Abstract
Reproductive cooperation in social animals has been the focus of intensive research, yet the role of environmental factors in promoting such cooperation remains uncertain. A recent global analysis suggested that cooperative breeding in birds is a ‘bet-hedging’ strategy associated with climatic uncertainty, but it is unclear whether this mechanism applies generally or is restricted to the insectivorous passerines that predominate as cooperative breeders at the global scale. Here, we use a phylogenetic framework to assess the effect of climate on the evolution of cooperation in hornbills (Bucerotidae), an avian family characterized by frugivory and carnivory. We show that, in contrast to the global pattern, cooperative reproduction is positively associated with both inter- and intra-annual climatic stability. This reversed relationship implies that hornbills are relatively insensitive to climatic fluctuations, perhaps because of their dietary niche or increased body mass, both of which may remove the need for bet-hedging. We conclude that the relationship between climatic variability and cooperative breeding is inconsistent across taxa, and potentially mediated by life-history variation. These findings help to explain the mixed results of previous studies and highlight the likely shortcomings of global datasets inherently biased towards particular categories.
Keywords: climate, comparative analysis, cooperation, ecological traits, social behaviour
1. Introduction
Cooperative breeding behaviour involves non-reproductive members of a social group providing aid to the offspring of other individuals, an apparent paradox that raises important questions about the evolution of cooperation and sociality [1–3]. Cooperatively breeding groups are typically formed by the retention of young on the natal territory, and thus the dominant explanation for this breeding strategy is that helpers may gain indirect fitness benefits from raising kin [4–7]. However, breeding groups in many species contain non-kin, suggesting that cooperative breeding may also be promoted by direct benefits of group-living (e.g. improved resource defence, increased predator detection and territory inheritance) [8–12]. Regardless of whether the fitness benefits of helping are direct (natural selection) or indirect (kin selection), many studies have suggested a role for the environment in mediating the decision to cooperate, yet this has led to little consensus about the mechanisms involved [13].
A longstanding view is that cooperative breeding is more frequent when climatic conditions are stable as this allows populations to persist at carrying capacity, leading to ‘habitat saturation’ [14–17]. Stable climates may therefore promote cooperative breeding because young individuals have little opportunity to establish independent territories and are thus more likely to remain as helpers [18]. In support of this idea, some early comparative analyses reported a higher incidence of cooperative breeding in birds inhabiting aseasonal environments with relatively constant temperature and rainfall [17,19]. Moreover, Ligon & Burt [5] presented evidence that environmental unpredictability and strong seasonality of food supply typically reduced cooperative breeding behaviour. These findings led to a widespread assumption that the likelihood of reproductive cooperation peaks in aseasonal environments [8,20].
An opposing view is that the evolution of cooperative breeding is promoted by ‘temporal variability’ of climates [21] or ‘environmental uncertainty’ [22]. In this case, cooperation over reproduction is not viewed as the result of habitat saturation, but as a ‘bet-hedging’ strategy that maximizes fitness by reducing environmentally mediated variance in fecundity [13]. Helpers incur reduced fitness under ideal conditions, but this may be offset over time by gains in fitness when conditions are poor. Support for this idea has been provided by long-term field studies of African starlings (Sturnidae) [21,23], as well as a broad survey demonstrating a positive relationship between cooperative breeding and environmental uncertainty across approximately 95% of the world's birds [22]. This global analysis revealed that high inter-annual variability in rainfall predicted reproductive cooperation, potentially explaining the unusual prevalence of cooperatively breeding passerines in non-forest habitats of Africa and Australia.
Although general patterns across birds [22] suggest that bet-hedging may be the dominant environmental explanation for cooperative breeding, the contrasting results of many studies focusing on smaller geographical or taxonomic scales [5,17,19,24] imply that explanations may vary across clades or regions. Even comparing across all birds (more than 9000 species), the evidence for a correlation between fluctuating rainfall patterns and cooperative breeding was relatively weak, and restricted to passerines, while in non-passerines cooperative breeding was less frequent and apparently unrelated to inter-annual variation in precipitation [22]. Moreover, the inclusion of ecological traits such as diet improved the fit of global-scale models, suggesting that the incidence of cooperative breeding in birds is best explained by interactions between climate and ecology [22]. A better understanding of these interactions requires a focus on the incidence of cooperation in smaller clades, containing less extreme variation in ecology, evolutionary history and geography [13].
In this study, we test the hypothesis that environmental uncertainty drives the evolution of cooperative breeding in hornbills (Bucerotidae), a radiation of non-passerine bird species restricted to the Old World tropics. Hornbills offer an ideal system because cooperative breeding occurs from the smallest species (Lophoceros camurus, 30 cm) to the largest (Bucorvus leadbeateri, 120 cm), with an incidence (approx. 40% of species) about fivefold higher than in birds in general [22]. They also represent a useful counterpoint to previous studies because all members of the family are primarily frugivorous or ‘faunivorous’, the latter term having been coined for hornbills that consume animals, typically including a broad variety of both vertebrates and invertebrates [25]. By contrast, almost all intensive studies of avian cooperative breeding have focused on relatively ecologically homogeneous samples of species that are either insectivorous or feed their nestlings mainly with insects and their larvae.
Despite the potential of hornbills as a study system for understanding the evolution of sociality, hypotheses for cooperative breeding remain largely untested in the family [25]. This has partly reflected the lack of a comprehensive phylogeny [26], as previous published trees for hornbills contain only genetic data from roughly half of the family [27], leading to much uncertainty regarding the historical relationships between species. Here, we use a recently published multi-locus phylogenetic tree for all hornbill species [28] as a framework for three complementary analytical techniques: ancestral state reconstructions, Bayesian analyses of evolutionary transitions and phylogenetic comparative methods. Our goal is to trace the origins of cooperative breeding, and to examine the relationship between sociality and climatic variability. In particular, we explore the potential role of interactions between climate and ecological traits previously proposed to be linked with cooperative breeding in birds, including primary habitat and diet, body mass, nest availability and territoriality.
