Abstract
Objective
To assess the safety and efficacy of ABT-089, a novel α4β2 neuronal nicotinic receptor partial agonist, vs. placebo in children with attention-deficit/hyperactivity disorder (ADHD).
Method
Two multicenter, randomized, double-blind, placebo-controlled, parallel-group studies of children 6–12 years old were conducted. Study 1 (n=274) assessed six treatment groups over 8 weeks: 4 once-daily (QD) ABT-089 doses (0.085–0.700 mg/kg), QD atomoxetine, and placebo.Study 2 (n=119) assessed three treatment groups over 6 weeks: 2 QD ABT-089 doses (0.7 mg/kg, 1.4 mg/kg) and placebo. The primary efficacy variable was the investigator-administered ADHD Rating Scale-IV: Home Version (ADHD-RS-IV [HV]) Total Score. Safety was assessed by adverse event (AE) monitoring, lab tests, vital signs, physical exams, and electrocardiogram measures.
Results
There was no statistically significant difference between ABT-089 and placebo in mean change from baseline to final evaluation of ADHD-RS-IV (HV) Total Score or other outcome measures at any dose in either study; in Study 1, atomoxetine showed statistically significant improvement for the primary and most secondary endpoints. ABT-089 was generally safe and well tolerated, with no statistically significant difference between any ABT-089 dose and placebo in the overall incidence of any specific AE, and no clinically significant changes in other safety measures.
Conclusions
ABT-089 did not show efficacy on the primary efficacy variable, the ADHD-RS-IV (HV) Total Score, or other measures of ADHD symptomatology in children with ADHD, and had a safety profile similar to placebo. These results contrast with published reports of efficacy of nicotinic modulators in adults with ADHD.
Keywords: ADHD, pharmacologic treatment, α4β2, neuronal nicotinic receptor, children
INTRODUCTION
Increasing interest has been focused on the role of the cholinergic system in cognitive disturbances, including attention-deficit/hyperactivity disorder (ADHD). There is a large body of evidence demonstrating that cholinergic signaling is involved in cognition,1 and studies have shown that cholinergic dysregulation, particularly of the nicotinic cholinergic system, may be involved in ADHD pathophysiology.2 Additionally, in utero nicotine exposure, which may influence development of the cholinergic system, has been implicated as a risk factor for ADHD.3 Furthermore, ADHD is also linked to a higher risk of nicotine use, including an earlier-onset, more severe and persistent course of cigarette smoking.4,5 Conversely, nicotine has been shown to ameliorate ADHD symptoms,6,7,2 potentially explaining the link with excess cigarette smoking in ADHD. Recently, a class of agents has been developed to selectively modulate nicotinic cholinergic signaling that may provide a treatment alternative with a favorable efficacy and side effect profile compared with existing treatments.
The nicotinic cholinergic system includes pharmacologically and functionally distinct neuronal nicotinic receptor (NNR) subtypes that are multimeric complexes composed of structurally related subunits.8 NNRs of varying subunit compositions are responsible for mediating the broad effects of nicotine, including positive effects (analgesia and cognitive enhancement)9,10 and negative effects (physical dependence, nausea, and increased heart rate).11 The α4β2 NNR is primarily distributed in brain regions involved in cognition and is thought to mediate cognitive processes.1 By contrast, other NNR subtypes, such as α3β4 NNRs, are predominantly distributed in the autonomic nervous system, suggesting involvement in the adverse effects of NNR agonists.11 ABT-089 is a novel NNR partial agonist with high in vitro binding affinity and selectivity for the α4β2 NNR subtype (Ki~15 nM) and weak agonism at other NNR subtypes, particularly α3β4 NNRs.12 In preclinical studies, ABT-089 has shown minimal effects on dopaminergic transmission, suggesting it may not be associated with concerns often seen with NNR activation by nicotine, such as abuse liability and insomnia.13
In a previous proof-of-concept study in adults with ADHD that evaluated three doses of ABT-089 using a crossover design, ABT-089 was associated with significant improvement in ADHD symptomatology vs. placebo.14 Recently, a larger crossover study in adults with ADHD compared five doses of ABT-089 with placebo. In this study, ABT-089 was significantly superior to placebo on ADHD outcome at 40 mg given once or twice daily and was generally well tolerated (submitted). Given these positive findings in adults, the current studies examined the efficacy and tolerability of ABT-089 in pediatric ADHD. To fully explore the dose-response relationship, the doses administered in these studies were the pediatric equivalents of doses evaluated in the recent adult ADHD study, as well as the pediatric equivalent of an adult 30 mg once-daily (QD) dose.
To our knowledge, this is the first report of a nicotinic analog in treating pediatric ADHD. We present the results of two controlled Phase 2 trials comprising 393 pediatric subjects, aged 6 to 12 years, treated with ABT-089. We hypothesized that ABT-089 would be superior to placebo in the treatment of ADHD symptomatology. Secondarily, we hypothesized improvement in functional outcomes and examined the tolerability and safety of ABT-089 in this pediatric population.
METHOD
Study Patients
Males and females, aged 6–12 years (inclusive), with a DSM-IV diagnosis of any ADHD subtype, confirmed by the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL),15 and a rating of 4 or higher on the Clinical Global Impression-ADHD-Severity Scale (CGI-ADHD-S) were enrolled at 23 sites (Study 1: 10; Study 2: 3; Studies 1 and 2: 10) in the United States (September 2007 - July 2008). For all sites, an institutional review board approved the study protocol. A parent/caregiver of each youth provided informed consent, and subjects ages 7–12 provided written assent.
Exclusion criteria included: current or past diagnosis of bipolar I, II, or NOS (Not Otherwise Specified) disorder; psychotic disorder; autism, Asperger’s syndrome or pervasive developmental disorder; tics or Tourette syndrome; seizure disorder; traumatic brain injury; current diagnosis of obsessive-compulsive disorder, eating disorder, anxiety disorder, or depressive disorder requiring treatment of any kind; psychotropic medications within 14 days or 5 half-lives (7 days for stimulants), whichever was longer, prior to the Day −1 ADHD Rating Scale-IV: Home Version (ADHD-RS-IV [HV]) assessment; in Study 1, atomoxetine within 3 months of randomization or not a suitable candidate to receive atomoxetine. Failure to respond to two or more adequate trials of U.S. Food and Drug Administration-approved ADHD medication was also exclusionary.
