Abstract
Why females assess ornaments when choosing mates remains a central question in evolutionary biology. We hypothesize that the imperative for a choosing female to find a mate with nuclear oxidative phosphorylation (OXPHOS) genes that are compatible with her mitochondrial OXPHOS genes drives the evolution of ornaments. Indicator traits are proposed to signal the efficiency of OXPHOS function thus enabling females to select mates with nuclear genes that are compatible with maternal mitochondrial genes in the formation of OXPHOS complexes. Species-typical pattern of ornamentation is proposed to serve as a marker of mitochondrial type ensuring that females assess prospective mates with a shared mitochondrial background. The mitonuclear compatibility hypothesis predicts that the production of ornaments will be closely linked to OXPHOS pathways, and that sexual selection for compatible mates will be strongest when genes for nuclear components of OXPHOS complexes are Z-linked. The implications of this hypothesis are that sexual selection may serve as a driver for the evolution of more efficient cellular respiration.
Keywords: ornamentation, cellular respiration, mate choice, good genes, lek paradox
1. Introduction
Why do animals have ornaments? Under what circumstances did the immense train of a peacock, the cumbersome antlers of an elk and the conspicuous red coloration of a cardinal evolve? Biologists now accept that the primary force for the evolution of many such display traits in male animals is the benefit of mate attraction [1–3]. There remains, however, the bigger question that has eluded solution for more than a century: why do females pay attention to male ornaments? Explanations invariably fall into two camps. Either ornaments fulfil an arbitrary attraction by females or they signal important aspects of male condition, including male genetic quality [2,4].
Models of arbitrary mate choice posit that females are innately attracted to specific but esoteric characteristics of males [5]. These innate preferences are proposed to evolve through a runaway process whereby the benefits of producing attractive sons leads to a reinforcing cycle that increases both the trait and the preference for the trait [6,7]. The runaway model of arbitrary mate choice works very well in mathematical models [8], but finds little support in nature beyond the existence of highly elaborate traits [9].
Under models of adaptive mate choice, ornamental traits are postulated to evolve because they are reliable signals of male quality [10,11]. Female choice drives the evolution of these traits and, in turn, female choice for ornaments evolves because of the benefits of mating with high-quality males [12]. Benefits to females for choosing highly ornamented males can be resources such as more food or access to a better territory [13], but in many species, males convey no apparent resources to females. Thus, ornamental traits are proposed to signal good genes [5]. By this good genes hypothesis, ornaments are challenging to produce [14] or invoke a fitness cost to maintain [15], and only males with good genotypes are capable of meeting the challenge of ornamentation. Hence, by choosing ornamented males, females choose males with better-than-average genes [11]. In contrast to the lack of empirical support for models of runaway sexual selection, there is abundant evidence in nature that ornamental traits are associated with aspects of male condition [16–18]. Very few studies, however, have provided compelling evidence that ornamentation is linked to specific good genes [19,20].
(a). The mitonuclear compatibility model of sexual selection
Here, we present a new hypothesis for the evolution of ornamental traits founded on the premise that the greatest imperative for a choosing female is identification of a male that is genetically compatible. Discussions of good genes associated with ornaments have focused exclusively on the nuclear genome, but eukaryotes also have mitochondrial genes. Among the primary products of these mitochondrial genes are proteins that form complexes with proteins encoded by nuclear genes [21]. The mitonuclear complexes include four of the five functional units of the electron transport chain, the machinery in the inner-mitochondrial membrane that carries out oxidative phosphorylation (OXPHOS) reactions [22,23]. Mitochondria-encoded proteins and nuclear-encoded proteins must work in concert in forming functional complexes for efficient OXPHOS and energy production [24,25]. Any incompatibility between mitochondrial and nuclear genes leads to loss of respiratory efficiency and an increase in free radical production [26,27]. Serious incompatibility leads to loss of functionality and system failure [28,29].