2. Material and methods
(a). Study system and phylogeny
The hornbills occur in tropical forests and savannahs of Asia and Africa [25], and belong to an avian lineage predisposed to sociality [29]. However, in comparison with related clades more widely known for cooperative breeding—such as woodpeckers (Melanerpes; 7 of 23 species cooperative, 30%), bee-eaters (Meropidae; 19 of 25 species cooperative, 76%) and wood-hoopoes (Phoeniculidae; 5 of 8 species cooperative, 62%)—they provide a larger mixed sample for comparative analyses (25 of 61 species cooperative, 41%). Some parallels can be drawn between hornbills and the distantly related African starlings (table 1), the main system for previous studies of climatic effects on cooperation [21,23]. In particular, both groups contain similar proportions of cooperative and non-cooperative breeders, occupy a similar range of dry and humid habitats, and select comparable breeding sites (mainly cavities). The main points of difference are that hornbills are far larger in body size, with a higher incidence of frugivory and lower incidence of insectivory, particularly in the nestling diet (table 1). In addition, although 100% of cooperatively breeding starlings (Sturnidae) are restricted to Africa [21], cooperatively breeding hornbills are distributed across biogeographic regions, with eight species (32%) being African, and the remainder Asiatic.
Table 1.
Comparison of life-history traits in African starlings (Sturnidae) and hornbills (Bucerotidae) based on data from [21,25,30,31].
| trait |
African starlings (n = 45)a | hornbills (n = 61) | |
|---|---|---|---|
| breeding system | cooperative | 17 (38%) | 25 (41%) |
| breeding site | cavities | 38 (84%) | 61 (100%) |
| preferred habitat | humid | 20 (45%) | 42 (69%) |
| dry | 25 (55%) | 19 (31%) | |
| primary adult diet | frugivory | 19 (42%) | 43 (70%) |
| primary nestling diet | insectivory | 45 (100%) | 7 (11%) |
| size | mean body mass (g) | 78.8 | 975.7 |
aSample size was slightly lower (n = 43) for mean body mass because data were unavailable for two species.
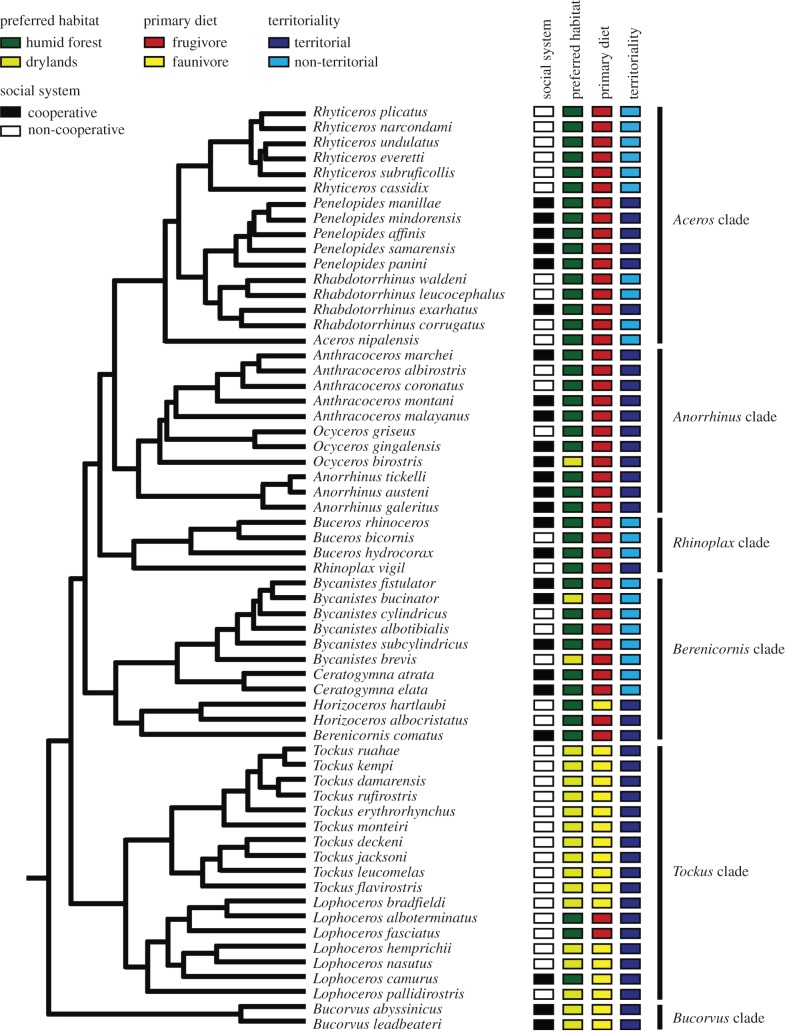
As part of a parallel study [28], we resolved the evolutionary relationships of hornbills by constructing a comprehensive molecular phylogeny. In brief, this involved sequencing one nuclear (adenylate kinase 1 intron) and one mitochondrial (cytochrome b) gene, and then using a concatenated dataset of both genes to build a single maximum-credibility clade (MCC) tree with Bayesian Markov chain Monte Carlo (MCMC) simulations (figure 1). Branch lengths were calibrated using both fossil and molecular anchor-points implemented in the program BEAST v. 1.6.1 [32]. After trimming to remove out-groups, the MCC tree was tested to be ultrametric in the R package ‘Ape’ [33]. For full details of phylogenetic reconstruction, see [28].