Study Design and Treatments
Study 1 consisted of a screening/wash-out period of up to two weeks and an 8-week, double-blind treatment period (study visits on Days 7, 14, 28, 42, 56). Doses were chosen to evaluate pediatric equivalent doses (on a mg/kg basis) of the doses chosen for the adult crossover study of ABT-089 in ADHD. At the time Study 1 was initiated, the available safety and tolerability data for ABT-089 in children limited the highest absolute dose to 40 mg QD. Patients were randomized in equal proportion to one of six treatment groups: four QD doses of ABT-089 (0.085 mg/kg, 0.260 mg/kg, 0.520 mg/kg, 0.700 mg/kg), QD atomoxetine (included as a measure of assay sensitivity; initiated at 0.5 mg/kg/day and increased after two weeks to 1.2 mg/kg/day), or placebo. When results from the adult crossover study in ADHD indicated that total daily doses of 40 mg and 80 mg were efficacious, additional safety and tolerability data were obtained to support a second pediatric study to test doses up to 80 mg QD. Study 2 consisted of a screening/wash-out period of up to two weeks and a 6-week double-blind treatment period (study visits on Days 7, 14, 28, 42). Patients were randomized in equal proportion to 0.7 mg/kg ABT-089 QD, 1.4 mg/kg ABT-089 QD, or placebo. In both studies, a central interactive voice response system (IVRS) was used for subject enrollment and treatment allocation. The sponsor, investigative sites, and subject were blinded to each subject's treatment. Blind-breaking information was available via the IVRS, as required in an emergency. For each study, study drug and placebo were identical in appearance.
Assessments
The primary outcome variable was the investigator-administered ADHD-RS-IV (HV) Total Score (assessed at each study visit). The ADHD-RS-IV (HV) is an 18-item scale based on the DSM-IV criteria that produces three scale scores: Inattention, Hyperactivity/Impulsivity, and Total.16 Each item on the scale was scored from 0 to 3 (0 = never or rarely, 1 = sometimes, 2 = often, 3 = very often) and ratings were based on the severity of symptoms over the previous seven days. Secondary efficacy was assessed by the following measures: ADHD-RS-IV (HV) Inattention and Hyperactivity/Impulsivity subscale scores; Clinical Global Impression ADHD Severity (CGI-ADHD-S)17; Conners’ Global Index-Parent Version (CGI-P)18; Behavior Rating Inventory of Executive Function-Parent Questionnaire (BRIEF)19; Child’s Sleep Habits Questionnaire (CSHQ)20; ADHD Impact Module-Child (AIM-C)21; and the Child Health Questionnaire (CHQ).22 Study 1 also included the ADHD-RS-IV (School Version).16
The ADHD-RS-IV (HV) and CGI-ADHD-S were administered by raters with Doctoral degrees with at least 2 years previous clinical experience with the patient population and who had completed didactic training on scale administration and scoring. In addition, ADHD-RS-IV (HV) raters must have correctly answered ≥ 80% of the items on the ADHD-RS-IV (HV) on a standardized assessment. The protocols specified that each subject was to be evaluated by the same rater at each assessment.
Plasma concentrations of ABT-089 were assessed on Days 14 and 28 (both studies), Day 42 (Study 2) and Day 56 (Study 1), prior to the morning dose of drug whenever possible, using a liquid chromatography method with mass spectrometric detection (internally validated technique, Abbott, Abbott Park, IL). Plasma ABT-089 concentrations were expected to reach steady state by Day 14 of dosing; therefore, for calculation of summary statistics, plasma concentrations of ABT-089 for each dose level were combined across all study visits and summarized by dose group and time since previous dose window.
Treatment-emergent AEs, laboratory tests (including clinical chemistry, hematology and urinalysis panels), vital signs and electrocardiogram (ECG) measurements were monitored throughout both studies. A treatment-emergent AE was defined as any AE that began or worsened in severity on or after the first day of study drug dosing. Laboratory, vital sign and ECG abnormalities were considered AEs if they resulted in study discontinuation, necessitated therapeutic medical intervention, or the investigator considered them to be AEs.
Statistical Methods
Sample sizes were based on an assumed effect of size of 0.7 (comparable to atomoxetine effect sizes in earlier studies23,24), with a treatment difference for ABT-089 vs. placebo of 9.1 in the ADHD-RS-IV (HV) Total Score and a pooled standard deviation of 13. In Study 1, a sample size of 45 subjects per treatment group provided at least 90% power for a onesided test of ABT-089 vs. placebo (α = 0.05), and in Study 2, a sample size of 35 subjects per treatment group provided at least 85% power for a one-sided test of ABT-089 vs. placebo (α = 0.05), assuming 5% of subjects would not have efficacy assessments after randomization. The one-sided test was chosen because ABT-089 had to demonstrate improvement compared to placebo to be considered effective.
Efficacy analyses were conducted on the intent-to-treat (ITT) dataset, defined as randomized subjects who took at least one dose of study drug and had at least one on-treatment ADHD-RS-IV (HV) evaluation. The primary comparisons were each dose of ABT-089 vs. placebo. In Study 1, atomoxetine vs. placebo was also evaluated. No adjustments for multiple comparisons were made because these were Phase 2 proof-of-concept studies with the primary objective of evaluating each ABT-089 dose relative to placebo. Baseline was the last non-missing observation that occurred on or before the first day of study drug administration, and “final measure” was the last non-missing observation in the double-blind treatment period. Subjects with a baseline evaluation and at least one post-baseline evaluation for a variable of interest were included in the analyses.
The primary efficacy variable was change from baseline to final evaluation in the ADHD-RS-IV (HV) Total Score. The primary analysis was an analysis of covariance (ANCOVA) with factors of treatment group and site, with baseline score as a covariate. Estimates of treatment difference for active treatment vs. placebo, as well as associated confidence intervals, were obtained within the ANCOVA framework. Change from baseline to each evaluation in ADHD-RS-IV (HV) Total Score was also evaluated with a repeated measures analysis using a model that included the fixed categorical effects of treatment group, site, visit, and treatment group by visit interaction, as well as the continuous fixed covariates of baseline score and baseline score-byvisit interaction. Analyses for change from baseline to final evaluation and repeated measures analysis of each of the secondary efficacy endpoints, with the exception of CGI-P, were performed using the same models as the primary efficacy variable. Because the CGI-P was to be completed at multiple time points on the same day, it was analyzed using a repeated measures model with factors of treatment group, site and time of day, an interaction term of treatment group by time of day, and time-matched baseline score as covariate.
Safety analyses were performed on the safety dataset, defined as randomized subjects who took at least one dose of study drug. Statistical tests for safety were two-sided (α = 0.05). AEs were coded using the Medical Dictionary for Regulatory Activities Version 10.0,25 and the percentage of subjects in each active dose group reporting each preferred term was compared with placebo using Fisher's exact test. Treatment differences in change from baseline to minimum, maximum, and final evaluation for clinical laboratory evaluations and vital sign variables were analyzed using a one-way analysis of variance (ANOVA).