These mitonuclear complexes arise anew each generation as the products of maternally inherited mitochondrial DNA combined with the products of the new diploid nuclear genome. Thus, selection must take place each generation after zygote formation [24]. A fundamental challenge to females in choosing mates is to find males with nuclear genes that are compatible with their maternal mitochondrial genes. Inefficient cellular respiration is likely an indication of nuclear genes that are incompatible with mitochondrial genes [30,31] and that will be passed on to offspring, so females should be selected to assess potential mates based on efficiency of cellular respiration. Because cellular respiration cannot be directly assessed, we propose that ornamental traits evolved as indicators of OXPHOS functionality and of signals that males carry common mitochondrial genes. By this mitonuclear compatibility hypothesis of sexual selection, the critical need for females to assess the compatibility between mitochondrial and nuclear genes in prospective mates is a motive force in the evolution of ornamental traits.
Any change in the structure of the proteins that form OXPHOS complexes might lead to reduced energy production [26,28] or a host of other diseases [21], so mutation should perpetually introduce incompatible nuclear genes into the population of prospective mates. Ornamentation that reveals cellular respiratory efficiency will reveal such mutations, providing a tangible benefit for choosing well-ornamented males. Incompatibilities are also expected to occur if females mate with heterospecific males [32], and avoidance of mitonuclear incompatibilities should be a major form of selection opposing hybridization [33,34].
2. Predictions
(a). Ornaments as signals of cellular respiration
A critical assumption of the mitonuclear compatibility model of sexual selection is that components of ornamentation will be linked to OXPHOS pathways. By current indicator models of sexual selection, ornamental traits are proposed to reveal the condition or the quality of prospective mates, allowing females to make a better mate choice [2,10,35]. Here, we propose that condition (sensu [14]) is the efficiency of production of ATP via OXPHOS, and that it is OXPHOS efficiency that is signalled by many condition-dependent ornaments. Many dozens of studies conducted over the last 10 years have linked ornamental traits in animals to an array of proxies for condition, including mass corrected for body size (adiposity) [36], aerobic capacity [37], oxidative stress [38], the ability to synthesize protein structures [39], immunocompetence [40] and cognition [41]. Such a diverse range of individual properties linked to ornamentation has led to the perception that there is no most-important core aspect of individual performance that is linked to ornamentation [8]. Building on the ideas of Velando et al. [42] and von Schantz et al. [43], we propose that the most fundamental aspect of a prospective mate lies in its ability to generate ATP from OXPHOS [44]. Moreover, the molecular machinery that enables OXPHOS is subject to disruption by both environmental [43] and genetic [45] effects making information about the state of OXPHOS of critical relevance to a prospective mate.
Some of the best evidence that ornamentation is intimately linked to OXPHOS reactions comes from studies of ornamental carotenoid coloration in birds, which is one of the most widely cited examples of a sexually selected display trait. It has recently been observed that red ketolated carotenoids concentrate within hepatic mitochondria of male house finches (Haemorhous mexicanus) that are moulting red feathers (J. D. Johnson & G. E. Hill 2013, unpublished data) suggesting that the production of red ornamental coloration is closely tied to oxidative state of the inner-mitochondrial membrane [46,47]. These studies suggest that by assessing red ornamental coloration, females are directly assessing the efficiency of OXPHOS. Any malfunction or poor function of the protein complexes needed for OXPHOS reactions are revealed in quality red ornamentation, which is well established as a criterion in mate choice [48]. The same basic arguments should also apply to non-ketolated carotenoids, such as yellow canary xanthophylls displayed in the feathers of many birds [46], and a recent study showed that the brightness of canary xanthophyll coloration of feathers predicted resting metabolic rate [49].
Production of other types of ornamental traits may also link to OXPHOS pathways. The synthesis of proteins is among the most energy-demanding processes in organisms [26], so OXPHOS efficiency might be signalled through the many large structures with a high protein content that serve as ornamental traits, such as elongated tails, crests, wattles and dewlaps. Likewise, behavioural displays such as loud calls, courtship dances or flight displays demand high aerobic capacity and might signal efficient energy production [49–54]. Displays requiring high neural function as songs or bower construction could also link to access to OXPHOS efficiency during development [55].