Figure 1.
Molecular phylogeny of hornbills and their social system in relation to preferred habitat, primary diet and territoriality. The topology shown is an ultrametric Bayesian tree based on a combination of nuclear and mitochondrial DNA sequences. All characters are discrete and treated as binary.
(b). Social system
Hornbills exhibit typical helper-at-the-nest cooperative breeding wherein non-reproductive individuals provide aid to a breeding pair [25,26]. We categorized species as either cooperative or non-cooperative breeders following Cockburn [34], with updates from Jetz & Rubenstein [22] (see the electronic supplementary material, table S1). In brief, these classifications pool species with obligate and facultative forms of cooperative breeding, and include those species strongly suspected to be cooperative breeders. An underlying assumption of such classifications is that resource defence by social groups during breeding is indicative of reproductive cooperation. We note that group defence behaviour is sometimes viewed as equivalent to cooperative breeding because reproductive output can be promoted by help during contests over nest sites or territories even when there is no help at the nest [10,11]. While this view may not apply universally across species, cooperative resource defence by social groups is a form of complex sociality, and thus accords with our hypotheses. Our dataset included some species absent from earlier classifications, including the recent split of Penelopides samarensis from P. affinis [28]. In this case, the parent species breeds cooperatively, along with all other members of the genus Penelopides. We therefore categorized the daughter species as a cooperative breeder, following methods outlined by Cockburn [34].
(c). Ecological traits
We surveyed literature to compile data on five traits proposed to influence the likelihood of cooperative breeding in birds: (i) primary habitat (1 = humid forest, 0 = drylands, including grasslands and thorn forest), (ii) primary diet (1 = frugivorous, 0 = faunivorous), (iii) territoriality (1 = species defending territories as pairs or groups, including year-round territories and weak/seasonal territories, 0 = non-territorial), (iv) body mass (in grams) and (v) nest-hole size (diameter in centimetres). Habitat, diet and territorial behaviour (i)–(iii) were classified as binary variables, whereas mass and nest-hole size (iv)–(v) were continuous variables. Nest data were only available for 15 species, whereas body mass was available for all 61 species. Because body mass and nest-hole size were positively correlated (R2 = 0.37, F1,13 = 7.76, p = 0.015), we dropped nest-hole data from our analyses and assumed that body mass was a proxy for nest-hole size.
(d). Climatic niche
We quantified the climatic niche of each species in terms of six variables: (i) mean annual temperature (in °C), (ii) total annual precipitation (in millilitres), (iii) intra-annual temperature variation, (iv) intra-annual variation in precipitation, (v) inter-annual temperature variation and (vi) inter-annual variation in precipitation. Climate data for 55 hornbill species derive from Jetz & Rubenstein [22], and data for the remaining six species were generated using the same methodology. In brief, this involved preparing environmental GIS layers at 0.01° spatial resolution, extracting variables (i) and (ii) from a climatic database of mean monthly values for the period 1961–1990 [35], and the remaining variables (iii)–(vi) from a related dataset covering 1963–2002 in which data are aggregated in three-month periods (CRU TS 2.1 [36]). We calculated (iii) and (iv) as the mean among all years of the within-year standard deviations of the three-month values, and (v) and (vi) as the standard deviation within the same three-month period across all years, averaged across four three-month periods. We calculated climatic niche as the mean of log10-transformed values across all 55 × 55 km grid cells occupied by a species, as determined from its range polygon [37].
For a full list of species included in the study, together with data on intrinsic traits and climatic niches, see the electronic supplementary material, tables S1 and S2.
(e). Analytical approach
To explore the evolutionary history of cooperative breeding in hornbills, we used ancestral state reconstruction in Mesquite v. 2.74 [38]. We then evaluated the evolutionary transitions generating the associations between ecological traits and cooperative breeding using Pagel's discrete algorithm with MCMC, implemented in BayesTraits [39]. This method tests whether the evolution of two binary traits are correlated by comparing independent and dependent models using a likelihood ratio (LHR) test. Discrete also estimates how evolutionary transitions occur among the four possible states of two binary traits, assuming correlated evolution. As the software requires traits to be binary, we dichotomized body mass data around the mean as 0 ≤ 0.5 kg, 1 ≥ 0.5 kg.
We then assessed whether intrinsic (ecological) and extrinsic (climate) traits predicted the occurrence of cooperative breeding in hornbills using Bayesian phylogenetic mixed-effect models (BPMM) with MCMC estimation in MCMCglmm [40,41]. This allowed us to test for the effect of both continuous and dichotomous traits on the distribution of a binary trait (cooperative breeding versus non-cooperative breeding), including the phylogenetic similarity of species as a covariance matrix (see the electronic supplementary material for details). Using binary response models and logit link function [41], we compared models with each ecological and climatic trait fitted separately with a null model that included only cooperative breeding as a random effect. Estimates of the posterior mean with 95% lower and upper confidence intervals were reported. We also reported p-values for correlations between traits and the occurrence of cooperative breeding; we refer to this as pMCMC, the Bayesian equivalent of a p-value [40,41].
To assess whether our results were robust to alternative phylogenetic modelling approaches, we ran our analyses using phylogenetic independent contrasts analysis (PIC) and phylogenetic least-squares regression (PGLS) (see the electronic supplementary materials). The distribution of some continuous variables was normalized by log-transformation prior to analysis; all analyses were conducted in R [42].