RESULTS
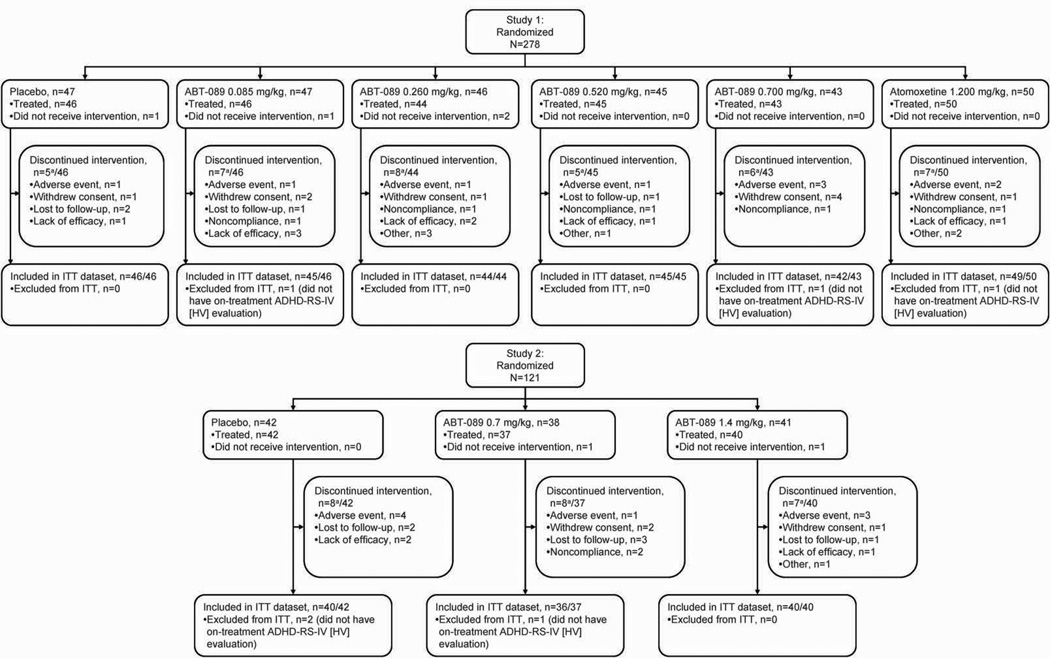
Two-hundred seventy-eight subjects were randomized into Study 1, and 274 of these subjects received at least one dose of study drug (safety dataset). Two-hundred seventy-one subjects (99% of treated subjects) were included in the ITT dataset, and 236 subjects (86% of treated subjects) completed Study 1. One-hundred twenty-one subjects were randomized into Study 2, and 119 of these subjects received at least one dose of study drug (safety dataset). One-hundred sixteen subjects (97% of treated subjects) were included in the ITT dataset, and 96 subjects (81% of treated subjects) completed Study 2. There were no differences in the reasons leading to premature discontinuation between treatment groups in either study (Figure 1).
Figure 1.
Subject disposition and flow through studies
Note: aSubjects may have reported more than one reason for premature discontinuation, but are counted only once in the total
Baseline characteristics did not differ between treatment groups within or between studies (Table 1). Most subjects were male (66%, both studies) and the mean ages were 8.6 (Study 1) and 8.5 (Study 2) years. Most subjects were diagnosed with the combined ADHD subtype (Study 1: 80%; Study 2: 76%). The proportion of subjects who had taken any ADHD medication or had taken stimulants was similar across treatment groups in both studies (data not shown).
Table 1.
Baseline Characteristics (Intent-to-Treat Dataset)
| Study 1, n=271 |
Study 2, n=116 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PBO (n=46) |
ABT-089 (mg/kg) |
ATM (n=49) |
pa | PBO (n=40) |
ABT-089 (mg/kg) |
pa | |||||
| 0.085 (n=45) |
0.260 (n=44) |
0.520 (n=45) |
0.700 (n=42) |
0.7 (n=36) |
1.4 (n=40) |
||||||
| Mean age, years (SD) | 8.6 (1.88) | 8.7 (1.75) | 8.4 (1.75) | 8.6 (1.82) | 8.7 (1.90) | 8.7 (2.02) | .956 | 8.4 (2.12) | 8.7 (1.77) | 8.4 (1.90) | .669 |
| Gender | .630 | .829 | |||||||||
| Male, n (%) | 28 (61) | 32 (71) | 25 (57) | 32 (71) | 27 (64) | 34 (69) | 25 (63) | 25 (69) | 27 (68) | ||
| Female, n (%) | 18 (39) | 13 (29) | 19 (43) | 13 (29) | 15 (36) | 15 (31) | 15 (38) | 11 (31) | 13 (33) | ||
| Race | .606b | .685b | |||||||||
| White, n (%) | 28 (61) | 32 (71) | 33 (75) | 27 (60) | 27 (64) | 34 (69) | 27 (68) | 26 (72) | 25 (63) | ||
| Black, n (%) | 14 (30) | 11 (24) | 9 (21) | 13 (29) | 12 (29) | 15 (31) | 8 (20) | 9 (25) | 13 (33) | ||
| Other, n (%) | 4 (9) | 2 (4) | 2 (5) | 5 (11) | 3 (7) | 0 | 5 (13) | 1 (3) | 2 (5) | ||
| ADHD subtype diagnosed at Screening, n (%) | |||||||||||
| Combined | 39 (85) | 37 (82) | 37 (84) | 36 (80) | 30 (71) | 38 (78) | .668c | 33 (83) | 23 (64) | 32 (80) | .133c |
| Hyperactive-Impulsive | 2 (4) | 0 | 2 (5) | 0 | 0 | 2 (4) | 0 | 1 (3) | 0 | ||
| Inattentive | 5 (11) | 8 (18) | 5 (11) | 9 (20) | 12 (29) | 9 (18) | 7 (18) | 12 (33) | 8 (20) | ||
| Diagnosis and treatment status | |||||||||||
| Not previously diagnosed, n (%) | 20 (43) | 24 (53) | 17 (39) | 27 (60) | 24 (57) | 24 (49) | .317d | 22 (55) | 21 (58) | 22 (55) | .945d |
| Previously diagnosed, untreated, n (%) | 6 (13) | 5 (11) | 5 (11) | 1 (2) | 1 (2) | 3 (6) | 3 (8) | 2 (6) | 4 (10) | ||
| Previously diagnosed, treated, n (%) | 20 (43) | 16 (36) | 22 (50) | 17 (38) | 17 (40) | 22 (45) | 15 (38) | 13 (36) | 14 (35) | ||
Note: ABT-089 = a novel α4β2 neuronal nicotinic receptor partial agonist; ADHD=attention-deficit/hyperactivity disorder; ATM = atomoxetine; PBO = placebo.