(b). Ornamental displays as signals of mitochondrial type
A choosing female must evaluate a prospective mate against a shared mitochondrial genotype, or she gains no useful information regarding mitonuclear compatibility through assessment of ornamentation. Thus, a second critical assumption of the mitonuclear compatibility hypothesis of sexual selection is that females share a common set of mitochondrial haplotypes (a common haplogroup) with prospective mates. Because of the need for mitonuclear coadaptation [56] there is little diversity of functional mitochondrial types in most animal populations [22,57], so the assumption of shared mitochondrial types should hold within population boundaries. But how do females recognize population boundaries? The species recognition hypothesis was proposed in the nineteenth century as an explanation for female choice for species-typical ornament types [58]. By mating with males with species-typical ornaments, females are hypothesized to avoid novel nuclear genes and chromosomal arrangements [59]. The mitonuclear compatibility hypothesis clarifies the benefits to females of avoiding hybrid pairings [33]. We propose that species-typical sexual ornaments arise and are maintained in response to selection on females to mate with males with a common mitochondrial type and hence to avoid the incompatibility problems that arise with hybridization [32,60]. We propose that it is no coincidence that taxonomists designate species-level differences among animals based on the same traits that females use in mate choice. The widespread use of mitochondrial DNA sequences in systematics [61] supports the assumption that there is concordance between mitochondrial types and mating populations [62].
Putting these ideas together: the imperative of choosing a sire for offspring that provides nuclear genes that are compatible with female mitochondrial genes leads to the evolution of two types of ornamental displays in males. Indicator traits signal cellular respiration. To judge OXPHOS competency, females assess how well such indicator traits are executed by an individual (e.g. how vibrant are colour displays or how vigorous are behavioural displays). Signals of species identity, on the other hand, convey to the female that she is dealing with a male that has a common mitochondrial background. The same trait might serve as both an indicator of cellular respiration and a signal of species identity, but in general we would expect the two ornament types to be distinct. Indicator traits must be variable in expression to signal male condition, while traits used in species recognition should be relatively invariant [35]. In support of these ideas, birds choose species-typical patterns of plumage pigmentation over heterospecific patterns even when the latter are bigger and brighter [63]. Within species-typical plumage types, however, females choose the males with the reddest carotenoid displays [48] (figure 1).
Figure 1.
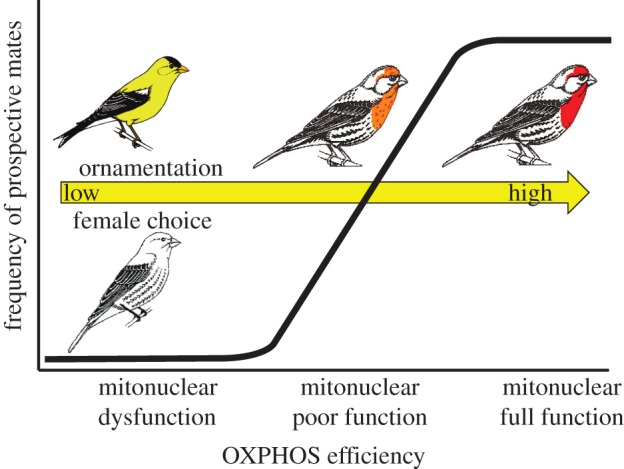
A graphical representation of the mitonuclear hypothesis of sexual selection. OXPHOS efficiency describes cellular respiration of offspring produced by a female, in this example a house finch (Haemorhous mexicanus), who chooses various ornament expressions in mates. Choosing an ornament type different than that of a typical male (in this case American goldfinch; Spinus tristis) results in severe mitonuclear incompatibility and dysfunction. Drab plumage coloration among conspecifics may indicate poor mitonuclear compatibility in a male, and hence genes that are incompatible with female mitotype resulting in offspring with poor OXPHOS efficiency. Most males have mitonuclear compatibility and fine ornamentation. These are the preferred mates of females and are likely to sire healthy offspring. (Online version in colour.)