3. Results
(a). Distribution, origins and evolution of cooperative breeding
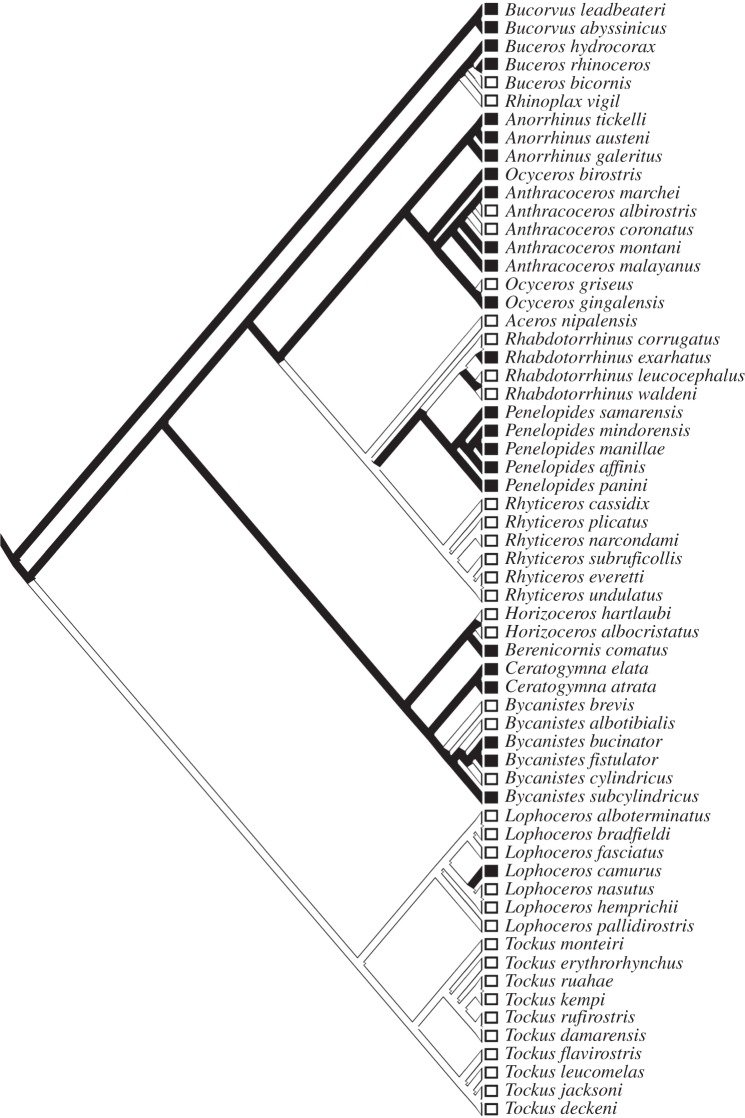
Cooperatively breeding hornbills are taxonomically diverse, being distributed in both subfamilies and all five major clades within the subfamily Bucerotinae (figure 1). Of 14 hornbill genera, four (Bucorvus, Ceratogymna, Anorrhinus and Penelopides) are exclusively composed of cooperatively breeding species. Ancestral state reconstructions confirmed that cooperative breeding is ancestral in hornbills (figure 2), which accords with earlier assessments [25], and appears to be the case in other closely related Coraciiforme clades (wood-hoopoes, bee-eaters and kingfishers) [5,34].
Figure 2.
Ancestral state reconstruction of social system in hornbills using stochastic character mapping. Black squares and branches indicate cooperative breeding species and lineages; white squares and branches indicate non-cooperative breeding species and lineages.
Posterior probabilities (PP) of root characters suggested that the origins of cooperative breeding in hornbills coincide with large body mass (more than 500 g, PP = 1.00), and to a lesser extent with occupancy of humid forests and territorial behaviour (PP = 0.50). However, this result is presumably influenced by the ancient node linking the ground-hornbills (two large, cooperatively breeding species) with the rest of the family. Models of evolutionary transitions throughout the family revealed that the evolution of cooperative breeding is independent from that of body mass, habitat and primary diet (table 2). However, we found evidence of correlated evolution between the occurrence of cooperative breeding and territoriality; that is, the model in which the evolution of cooperative breeding was dependent on territoriality had a better fit than the model in which cooperative breeding and territoriality were evolutionarily independent (table 2).
Table 2.
LHR tests for correlated evolution between cooperative breeding and four intrinsic traits. Ind., independent; Dep., dependent; LH, likelihood; LLH, log likelihood; LLHR, log likelihood ratio; Coop, cooperative breeding; Non, non-cooperative breeding.
| trait | Ind. LL | Dep. LL | LLRa | p-value | root posterior probabilityb |
|---|---|---|---|---|---|
| habitat | −79.15 | −80.41 | −2.51 | 0.64 | 0.5 (Coop, humid forest) |
| primary diet | −74.03 | −71.37 | −5.32 | 0.26 | 0.5 (Non, faunivore) |
| territoriality | −81.57 | −74.69 | −13.76 | 0.008 | 0.5 (Coop, territorial) |
| body mass | −72.92 | −70.69 | −4.46 | 0.35 | 1.0 (Coop, large) |
aLLHR tests compared a model in which evolution of cooperative breeding is independent of a given trait with a model in which it is dependent on that trait, based on the differences in maximum LLH values between models (2 × LH Ind. model − LH Dep. model). p-values were calculated from χ2-distributions of the LLHR, with degrees of freedom equal to the difference in number of parameters estimated [43].
bValues approaching 1.0 have strong support.
