adolescent Psychopharmacology. 2003;13:495. doi: 10.1089/104454603322724887. [DOI] [PubMed] [Google Scholar]
- 172.Wilens T, Biederman J, Milberger S, et al. Is bipolar disorder a risk for cigarette smoking in ADHD youth? American Journal on Addictions. 2000;9:187. doi: 10.1080/10550490050148017. [DOI] [PubMed] [Google Scholar]
- 173.Wilens T, Martelon M, Kruesi M. Journal of Clinical Psychiatry. Does Conduct Disorder Mediate the Development of Substance Use Disorders in Adolescents With Bipolar Disorder? A Controlled Study. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wilens T, Prince J, Spencer T, et al. An Open Trial of Bupropion for the Treatment of Adults with Attention Deficit Hyperactivity Disorder and Bipolar Disorder. Biological Psychiatry. 2003;54:9. doi: 10.1016/s0006-3223(02)01664-5. [DOI] [PubMed] [Google Scholar]
- 175.Wilens TE, Biederman J, Adamson JJ, et al. Further evidence of an association between adolescent bipolar disorder with smoking and substance use disorders: A controlled study. Drug Alcohol Depend. 2008;95:188. doi: 10.1016/j.drugalcdep.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wilens TE, Biederman J, Millstein R, et al. Risk for substance use disorders in youth with child- and adolescent-onset bipolar disorder. J Am Acad Child Adolesc Psychiatry. 1999;38:680. doi: 10.1097/00004583-199906000-00014. [DOI] [PubMed] [Google Scholar]
- 177.Wilens TE, Gignac M. Treatment of Bipolar Disorder in Children and Adolescents. In: Geller B, DelBello MP, editors. Pediatric Bipolar Disorder and Substance Use Disorders. 2008. [Google Scholar]
- 178.Wing L. Asperger’s syndrome: a clinical account. Psychol Med. 1981;11:115. doi: 10.1017/s0033291700053332. [DOI] [PubMed] [Google Scholar]
- 179.Winokur G, Coryell W, Akiskal HS, et al. Alcoholism in manic-depressive (bipolar) illness: Familial illness, course of illness, and the primary-secondary distinction. American Journal of Psychiatry. 1995;152:365. doi: 10.1176/ajp.152.3.365. [DOI] [PubMed] [Google Scholar]
- 180.Wolf ME, Alavi A, Mosnaim AD. Posttraumatic stress disorder in Vietnam veterans clinical and EEG findings; possible therapeutic effects of carbamazepine. Biol Psychiatry. 1988;23:642. doi: 10.1016/0006-3223(88)90011-x. [DOI] [PubMed] [Google Scholar]
- 181.Wozniak J, Biederman J. A pharmacological approach to the quagmire of comorbidity in juvenile mania. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:826. doi: 10.1097/00004583-199606000-00023. [DOI] [PubMed] [Google Scholar]
- 182.Wozniak J, Biederman J, Faraone S, et al. Mania in children with pervasive developmental disorder revisited. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1552. doi: 10.1016/S0890-8567(09)66564-3. [DOI] [PubMed] [Google Scholar]
- 183.Wozniak J, Biederman J, Faraone SV, et al. Heterogeneity of childhood conduct disorder: further evidence of a subtype of conduct disorder linked to bipolar disorder. Journal of Affective Disorders. 2001;64:121. doi: 10.1016/s0165-0327(00)00217-2. [DOI] [PubMed] [Google Scholar]
- 184.Wozniak J, Biederman J, Monuteaux MC, et al. Parsing the comorbidity between bipolar disorder and anxiety disorders: A familial risk analysis. Journal of Child and Adolescent Psychopharmacology. 2002;12:101. doi: 10.1089/104454602760219144. [DOI] [PubMed] [Google Scholar]
- 185.Young L, Cooke R, Robb J, et al. Anxious and non-anxious bipolar disorder. Journal of Affective Disorders. 1993;29:49. doi: 10.1016/0165-0327(93)90118-4. [DOI] [PubMed] [Google Scholar]