Ornamental displays often include suites of behaviours and morphological traits. A conundrum in discussions of mechanisms for the evolution of such ornamentation is how these gaudy displays can appear to be arbitrary when considered in total and yet execution of specific elements consistently link to individual condition [4,8]. In proposing that mate choice for mitonuclear compatibility operates at two distinct levels—choice for mitochondrial type and choice of efficient OXPHOS pathways within type—we provide an explanation for this dual nature of animal ornamentation (figure 1). An indicator component must be closely linked to OXPHOS processes to reveal incompatibility, but signals of mitochondrial type are arbitrary with respect to male condition. We propose no novel mechanisms for extreme elaboration of ornamentation, but existing models [6,7] can explain how arbitrary signals of species identity can be elaborated.
3. Chromosomal position and male influence
(a). Sex linkage of oxidative phosphorylation genes
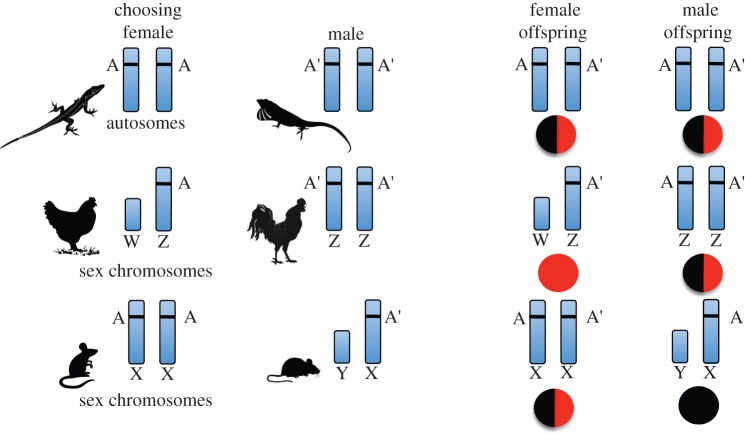
The imperative for females to find mates with nuclear genes that are compatible with mitochondrial genes arises when male genotype is expressed regardless of female genotype. Incomplete dominance of female autosomal alleles that encode for components of respiratory complexes would mean that a fully functional female allele would be impaired if matched to a poorly functioning male allele (figure 2), and thus that the negative effects of the male allele could exert selective pressure on females to avoid choosing such incompatible males. The necessity for females to find compatible males would be stronger, however, if the contribution of male genes to OXPHOS proteins was greater. Such male influence is possible when males are the homogametic sex (ZZ), as in taxa like birds and butterflies, and OXPHOS genes are positioned on the Z chromosome. In such systems, female (WZ) offspring are viable only if the male sire contributes functional Z-linked genes. Moreover, the total influence of the male sire on the OXPHOS systems of offspring would be large because males contribute 67% of Z-linked genes to offspring (figure 2). When males are the heterogametic sex (XY), as in mammals and fruit flies, sex linkage of nuclear OXPHOS genes does not impose the same selective pressure for mate choice. X chromosomes are 67% maternally inherited so the dilution of incompatible male genes would be greater on the X chromosome than on autosomes [22] (figure 2). The prediction that emerges is Z-linkage of OXPHOS genes will promote female mate choice and the evolution of ornaments but X-linkage will not.
Figure 2.
The potential effects of male genotype on offspring viability in relation to chromosomal position of OXPHOS genes. Allele A from the female is compatible with maternal mitochondrial genotype; allele A’ from the male is incompatible with maternal mitochondrial genotype. Proportion of pie chart that is not black indicates potential loss of functionality in offspring from the incompatible male OXPHOS gene. Co-dominance of alleles is assumed. When males are the homogametic sex, ZZ, placement of OXPHOS genes on Z chromosomes increases the influence of paternal alleles (a smaller proportion of the pies in black). By contrast, when females are homogametic, XX, placement of OXPHOS genes on X chromosome decreases the influence of paternal alleles (a larger proportion of the pies is black). (Online version in colour.)