(b). Intrinsic and extrinsic predictors of cooperative breeding
When we plotted uncorrected species-level data, it appeared that cooperative breeding in hornbills was more frequent in species inhabiting tropical humid forest than drylands, and in frugivores rather than faunivores (figure 3). In particular, we found a higher proportion of cooperatively breeding species in humid forests (21/42 species, 50%) than in grasslands and deciduous forests (4/19 species, 21%). However, controlling for the effects of shared evolutionary history, the apparent influence of habitat and primary diet disappeared, and the only intrinsic trait significantly associated with cooperative breeding was territoriality (table 3 and figure 3).
Figure 3.
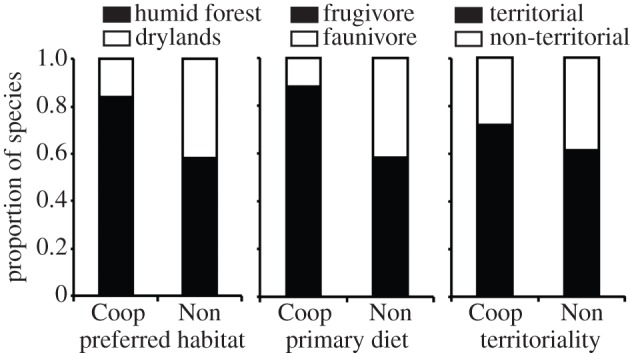
Proportion of cooperative and non-cooperative breeding hornbills associated with different preferred habitats, primary diets and territorial behaviour. All characters are discrete and treated as binary, with columns separated into cooperative (‘Coop’, n = 25) and non-cooperative (‘Non’, n = 36) species.
Table 3.
Ecological and environmental correlates of cooperative breeding in hornbills (n = 61 species). Temp., temperature; Ppt., precipitation; Var., variation. Shown are the posterior estimates of the effect (plus the 95% credibility interval) and the probability that the effect is different from the null hypothesis (i.e. effect = 0). ΔDIC is difference in deviance information criterion between model including variable in question and the null model in which only ‘cooperative breeding’ is included as a random effect.
| predictor | posterior mean | lower 95% CI | upper 95% CI | pMCMC | ΔDIC |
|---|---|---|---|---|---|
| preferred habitat | 59.6 | −174.2 | 333.7 | 0.572 | 2.354 |
| primary diet | 119.5 | −262.6 | 557.7 | 0.515 | 2.592 |
| territoriality | 247.7 | −23.0 | 596.6 | 0.048 | 2.589 |
| body mass | −55.5 | −215.3 | 78.0 | 0.389 | 2.522 |
| mean annual temperature | 876.9 | −452.4 | 2533.2 | 0.174 | 2.599 |
| mean annual precipitation | 153.6 | −114.5 | 484.8 | 0.236 | 2.613 |
| temp. var. within years | −126.9 | −277.8 | 0.363 | 0.031 | 2.807 |
| temp. variation among years | −298.8 | −704.9 | 83.42 | 0.115 | 2.435 |
| Ppt. variation within year | −124.8 | −124.8 | −276.76 | 0.029 | 2.701 |
| Ppt. variation among years | −202.3 | −202.3 | −456.96 | 0.030 | 2.706 |
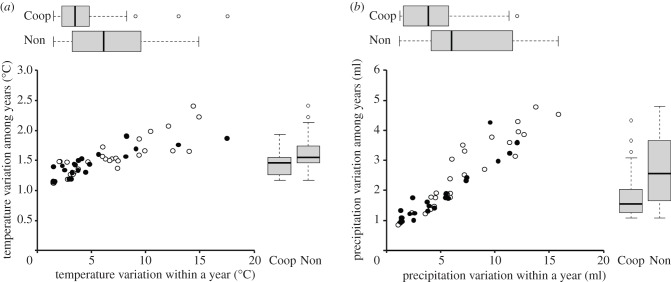
Focusing on environmental variables, we found that reproductive cooperation in hornbills was positively associated with climatic stability (figure 4). Direct comparisons of uncorrected values revealed that cooperatively breeding species were associated with lower mean climatic variation within and between years, both for temperature (figure 4a) and precipitation (figure 4b). Similar results were retained after phylogenetic correction, with cooperative breeding being significantly negatively related to intra-annual variation in temperature and precipitation, and inter-annual variation of rainfall (table 3). However, the occurrence of cooperative breeding was not associated with mean annual temperature and precipitation, nor with inter-annual variation in temperature. Note that qualitatively similar results were obtained when analysing associations between traits and cooperative breeding using alternative phylogenetic modelling techniques (PIC and PGLS; see electronic supplementary material, table S3).
Figure 4.
Relationship between cooperative breeding in hornbills and environmental uncertainty as indicated by variation in (a) temperature and (b) precipitation. Individual circles in the scatterplots represent the climatic niche position of a single species, with black circles indicating species with cooperative reproduction (‘Coop’) and white circles indicating species with non-cooperative reproduction (‘Non’). Tukey box plots summarize these data for a single axis.
(c). Cavity nesting
It is worth considering whether cooperative breeding is favoured in hornbills because they are specialized cavity-nesters, with successful reproduction constrained by the availability of ample-sized, safe nest-holes [25]. Many other cavity-nesting non-passerine birds share a high incidence of cooperatively breeding species, including parrots, woodpeckers, African barbets, aracaris, jacamars, todies, bee-eaters, hoopoes and wood-hoopoes [34], perhaps because nest-holes are a limiting resource accentuating any shortage of available breeding territories [24]. Given that larger cavities are theoretically more limiting than small cavities, the nest-hole hypothesis predicts greater incidence of cooperation in large hornbills, since hole size and body size are correlated. However, although the body mass of cooperatively breeding hornbills (mean ± s.e. = 1096 ± 185 g) tends to be greater than that of non-cooperative hornbills (891 ± 154; t = 1.66, p = 0.10; see electronic supplementary material, figure S1), we detected no such relationship after phylogenetic control (table 3).