P-values for differences between treatment groups (within-study) from Fisher’s Exact Test;
Non-white races combined for analysis;
P-value for number of subjects with Combined subtype vs. non-Combined subtype;
P-value for number of subjects previously diagnosed vs. not previously diagnosed.
Efficacy
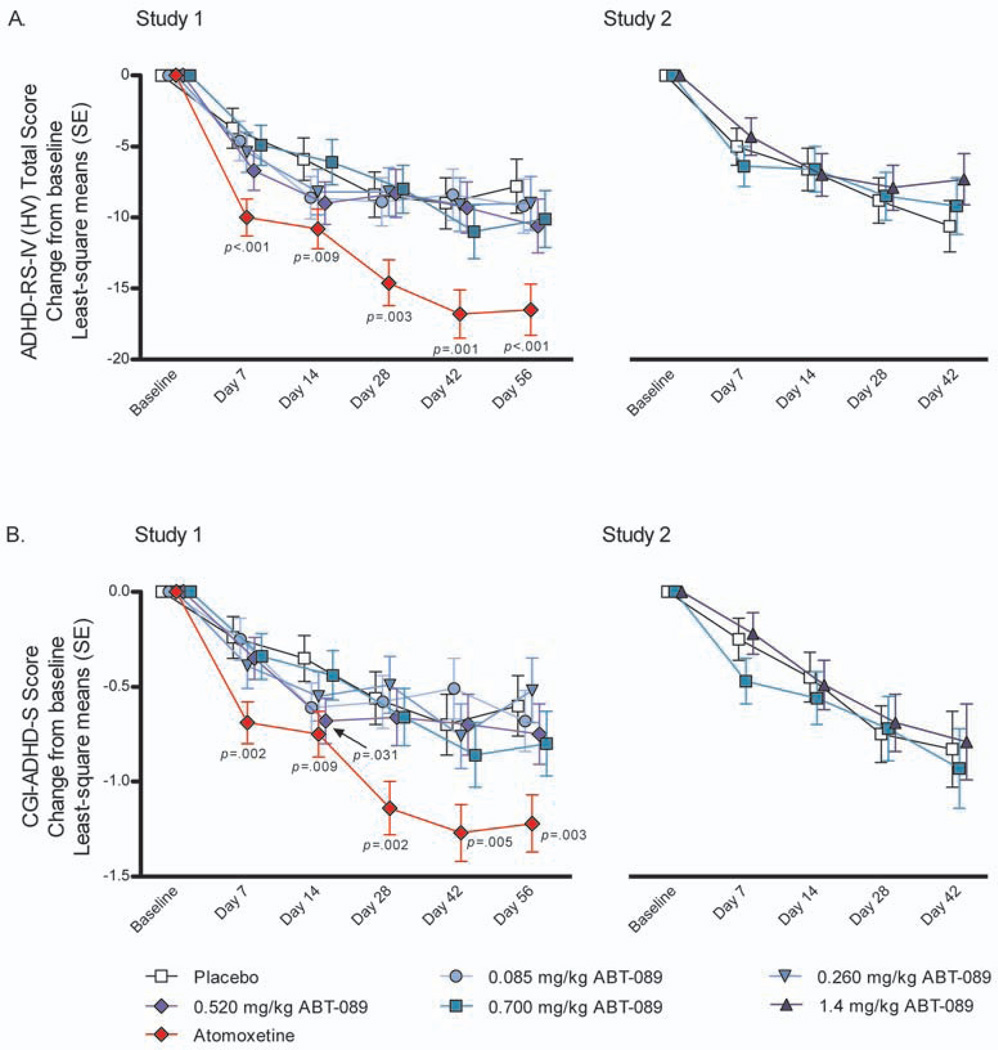
At all ABT-089 doses examined in both studies, there was no statistically significant difference between ABT-089 and placebo in the primary efficacy analysis of mean change from baseline to final evaluation of the ADHD-RS-IV (HV) Total Score (Table 2), or on the secondary analysis of mean change from baseline to each evaluation (Figure 2A). In contrast, a statistically significant treatment effect was observed for atomoxetine compared with placebo at each evaluation in Study 1 (Table 2, Figure 2A).
Table 2.
Mean Change from Baseline to Final Evaluation and Difference from Placebo for Attention-Deficit/Hyperactivity Disorder Rating Scale-IV: Home Version Total Score and Clinical Global Impression-Attention-Deficit/Hyperactivity Disorder-Severity Scale (Intent-to-Treat Dataset)
| Efficacy measure | Study 1, n=271 |
Study 2, n=116 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PBO (n=46) |
ABT-089 (mg/kg) |
ATM (n=49) |
PBO (n=40) |
ABT-089 (mg/kg) |
|||||
| 0.085 (n=45) |
0.260 (n=44) |
0.520 (n=45) |
0.700 (n=42) |
0.7 (n=36) |
1.4 (n=40) |
||||
| ADHD-RS-IV (HV) Total Score | |||||||||
| Baseline, mean (SE) | 42.9 (1.0) | 42.0 (1.1) | 43.7 (1.1) | 42.4 (1.1) | 42.8 (1.1) | 42.9 (1.0) | 42.7 (1.3)a | 39.6 (1.4) | 41.8 (1.3) |
| Mean change to final (SE) | −7.8 (1.8) | −9.0 (1.8) | −8.6 (1.8) | −10.1 (1.8) | −9.7 (1.9) | −15.9 (1.7) | −9.9 (1.7)a | −8.5 (1.8) | −7.4 (1.7) |
| Mean difference vs. placebo (90% CI) | −1.2 (−5.4, 2.9) | −0.8 (−5.0, 3.4) | −2.3 (−6.5, 1.8) | −1.9 (−6.1, 2.3) | −8.2 (−12.2, −4.1) | 1.4 (−2.7, 5.5) | 2.5 (−1.5, 6.5) | ||
| p | .310 | .374 | .177 | .231 | <.001*** | .711 | .848 | ||
| CGI-ADHD-S | |||||||||
| Baseline, mean (SE) | 4.71 (0.08) | 4.57 (0.08) | 4.71 (0.08) | 4.58 (0.08) | 4.72 (0.09) | 4.65 (0.08) | 4.71 (0.09) | 4.47 (0.09) | 4.73 (0.09) |
| Mean change to final (SE) | −0.59 (0.15) | −0.64 (0.15) | −0.51 (0.16) | −0.73 (0.15) | −0.77 (0.16) | −1.18 (0.15) | −0.77 (0.18) | −0.82 (0.19) | −0.78 (0.18) |
| Mean difference vs. placebo (90% CI) | −0.06 (−0.41, 0.30) | 0.07 (−0.28, 0.43) | −0.14 (−0.50, 0.22) | −0.19 (−0.55, 0.18) | −0.60 (−0.95, −0.25) | −0.05 (−0.49. 0.38) | −0.01 (−0.43, 940) | ||
| p | .400 | .633 | .257 | .198 | .003** | .419 | .470) | ||
Note: Least square model-based means presented. ABT-089 = a novel α4β2 neuronal nicotinic receptor partial agonist; ADHD-RS-IV (HV) = Attention-deficit/hyperactivity disorder Rating Scale-IV: Home Version; ATM = atomoxetine; CGI-ADHD-S = Clinical Global Impression-Attention-deficit/hyperactivity disorder-Severity scale; CI = confidence interval; PBO = placebo; SE = standard error.