What is the distribution of OXPHOS genes on Z and X chromosomes and could patterns of sex linkage affect sexual selection and ornamentation? In a survey of 14 mammal species, nuclear OXPHOS genes were found to be highly under-represented on the X chromosome [64]. In the two bird species included in the survey, by contrast, OXPHOS genes were located on Z chromosomes at the frequency expected by chance but at a frequency that was much higher than the frequency observed for OXPHOS genes on the X chromosome in mammals [64]. Moreover, among the OXPHOS genes encoded on the zebra finch (Taeniopygia guttata) Z chromosome are the Complex I sub-units NDUFS4 and NDUFAF2 (J. D. Johnson & G. E. Hill 2013, unpublished data), both of which play an essential role in the functionality of electron transport, including ATP synthesis and the modulation of apoptosis. The location of key OXPHOS genes on the Z chromosome in birds means that female birds will produce viable offspring only if they mate with compatible males.
A complication to the idea that Z linkage promotes female mate choice is that in males the Z chromosome is diploid, and therefore deleterious recessive mutations that are Z linked can be masked, just as in autosomes. However, many human disorders appear to arise from incompatible (defective) nuclear-encoded mitochondrial genes [21] that are the result of rare mutations on autosomes, suggesting that deleterious OXPHOS alleles may not always be recessive.
(b). ZW versus XY taxa
While birds are characterized by colours, songs and courtship displays that function in female choice, mammals much more commonly have armaments, physical contests and dimorphism that result from male–male competition [65]. Across a broader survey of taxa, the pattern that emerges is that animals with ZW sex determination systems are more ornamented than animals with XY sex determination [66]. The protected invasion hypothesis provides a potential explanation for the association between ornamentation and ZW sex determination [66]. Sexual determination systems in which females are the heterogametic sex better shelter rare sex-linked genes from loss due to genetic drift, hence promoting the evolution of novel ornaments [66] (but see [67]). Alternatively, mathematical models show that, compared with autosomal or X-linkage, Z-linkage of preference genes substantially enhances conditions for ornament elaboration through a runaway process. Models further indicated that X-linkage of preference genes and autosomal positioning of ornamentation genes is conducive to good genes processes [67].
The observation that OXPHOS genes are positioned on Z chromosomes suggests additional connections between sexual selection and sex determination systems. It is interesting to speculate that—regardless of the reason for placement of OXPHOS genes on the Z chromosome of birds—Z-linkage of OXPHOS genes in birds makes selection on mate choice for mitonuclear compatibility stronger in birds than in mammals. If this assumption is correct then sex linkage of OXPHOS genes present an alternative—or more likely complementary—explanation to the protection invasion hypothesis and runaway models for the ubiquity of ornamentation in ZW taxa compared with XY taxa. Our hypothesis that placement of OXPHOS genes on Z chromosomes leads to selection for female choice and the evolution of ornamentation could be more rigorously tested with comparative studies of ornamentation and Z-linkage of OXPHOS genes across diverse taxa.
Another potential consequence of location of OXPHOS genes on Z chromosomes is that it increases the importance of male genotype in mitonuclear compatibility and hence increases the necessity of mate choice and the intensity of sexual selection. If ornamental traits evolve as signals of respiratory efficiency and mitochondrial type, then sexual selection becomes an important force in the evolution of OXPHOS systems of animals. Because females can assess poor OXPHOS function and inappropriate mates by choosing specific ornament displays, there would be stronger purifying selection in ornamented species that would efficiently purge both deleterious mutations and immigrant genes each generation. Z-linked genes have been shown to evolve faster than autosomal or X-linked genes [68,69] and strong sexual selection would further enhance the evolutionary process. Such selection could also lead to more rapid fixation of evolutionary novelties that would increase respiratory efficiency. The strength of purifying selection on OXPHOS systems in birds is indeed indicated by relatively slow rates of mitochondrial DNA evolution in birds compared with mammals [70]. Magnified across evolutionary time, the result would be fundamentally better respiratory processes in animals with female mate choice for mitonuclear compatibility. It is interesting to speculate that sexual selection for compatibility and the greater potential for evolved coadaptation afforded by ZW sex determination are factors in the more efficient cellular respiration of birds compared with mammals [28,71] and perhaps even in the evolution of flight in birds [24]. Our general prediction is the animals with ZW sex determination will have more efficient cellular respiration than animals with other forms of sex determination.