4. Discussion
Our results indicate that cooperative breeding in hornbills is negatively related to climatic variability, and in particular inter-annual variation in rainfall, suggesting that sociality is associated with stable rather than variable climatic conditions. This pattern is opposite to that detected in African starlings [21,23], confirming that the relationship between inter-annual climatic variability and cooperative breeding varies across clades. One interpretation of these findings is that the association between environmental conditions and cooperative behaviour may be spurious and simply related to chance; however, given that only a small number of taxa have been investigated, this may be unlikely. Alternatively, different families may be subject to contrasting selective environments because they vary in ecological traits, leading to fundamental differences in the mechanisms promoting cooperative breeding.
To understand why ecological traits may be critical, it is useful to consider the two contrasting scenarios proposed to link climate and cooperation in birds. The ‘habitat saturation’ hypothesis [14–17] is based on young birds remaining as helpers because of extrinsic constraints (e.g. shortage of territories in predictable environments), whereas the ‘hard life’ hypothesis is based on cooperation being favoured by intrinsic constraints (e.g. reduced fecundity in fluctuating environments) [18]. Intrinsic constraints appear to outweigh extrinsic constraints in African starlings, leading to greater cooperation in unpredictable habitats [21,23]. The question becomes: why might cooperative breeding be a bet-hedging strategy for maximizing reproductive success in African starlings, but not in hornbills?
The most obvious explanation is that hornbills are simply less susceptible to climatic fluctuations. This may be because of their larger size (table 1), which in turn is linked to lower reproductive output and longer generation spans, or because of differences in dietary ecology. Environmental variability is well known to affect seasonal insect abundance, for example, and thus may promote bet-hedging in insectivores [19,23,24]. Indeed, all cases providing direct evidence of cooperative breeding as a bet-hedging strategy [21,23] derive from species for which insects are important in the nestling diet. By contrast, most hornbills do not feed their offspring on insects, but rather on fruit or (at least partially) vertebrate animals, the supply of which is perhaps less likely to be affected by variation in climate. Most importantly, hornbill species in tropical dryland habitats feed their nestlings with many small vertebrates (e.g. rodents, reptiles, birds) that are less susceptible than insects to population crashes during dry spells [25].
If the ‘hard life’ hypothesis is less relevant to hornbills, the primary mechanism linking climate to cooperation across the family may be ‘habitat saturation’, which predicts the opposite relationship with climatic stability [8,18]. Stable environments tend to increase survival and longevity, thus reducing territorial vacancies [2,44]. In addition, dispersal from natal territories may be delayed in predictable environments, not simply because of dispersal constraints, but because individuals may benefit from helping resident pairs to defend year-round territories in high-quality habitat [45,46]. For example, experiments have shown that a stable food supply leads to reduced dispersal of young from territories and increased helping at nests in carrion crows (Corvus corone) [47]. In further support of the idea that habitat saturation mediates the effects of climate in hornbills, we found that territoriality was the dominant predictor of cooperative breeding after phylogenetic correction. The link between territoriality and cooperation has been noted previously in hornbills, as all year-round territorial species are confirmed cooperative breeders [26].
Because detailed information is available from only two clades with contrasting life history and ecology (table 1), it is not possible to fully resolve the link between environmental conditions and cooperation in birds. However, our finding that the association with climate is reversed in these clades suggests that strikingly different mechanisms may predominate in different groups of organisms, with variation potentially driven by factors such as body size, territorial system and diet. This conclusion is consistent with previous comparative analyses showing that global patterns of sociality in birds are best explained by an interaction between climate and ecological traits [22]. We note that the majority of cooperatively breeding species worldwide (60%) are insectivores, whereas smaller proportions are listed as omnivores (generalists with broad dietary intake; 19%), herbivores (mainly eating fruit and seeds; 18%) and carnivores (preying mainly on vertebrates; 3%). The non-insectivorous (and often larger-bodied) categories make up most non-passerines and a relatively small proportion of passerines. Thus, if our findings in hornbills are representative of broader patterns, they may explain why the positive effect of fluctuating rainfall patterns on cooperative breeding detected by Jetz & Rubenstein [22] was weak, and present only in passerines.
At a more general level, the differences among avian families offer insights about common methodological approaches in evolutionary biology. It has long been acknowledged that broad patterns cannot be inferred from data on single families, yet the drawbacks of global datasets have rarely been discussed. On the one hand, such data-rich analyses are inherently biased by the dominant category of organisms (i.e. those with the most common life-history trait), and thus potentially conceal important nuances explaining variation in effects. On the other hand, testing hypotheses across thousands of species provides such large statistical power that minor correlations with uncertain biological significance can be overemphasized. Although global analyses are clearly of great value, these issues highlight the importance of careful interpretation, and further analysis based on data-points from multiple clades.
In conjunction with previous studies, the evidence from hornbills indicates that a combination of mechanisms gives rise to global patterns in social behaviour, with the effects of the external environment being mediated by ecological and life-history traits. In particular, we have shown that this interplay between ecology and environment may generate outcomes that are unpredictable based on ecology or environment alone, thus helping to explain the inconsistent results of previous studies [22,24,29,44]. Our findings reinforce the suggestion of Emlen [48] that cooperative breeding can only be understood in the context of both intrinsic and extrinsic constraints, which together provide a framework for understanding the evolution of sociality in animals.
Acknowledgement
We are grateful to C. Cooney, C. Cornwallis, J. Hadfield, A. Meade, D. Orme, A. Pigot, D. Rubenstein and N. Seddon for help with analyses and useful discussion, and W. Jetz for access to unpublished data on climatic niches.