n=39: one subject from Intent-to-treat dataset was excluded from analysis (subject completed too few items on scale to calculate final score);
Statistically significant at one-sided p=.01 vs. placebo (analysis of covariance with factors for site and treatment and with baseline scores as a covariate);
Statistically significant at one-sided p=.001 vs. placebo (analysis of covariance with factors for site and treatment and with baseline scores as a covariate);
Figure 2.
Mean change from baseline to each study visit on (A) Attention-deficit/hyperactivity disorder Rating Scale-IV: Home Version Total score and (B) Clinical Global Impression- Attention-deficit/hyperactivity disorder-Severity Scale score (Intent-to-treat dataset)
Note: One-sided P-values from mixed model repeated-measures analysis with factors for treatment group, site, visit, and treatment group-by-visit interaction, with continuous fixed covariates of baseline score and baseline score-by-visit interaction presented. ADHD-RS-IV (HV) = Attention-deficit/hyperactivity disorder Rating Scale-IV: Home Version; CGI-ADHD-S = Clinical Global Impression-Attention-deficit/hyperactivity disorder-Severity scale; SE = standard error.
There was no statistically significant difference between any ABT-089 dose and placebo for the mean change from baseline to final evaluation for the CGI-ADHD-S (Table 2), or on the mean change from baseline to each evaluation (Figure 2B), with the exception of the 0.520 mg/kg ABT-089 dose group in Study 1 at Day 14 (least-squares model-based mean, SE: −0.68, 0.12; P=.031). Since no corrections were made for Type I error rates and only one ABT-089 dose showed a statistically significant effect at a single time point, the overall effect of ABT-089 on the CGI-ADHD-S was not considered clinically relevant. Similarly, ABT-089 did not have clinically relevant effects on any other secondary endpoints (data not shown). In contrast, atomoxetine demonstrated efficacy on the CGI-ADHD-S (Table 2, Figure 2B) and almost all other secondary efficacy outcomes (data not shown). Subgroup analyses on ADHD-RS-IV (HV) Total scores and CGI-ADHD-S scores comparing treatment naïve subjects with previously-treated subjects indicated no statistical difference between the two groups (data not shown), indicating that both groups of subjects had consistent responses to treatment.
Pharmacokinetics
ABT-089 mean (SD) plasma concentrations during the window of 0–6 hours since previous dose were, in ng/mL: in Study 1, 11.4 (5.16), 37.0 (19.4), 77.9 (31.4), and 90.5 (46.5) for ABT-089 0.085 mg/kg, 0.260 mg/kg, 0.520 mg/kg, and 0.700 mg/kg, respectively; in Study 2, 140 (62.9) and 206 (134) for ABT-089 0.7 mg/kg and 1.4 mg/kg, respectively. The exposures of ABT-089 observed were consistent with values obtained from Phase 1 studies conducted in pediatric populations with ADHD and healthy adults, as well as a Phase 2 study conducted in adults with ADHD (unpublished data, Abbott, Abbott Park, IL).
Safety and Tolerability
The most common treatment-emergent AEs are listed in Table 3. There was no statistically significant difference in the overall incidence of treatment-emergent AEs between any ABT-089 dose and placebo, or between atomoxetine and placebo. Likewise, there were no specific AEs for which the incidence was statistically significantly higher for any ABT-089 dose or atomoxetine compared with placebo. There were no deaths, and there was only one serious treatment-emergent AE (accidental overdose of fluoxetine in Study 2; considered by the investigator to be not related to study drug). AEs that may be associated with NNR modulation are presented in Table S1, available online.
Table 3.
Summary of Adverse Events (Safety dataset)
| Study 1, N=274 |
Study 2, N=119 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PBO (n=46) |
ABT-089 (mg/kg) |
ATM (n=50) |
PBO (n=42) |
ABT-089 (mg/kg) |
|||||
| 0.085 (n=46) |
0.260 (n=44) |
0.520 (n=45) |
0.700 (n=43) |
0.7 (n=37) |
1.4 (n=40) |
||||
| Any AE, n (%) | 35 (76.1) | 30 (65.2) | 26 (59.1) | 30 (66.7) | 30 (69.8) | 41 (82.0) | 29 (69.0) | 25 (67.6) | 24 (60.0) |
| p | .360 | .115 | .360 | .634 | .616 | 1.000 | .490 | ||
| Most common treatment-emergent AEsa | |||||||||
| Cough, n (%) | 2 (4.3) | 1 (2.2) | 2 (4.5) | 4 (8.9) | 2 (4.7) | 1 (2.0) | 0 | 3 (8.1) | 1 (2.5) |
| p | 1.000 | 1.000 | .434 | 1.000 | .606 | .098 | .488 | ||
| Fatigue, n (%) | 2 (4.3) | 2 (4.3) | 3 (6.8) | 1 (2.2) | 0 | 8 (16.0) | 2 (4.8) | 2 (5.4) | 2 (5.0) |
| p | 1.000 | .673 | 1.000 | .495 | .094 | 1.000 | 1.000 | ||
| Headache, n (%) | 6 (13.0) | 1 (2.2) | 2 (4.5) | 2 (4.4) | 6 (14.0) | 5 (10.0) | 5 (11.9) | 6 (16.2) | 4 (10.0) |
| p | .111 | .267 | .267 | 1.000 | .753 | .747 | 1.000 | ||
| Insomnia, n (%) | 0 | 0 | 1 (2.2) | 3 (7.0) | 0 | 1 (2.4) | 2 (5.4) | 2 (5.0) | |
| p | 1.000 | 1.000 | .495 | .109 | 1.000 | .597 | .611 | ||
| Nausea, n (%) | 2 (4.3) | 1 (2.2) | 4 (9.1) | 2 (4.4) | 4 (9.3) | 5 (10.0) | 2 (4.8) | 2 (5.4) | 4 (10.0) |
| p | 1.000 | .429 | 1.000 | .424 | .438 | 1.000 | .427 | ||
| Upper abdominal pain, n (%) | 2 (4.3) | 1 (2.2) | 1 (2.3) | 5 (11.1) | 3 (7.0) | 3 (6.0) ) | 2 (4.8) | 6 (16.2) | 2 (5.0) |
| p | 1.000 | 1.000 | .267 | .670 | 1.000 | .138 | 1.000 | ||
Note: P-values from comparisons between placebo and active treatment using Fisher’s Exact Test. ABT-089 = a novel D4D2 neuronal nicotinic receptor partial agonist; AE = adverse event; ATM = atomoxetine; PBO = placebo.