Funding statement
This research was supported by the Ford Foundation International Fellowship Program, British Ornithologists’ Union and North of England Zoological Society.
References
- 1.Cockburn A. 1998. Evolution of helping behaviour in co-operatively breeding birds. Annu. Rev. Ecol. Syst. 29, 141–177 (doi:10.1146/annurev.ecolsys.29.1.141) [Google Scholar]
- 2.Covas R, Griesser M. 2007. Life history and the evolution of family living in birds. Proc. R. Soc. B 274, 1349–1357 (doi:10.1098/rspb.2007.0117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stacey PB, Koenig WD. 1990. Cooperative breeding in birds: long-term studies of ecology and behaviour. Cambridge, UK: Cambridge University Press [Google Scholar]
- 4.Charnov EL. 1981. Kin selection and helpers at the nest: effects of paternity and biparental care. Anim. Behav. 29, 631–632 (doi:10.1016/S0003-3472(81)80130-3) [Google Scholar]
- 5.Ligon JD, Burt DB. 2004. Evolutionary origins. In Ecology and evolution of cooperative breeding in birds (eds Koenig WD, Dickinson JL.), pp. 5–34 Cambridge, UK: Cambridge University Press [Google Scholar]
- 6.Hatchwell BJ. 2009. The evolution of cooperative breeding in birds: kinship, dispersal and life history. Phil. Trans. Roy. Soc. B 364, 3217–3227 (doi:10.1098/rstb.2009.0109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornwallis CK, Davis KE, West SA, Griffin AS. 2010. Promiscuity and the evolutionary transition to complex societies. Nature 466, 969–972 (doi:10.1038/nature09335) [DOI] [PubMed] [Google Scholar]
- 8.Hatchwell BJ, Komdeur J. 2000. Ecological constraints, life history traits and the evolution of cooperative breeding. Anim. Behav. 59, 1079–1086 (doi:10.1098/rspb.2005.3431) [DOI] [PubMed] [Google Scholar]
- 9.Seddon N, Tobias JA. 2003. Communal singing in the cooperatively breeding subdesert mesite Monias benschi: evidence of numerical assessment? J. Avian Biol. 34, 72–80 (doi:10.1034/j.1600-048X.2003.03067.x) [Google Scholar]
- 10.Seddon N, Tobias JA, Butchart SHM. 2003. Group-living, breeding behaviour and territoriality in the subdesert mesite Monias benschi. Ibis 145, 277–294 (doi:10.1046/j.1474-919X.2003.00150.x) [Google Scholar]
- 11.Theuerkauf J, Rouys S, Mériot JM, Gula R. 2009. Group territoriality as a form of cooperative breeding in the flightless Kagu (Rhynochetos jubatus) of New Caledonia. Auk 126, 371–375 (doi:10.1525/auk.2009.08092) [Google Scholar]
- 12.Kokko H, Johnstone RA, Clutton-Brock TH. 2001. The evolution of cooperative breeding through group augmentation. Proc. R. Soc. Lond. B 268, 187–196 (doi:10.1098/rspb.2000.1349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cockburn A, Russell AF. 2011. Cooperative breeding: a question of climate?. Curr. Biol. 21, R195–R197 (doi:10.1016/j.cub.2011.01.044) [DOI] [PubMed] [Google Scholar]
- 14.Selander RK. 1964. Speciation in wrens of the genus Campylorhynchus. Univ. Calif. Publ. Zool. 74, 1–224 [Google Scholar]
- 15.Brown JL. 1974. Alternative routes to sociality in jays—with a theory for the evolution of altruism and cooperative breeding. Am. Zool. 14, 63–80 [Google Scholar]
- 16.Ricklefs RE. 1975. The evolution of cooperative breeding in birds. Ibis 117, 531–534 (doi:10.1111/j.1474-919X.1975.tb04252.x) [Google Scholar]
- 17.Arnold KE, Owens IPF. 1999. Cooperative breeding in birds: the role of ecology. Behav. Ecol. 10, 465–471 (doi:10.1093/beheco/10.5.465) [Google Scholar]
- 18.Koenig WD, Walters EL, Haydock J. 2011. Variable helper effects, ecological conditions and the evolution of cooperative breeding in the acorn woodpecker. Am. Nat. 178, 145–158 (doi:10.1086/660832) [DOI] [PubMed] [Google Scholar]
- 19.Ford HA, Bell H, Nias R, Noske R. 1988. The relationship between ecology and the incidence of cooperative breeding in Australian birds. Behav. Ecol. Sociobiol. 22, 239–249 (doi:10.1007/BF00299838) [Google Scholar]
- 20.Ridley J, Yu DW, Sutherland WJ. 2005. Why long-lived species are more likely to be social: the role of local dominance. Behav. Ecol. Sociobiol. 16, 358–363 (doi:10.1093/beheco/arh170) [Google Scholar]
- 21.Rubenstein DR, Lovette IJ. 2007. Temporal environmental variability drives the evolution of cooperative breeding in birds. Curr. Biol. 17, 1414–1419 (doi:10.1016/j.cub.2007.07.032) [DOI] [PubMed] [Google Scholar]
- 22.Jetz W, Rubenstein DR. 2011. Environmental uncertainty and the global biogeography of cooperative breeding in birds. Curr. Biol. 21, 72–78 (doi:10.1016/j.cub.2010.11.075) [DOI] [PubMed] [Google Scholar]
- 23.Rubenstein DR. 2011. Spatiotemporal environmental variation, risk aversion, and the evolution of cooperative breeding as a bet-hedging strategy. Proc. Natl Acad. Sci. USA 108, 10 816–10 822 (doi:10.1073/pnas.1100303108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.du Plessis MA, Siegfried WR, Armstrong AJ. 1995. Ecological and life-history correlates of cooperative breeding in South African birds. Oecologia 102, 180–188 (doi:10.1007/BF00333250) [DOI] [PubMed] [Google Scholar]
- 25.Kemp AC. 2001. Family Bucerotidae (hornbills). In Handbook of the birds of the world. Vol. 6: mousebirds to hornbills (eds del Hoyo J, Elliott A, Sargatal J.), pp. 436–523 Barcelona, Spain: Lynx Edicions [Google Scholar]
- 26.Kinnaird MF, O'Brien TG. 2007. The ecology and conservation of Asian hornbills: farmers of the forest. Chicago, IL: University of Chicago Press [Google Scholar]
- 27.Viseshakul N, Charoennitikul W, Kitamura S, Kemp AC, Thong-aree S, Surapunpitak Y, Poonswad P, Ponglikitmongkol M. 2011. A phylogeny of frugivorous hornbills linked to the evolution of Indian plants within Asian rainforests. J. Evol. Biol. 24, 1533–1545 (doi:10.1111/j.1420-9101.2011.02285.x) [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez JCT, Sheldon BC, Collar NJ, Tobias JA. 2013. A comprehensive molecular phylogeny for the hornbills (Aves: Bucerotidae). Mol. Phylogenet. Evol. 67, 468–483 (doi:10.1016/j.ympev.2013.02.012) [DOI] [PubMed] [Google Scholar]
- 29.Arnold KE, Owens IPF. 1998. Cooperative breeding in birds: a comparative test of the life history hypothesis. Proc. R. Soc. Lond. B 265, 739–745 (doi:10.1098/rspb.1998.0355) [Google Scholar]
- 30.Craig AJ, Feare CJ. 2009. Family Sturnidae (Starlings). In Handbook of the birds of the world. Vol. 14: bush-shrikes to Old World sparrows. (eds del Hoyo J, Elliott A, Christie D.), pp. 654–709 Barcelona, Spain: Lynx Edicions [Google Scholar]
- 31.Dunning JB. 2008. CRC handbook of avian body masses. Boca Raton, FL: CRC Press [Google Scholar]
- 32.Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 (doi:10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (doi:10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 34.Cockburn A. 2006. Prevalence of different modes of parental care in birds. Proc. R. Soc. B 273, 1375–1383 (doi:10.1098/rspb.2005.3458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.New M, Lister D, Hulme M, Makin I. 2002. A high-resolution data set of surface climate over global land areas. Clim. Res. 21, 1–25 (doi:10.3354/cr021001) [Google Scholar]
- 36.Mitchell TD, Jones PD. 2005. An improved method of constructing a database of monthly climate observations and associated high-resolution grids. Int. J. Climatol. 25, 693–712 (doi:10.1002/joc.1181) [Google Scholar]
- 37.Pigot AL, Owens IPF, Orme CDL. 2010. The environmental limits to geographic range expansion in birds. Ecol. Lett. 13, 705–715 (doi:10.1111/j.1461-0248.2010.01462.x) [DOI] [PubMed] [Google Scholar]
- 38.Maddison W, Maddison D. 2010. Mesquite: a modular system for evolutionary analysis, v. 2.74. See http://mesquiteproject.org
- 39.Pagel M, Meade A. 2006. Bayesian analysis of correlated evolution of discrete characters by reversible-jump Markov chain Monte Carlo. Am. Nat. 167, 808–825 (doi:10.1086/503444) [DOI] [PubMed] [Google Scholar]
- 40.Hadfield JD. 2010. MCMC methods for multi-response generalised linear mixed models: the MCMCglmm R package. J. Statistic. Soft. 33, 1–22 [Google Scholar]
- 41.Hadfield JD, Nakagawa S. 2010. General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J. Evol. Biol. 23, 494–508 (doi:10.1111/j.1420-9101.2009.01915.x) [DOI] [PubMed] [Google Scholar]
- 42.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 43.Ekman J, Ericson PGP. 2006. Out of Gondwanaland: the evolutionary history of cooperative breeding and social behaviour among crows, magpies, jays and allies. Proc. R. Soc. B 273, 1117–1125 (doi:10.1098/rspb.2005.3431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Komdeur J. 1992. Importance of habitat saturation and territory quality for evolution of cooperative breeding in the Seychelles warbler. Nature 358, 493–495 (doi:10.1038/358493a0) [Google Scholar]
- 45.Tobias JA, Gamarra-Toledo V, Garcia-Olaechea D, Pulgarin PC, Seddon N. 2011. Year-round resource defence and the evolution of male and female song in suboscine birds: social armaments are mutual ornaments. J. Evol. Biol. 24, 2118–2138 (doi:10.1111/j.1420-9101.2011.02345.x) [DOI] [PubMed] [Google Scholar]
- 46.Baglione V, Marcos JM, Canestrari D, Griesser M, Andreotti G, Bardini C, Bogliani G. 2005. Does year-round territoriality rather than habitat saturation explain delayed natal dispersal and cooperative breeding in the carrion crow? J. Anim. Ecol. 74, 842–851 (doi:10.1111/j.1365-2656.2005.00983.x) [Google Scholar]
- 47.Baglione V, Canestrari D, Marcos JM, Ekman J. 2006. Experimentally increased food resources in the natal territory promote offspring philopatry and helping in cooperatively breeding carrion crows. Proc. R. Soc. B 273, 1529–1535 (doi:10.1098/rspb.2006.3481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emlen ST. 1982. The evolution of helping. I. An ecological constraints model. Am. Nat. 119, 29–39 (doi:10.1086/283888) [Google Scholar]