Defined as ≥ 5% and incidence higher with ABT-089 vs. placebo (within-study).
Nine subjects discontinued Study 1 prematurely due to an AE: one subject each in the placebo and ABT-089 0.085 mg/kg, 0.260 mg/kg, and 0.520 mg/kg groups (n=1, each: headache, emotional disorder, iron deficiency anemia, and dysphoria, respectively), three subjects in the ABT-089 0.700 mg/kg group (n=1, each: vomiting, abdominal pain upper, and negativism), and two subjects in the atomoxetine group (n=1: hepatic enzyme increased and Epstein-Barr virus infection; n=1: headache, fatigue and abdominal pain upper). Eight subjects discontinued Study 2 prematurely due to an AE: four subjects in the placebo group (n=1, rash; n=1, mood swings; n=1, auditory hallucination; n=1, agitation, anxiety, and affect lability), one subject in the ABT-089 0.7 mg/kg group (chest pain, abdominal pain upper, pain in extremity, and anorexia), and three subjects in the ABT-089 1.4 mg/kg group (n=1 each: abdominal pain, psychomotor hyperactivity, and accidental overdose).
There were no clinically meaningful differences between any ABT-089 dose and placebo based on mean change from baseline for any hematology, clinical chemistry, or urinalysis parameters. Overall, mean changes from baseline to maximum, minimum and final values in systolic blood pressure, diastolic blood pressure, pulse, and temperature were comparable between active treatment and placebo. There were no statistically significant differences between any ABT-089 dose group and placebo based on mean change from baseline to final evaluation for height, body weight or BMI. ECG results showed no conduction abnormalities associated with ABT-089. There were no apparent dose-related trends with ABT-089 for changes in laboratory values, vital signs or ECG measurements in either study.
The most commonly reported AEs (≥ 5% and incidence higher than placebo) in atomoxetine-treated subjects were, in decreasing rate of incidence, upper respiratory tract infection, fatigue, decreased appetite, irritability, nausea, somnolence, pharyngolaryngeal pain, upper abdominal pain, vomiting, tremor, and emotional disorder. Among subjects in the atomoxetine group, mean weight and BMI decreased by 0.1 kg and 0.2 kg/m2, respectively, over the 8-week study period (mean difference from placebo [95% confidence interval]: −1.3 [−1.99, −0.69] and −0.6 [−0.96, −0.19], respectively).
DISCUSSION
In two separate randomized, placebo-controlled studies that tested ABT-089 across a wide dose range in 393 children aged 6–12 years with ADHD, ABT-089 was not superior to placebo on the primary outcome measure of the ADHD-RS-IV (HV) Total score. Similarly, ABT-089 was not superior to placebo for most secondary efficacy outcomes. In contrast, in Study 1, atomoxetine performed significantly better than placebo on the primary and most secondary outcomes and displayed an efficacy and adverse event profile consistent with previously published data,23,26 indicating that the lack of response noted for ABT-089 was not due to improper study design or execution. The results seen with ABT-089 and placebo in Study 2 were similar to those observed in Study 1.
The current efficacy findings differ from results of previous studies examining nicotine and nicotinic analogs for ADHD in adults, as well as from recent Phase 2 studies of adults with ADHD treated with ABT-089 or other α4β2 NNR partial agonists. The earlier adult studies using nicotine and nicotine analogs demonstrated improvements in ADHD symptoms and neurological outcomes comparable to those seen with stimulants.27–29,7 Prior crossover studies of ABT-089 in adults with ADHD demonstrated significant improvement in ADHD symptoms compared with placebo on the Conners’ Adult ADHD Rating Scale–Investigator Rated (CAARS:Inv) Total score, as well as several secondary endpoints (submitted).14,30 Similarly, ispronicline, another α4β2 NNR partial agonist currently in development, also demonstrated significant improvement on the CAARS:Inv Total score in a crossover study in adults with ADHD.31 However, in a recent small parallel design study intended to provide safety and initial efficacy estimates of higher doses of ABT-089 in adults with ADHD, ABT-089 failed to show efficacy on the CAARS:Inv Total score.32 These data suggest that the disparity in efficacy between studies in adults could be due to differences in study design, which may also be a contributing factor to the lack of efficacy seen in the pediatric population in the current studies.
The possibility that ABT-089 failed to show efficacy in these studies because the study populations included high numbers of treatment resistant subjects or that these subjects were disproportionately distributed across treatment groups is not supported by the data. Both studies excluded subjects who had failed to show a therapeutic response to two or more prior trials of ADHD medications. Consequently, in both studies, the majority of subjects were treatment-naïve, with approximately 40% of subjects in either study with a history of ADHD medication use prior to study entry. The relative proportion of subjects with any prior ADHD medication use and the relative proportion of subjects with prior stimulant medication use was similar across treatment groups in each study. Subgroup analyses on ADHD-RS-IV (HV) Total scores and CGI-ADHD-S scores comparing treatment naïve subjects with previously treated subjects indicated no statistical difference between the two groups. In the crossover studies of ABT-089 in adults with ADHD in which more than half of the subjects had previous pharmacologic treatments, the drug was equally efficacious in subjects with or without previous medication treatment.
It is unlikely that the lack of effect seen in children is due to inadequate dosing of ABT-089. Comparison of the pharmacokinetic results from the current studies with those from earlier Phase 1 and 2 studies indicates that the target plasma concentrations of ABT-089 that demonstrated efficacy in adults with ADHD were obtained in the pediatric population. However, preclinical evidence suggests that permeability of the blood brain barrier may differ in pediatric and adult populations,33,34 therefore, the possibility of a difference in the brain penetration of ABT-089 between children and adults cannot be ruled out.
There are also several other possible reasons for differential efficacy between adults and children, including developmental and stage of illness factors, and heterogeneity of clinical outcome.35 For example, tricyclic antidepressants are more effective in adults with major depressive disorder than in pediatric depression. This disparity may be due to developmental differences in the neurobiological pathophysiology of major depression, chronic episodicity of depression in adults, or phenotypic differences in the disorder between age groups.36–38,35 Similar factors may underlie the disparity in results seen with ABT-089. Several preclinical studies have demonstrated developmental regulation of α4β2 NNR mRNA levels, as well as α4β2 NNR binding and function.39–41 Moreover, the clinical profile of ADHD changes with age, particularly with respect to comorbidities that are more prevalent in adults.42 Therefore, it is possible that the ADHD seen in adults may be distinct from pediatric ADHD, in terms of both the stage and the basic characteristics of the disorder.
Given that these studies are among the first to examine the use of nicotinic analogs in a pediatric population, the tolerability and short-term safety findings are promising. ABT-089 was well tolerated, with a profile of AEs comparable to that of placebo and similar to the AE profile of ABT-089 seen in adults in Phase 2 studies. No clinically significant trends or mean changes in clinical laboratory or vital sign values were observed, and evaluation of ECGs throughout the studies did not demonstrate any conduction abnormalities associated with ABT-089. Additionally, rates of adverse events that may be associated with nicotinic receptor activation, such as neurologic, psychiatric or gastrointestinal adverse events, were similar between ABT-089 and placebo.
The current studies had methodological limitations typical of clinical trials of investigational agents. Due to the need to establish subject inclusion and exclusion criteria to ensure subject safety and data integrity, these results may not generalize to the broader patient population. Although doses used in this study were the pediatric equivalents of doses that conferred efficacy in the previous study of ABT-089 in adults with ADHD and covered a wide dose range, it is possible that children and adults have different therapeutic indices for agents that modulate the NNR system. However, testing higher doses of ABT-089 in the current studies was not feasible, because the tolerable dose range established by earlier safety studies would have been exceeded.
While many studies have shown nicotine and nicotinic analogs to have procognitive effects in adults with or without ADHD,43,27,7 the procognitive and potential treatment effects of nicotinic agents in ADHD have yet to be demonstrated in a pediatric population. Further exploration of the characteristics of the response of children with ADHD to nicotinic compounds must take into consideration the central and peripheral effects of these agents and potential differences in pharmacokinetic or pharmacodynamic responses between adults and children.
Supplementary Material
Acknowledgments
These studies are funded by Abbott.
The authors thank the study subjects and their parents for participating in these studies, and the following contributors: Study 1 (M06-888), Edward A. Cherlin, M.D. of Valley Clinical Research, Inc., Andrea Corsino, R.N., M.S.N. of Consultants in Neurology, Ltd., Judith C. Fallon, M.D. of NeuroScience, Inc., James Grimm, M.D. of the Oregon Center for Clinical Investigators, David G. Krefetz, D.O., M.D. of CRI Worldwide, Alan J. Levine, M.D. of Alpine Clinical Research Center, Leslie vH. Taylor, M.D. of the Dean Foundation for Health Research and Education, Daniel Wynn, M.D. of Consultants in Neurology, Ltd.; Study 2 (M10-345), Michael S. Greenbaum, M.D. of Capstone Clinical Research, R. Bart Sangal, M.D. of the Attention Disorders Institute, Louise Thurman, M.D., M.P.H. of the IPS Research Company; both studies, Valerie Arnold, M.D. of the University of Tennessee Center for Health Science, Beal Essink, M.D. of the Oregon Center for Clinical Investigators, Linda S. Harper, M.D. and J. Mark Joyce of Clinical Neuroscience Solutions, Inc., and Bradley D. Vince, D.O. of Vince and Associates Clinical Research. Statistical experts were Weining Z. Robieson, Ph.D. (M06-888) and Coleen M. Hall, M.S. (M10-345), of Abbott. Medical writing support was provided in the development of this manuscript by Regula E. Egli, Ph.D., of Abbott.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material cited in this article is available online.
Clinical Trial Registry Information: M06-888 (Study 1): A Safety and Efficacy Study of ABT-089 in Children With Attention-Deficit/Hyperactivity Disorder (ADHD), Clinicaltrials.gov, NCT00528697; M10-345 (Study 2): Safety and Tolerability Study of ABT-089 in Children With Attention-Deficit/Hyperactivity Disorder (ADHD), Clinicaltrials.gov, NCT00640419.
REFERENCES
- 1.Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- 2.Potter AS, Newhouse PA, Bucci DJ. Central nicotinic cholinergic systems: a role in the cognitive dysfunction in attention-deficit/hyperactivity disorder? Behav Brain Res. 2006;175:201–211. doi: 10.1016/j.bbr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Linnet KM, Dalsgaard S, Obel C, et al. Maternal Lifestyle Factors in Pregnancy Risk of Attention Deficit Hyperactivity Disorder and Associated Behaviors: Review of the Current Evidence. Am J Psychiatry. 2003;160:1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- 4.Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and attentiondeficit/hyperactivity disorder symptoms in a population-based sample of young adults. Arch Gen Psychiatry. 2005;62:1142–1147. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- 5.Wilens TE, Vitulano M, Upadhyaya H, et al. Cigarette smoking associated with attention deficit hyperactivity disorder. J Pediatr. 2008;153:414–419. doi: 10.1016/j.jpeds.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Gehricke JG, Whalen CK, Jamner LD, Wigal TL, Steinhoff K. The reinforcing effects of nicotine and stimulant medication in the everyday lives of adult smokers with ADHD: A preliminary examination. Nicotine Tob Res. 2006;8:37–47. doi: 10.1080/14622200500431619. [DOI] [PubMed] [Google Scholar]
- 8.Wilens TE, Decker MW. Neuronal nicotinic receptor agonists for the treatment of attentiondeficit/hyperactivity disorder: focus on cognition. Biochem Pharmacol. 2007;74:1212–1223. doi: 10.1016/j.bcp.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decker MW, Rueter LE, Bitner RS. Nicotinic acetylcholine receptor agonists: a potential new class of analgesics. Curr Top Med Chem. 2004;4:369–384. doi: 10.2174/1568026043451447. [DOI] [PubMed] [Google Scholar]
- 10.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Wang N, Orr-Urtreger A, Korczyn AD. The role of neuronal nicotinic acetylcholine receptor subunits in autonomic ganglia: lessons from knockout mice. Prog Neurobiol. 2002;68:341–360. doi: 10.1016/s0301-0082(02)00106-5. [DOI] [PubMed] [Google Scholar]
- 12.Rueter LE, Anderson DJ, Briggs CA, et al. ABT-089: pharmacological properties of a neuronal nicotinic acetylcholine receptor agonist for the potential treatment of cognitive disorders. CNS Drug Reviews. 2004;10:167–182. doi: 10.1111/j.1527-3458.2004.tb00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan JP, Donnelly-Roberts D, Briggs CA, et al. ABT-089 [2-methyl-3-(2-(S)-pyrrolidinylmethoxy)pyridine]: I. A potent and selective cholinergic channel modulator with neuroprotective properties. J Pharmacol Exp Ther. 1997;283:235–246. [PubMed] [Google Scholar]
- 14.Wilens TE, Verlinden MH, Adler LA, Wozniak PJ, West SA. ABT-089, a neuronal nicotinic receptor partial agonist, for the treatment of attention-deficit/hyperactivity disorder in adults: results of a pilot study. Biol Psychiatry. 2006;59:1065–1070. doi: 10.1016/j.biopsych.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 16.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV Checklists, Norms and Clinical Interpretation. New York, NY: Guildford Press; 1998. [Google Scholar]
- 17.Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institutes of Mental Health; 1976. (revised 1976) [Google Scholar]
- 18.Conners C. Conners' Rating Scales- Revised, Technical Manual. Tonawanda, NY: Multi-Health Systems Inc; 2001. [Google Scholar]
- 19.Gioia GA, Isquith PK, Guy SC, Kenworthy L. BRIEF Behavior Rating Inventory of Executive Function, Professional Manual. Lutz: Psychological Assessment Resources, Inc; 2000. [Google Scholar]
- 20.Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–1051. [PubMed] [Google Scholar]
- 21.Landgraf JM, Rich M, Rappaport L. Measuring quality of life in children with attention-deficit/hyperactivity disorder and their families: development and evaluation of a new tool. Arch Pediatr Adolesc Med. 2002;156:384–391. doi: 10.1001/archpedi.156.4.384. [DOI] [PubMed] [Google Scholar]
- 22.Landgraf JM, Abetz L, Ware JE. The Child Health Questionnaire (CHQ): A User's Manual. Boston, MA: Health Institute, New England Medical Center; 1996. [Google Scholar]
- 23.Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry. 2002;159:1896–1901. doi: 10.1176/appi.ajp.159.11.1896. [DOI] [PubMed] [Google Scholar]
- 24.Spencer T, Heiligenstein JH, Biederman J, et al. Results from 2 proof-of-concept, placebo-controlled studies of atomoxetine in children with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2002;63:1140–1147. doi: 10.4088/jcp.v63n1209. [DOI] [PubMed] [Google Scholar]
- 25.Medical Dictionary for Regulatory Activities (MedDRA®): Version 10.0, MedDRA MSSO, VAR1/8A/MSSO. 12011 Sunset Hills Road, Reston, VA 20190-3285, USA.
- 26.Strattera Package Insert. 2008 [Google Scholar]
- 27.Levin ED, Conners CK, Sparrow E, et al. Nicotine effects on adults with attentiondeficit/hyperactivity disorder. Psycho pharmacology (Berl) 1996;123:55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
- 28.Wilens TE, Biederman J, Spencer TJ, et al. A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1931–1937. doi: 10.1176/ajp.156.12.1931. [DOI] [PubMed] [Google Scholar]
- 29.Levin ED, Conners CK, Silva D, Canu W, March J. Effects of chronic nicotine and methylphenidate in adults with attention deficit/hyperactivity disorder. Exp Clin Psychopharmacol. 2001;9:83–90. doi: 10.1037/1064-1297.9.1.83. [DOI] [PubMed] [Google Scholar]
- 30.Rajagopalan R, Anderson RT, Apostol G, Abi-Saab W. Quality of Life and Work Productivity Improvements Associated with ABT-089 in Adults with ADHD (#NR6-033); Washington, DC. Presented at the 161st Annual American Psychiatric Association Meeting; May 3–8, 2008.2008. [Google Scholar]
- 31.Targacept Announces Positive Top-Line Results from Phase II Study of AZD3480 in Adult ADHD [press release] Wnston-Salem, NC: Targacept; 2009. May 11, [Google Scholar]
- 32.Bain EE, Apostol G, Sangal RB, Robieson WZ, Abi-Saab WM, Saltarelli MD. Safety and efficacy of ABT-089, a novel α4β2 neuronal nicotinic receptor partial agonist, in the treatment of adults with attention-deficit/hyperactivity disorder; Honolulu, Hawaii. Presented at the 56th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; Oct 27-Nov 1, 2009. [Google Scholar]
- 33.Zeller K, Vogel J, Kuschinsky W. Postnatal distribution of Glut1 glucose transporter and relative capillary density in blood-brain barrier structures and circumventricular organs during development. Brain Res Dev Brain Res. 1996;91:200–208. doi: 10.1016/0165-3806(95)00177-8. [DOI] [PubMed] [Google Scholar]
- 34.Matsuoka Y, Okazaki M, Kitamura Y, Taniguchi T. Developmental expression of P-glycoprotein (multidrug resistance gene product) in the rat brain. J Neurobiol. 1999;39:383–392. doi: 10.1002/(sici)1097-4695(19990605)39:3<383::aid-neu5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 35.Kaufman J, Martin A, King RA, Charney D. Are child-, adolescent-, and adult-onset depression one and the same disorder? Biol Psychiatry. 2001;49:980–1001. doi: 10.1016/s0006-3223(01)01127-1. [DOI] [PubMed] [Google Scholar]
- 36.Goldman-Rakic PS, Brown RM. Postnatal development of monoamine content and synthesis in the cerebral cortex of rhesus monkeys. Brain Res. 1982;256:339–349. doi: 10.1016/0165-3806(82)90146-8. [DOI] [PubMed] [Google Scholar]
- 37.Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- 38.Hazell P, O'Connell D, Heathcote D, Henry D. Tricyclic drugs for depression in children and adolescents. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD002317. CD002317. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Liu C, Miao H, Gong ZH, Nordberg A. Postnatal changes of nicotinic acetylcholine receptor alpha 2, alpha 3, alpha 4, alpha 7 and beta 2 subunits genes expression in rat brain. Int J Dev Neurosci. 1998;16:507–518. doi: 10.1016/s0736-5748(98)00044-6. [DOI] [PubMed] [Google Scholar]
- 40.Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- 41.Doura MB, Gold AB, Keller AB, Perry DC. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res. 2008;1215:40–52. doi: 10.1016/j.brainres.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt S, Petermann F. Developmental psychopathology: Attention Deficit Hyperactivity Disorder (ADHD) BMC Psychiatry. 2009;9:58. doi: 10.1186/1471-244X-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conners CK, Levin ED, Sparrow E, et al. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD) Psychopharmacol Bull. 1996;32:67–73. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.