(c). Z-linkage and species introgression
If OXHPOS genes are Z-linked and there are strong negative fitness consequences for introgression of OXPHOS genes between species [33,72], then we would expect the flow of autosomal genes to be greater than Z-linked genes when hybridization occurs. Studies of birds [73–75] support this prediction. There are explanations besides the loss of mitonuclear compatibility for the restricted gene flow of Z-linked genes, but our suggestion that OXPHOS genes on Z chromosomes opposes introgression presents a novel hypothesis to explain why Z-linked genes intergrade less readily than autosomal genes.
(d). Haldane's rule
It has long been recognized that in heterospecific crosses, the heterogametic sex shows the greatest incidence of sterility, a phenomenon known as Haldane's rule [76]. In a review of the outcome of hybrid pairings in mammals (XY), Drosophila (XY), birds (ZW) and lepidopterans (ZW), there was nearly perfect agreement with Haldane's rule with regard to offspring sterility: XY males were sterile more often than XX females and ZW females were sterile more often than ZZ males [77]. By contrast, loss of viability was greater in the heterogamic sex only in birds and butterflies with ZW sex determination, not in mammals and Drosophila with XY sex determination. Loss of viability in fertile hybrid offspring can be a direct consequence of ATP production and free radical leakage, and we propose that the greater effects on ZW females compared with ZZ males in hybrid crosses is a consequence of key Z-linked OXPHOS genes coming exclusively from the male sire in female but not male offspring (figure 2). By contrast, few OXPHOS genes are positioned on the X chromosome in mammals [64], and even if there was X-linkage of OXPHOS genes, there should be slightly greater effects of mitonuclear incompatibility on XX females, which get one X from the male sire, compared with XY males, which get all X genes from their mother (figure 2). Hence, mitonuclear compatibility potentially explains heretofore confusing patterns of hybrid inviability [77].
4. The lek paradox
The mitonuclear compatibility hypothesis of sexual selection potentially helps to resolve one of the oldest and most persistent puzzles in evolutionary biology. So long as there is a stable adaptive peak, natural selection inevitably leads to the fixation of best-adapted alleles leaving no variation in the fitness of traits (no good genes) at equilibrium. Loss of heritable variation in fitness leads to the lek paradox, so named because in polygynous species that display at courts (leks) males provide females only with ejaculate; the benefits to females for choosing ornamented males seemingly must be good genes, which theoretically cannot exist [78,79]. Proposed solutions to the lek paradox include the argument that equilibrium is rarely achieved in changing biotic and abiotic environments [80] or with frequency-dependent selection [81], so there are perpetually good genes to be had [82]. Alternatively, the purging effects of sexual selection can increase mutation rates that generate sufficient genetic diversity for good genes models to work [83,84]. One of the simplest solutions is the suggestion that mutation alone can be sufficient to introduce heritable variation in fitness [85], and we propose that invoking the necessity of mitonuclear compatibility strengthens this argument. Via mutation and immigration, incompatible OXPHOS genes will be present every generation regardless of the strength of sexual selection. The benefits of assessing potential mates based on ornamental traits that reliably signal OXPHOS functionality will consequently never be exhausted. There is no paradox of choosing ornamented mates on leks if choice is for mitonuclear compatibility.
5. Mate quality beyond compatibility
In many species, even if selection for mitonuclear compatibility is the impetus for the evolution of female mate choice and male ornamentation, the benefits to females of assessing ornaments that signal the efficiency of OXPHOS will extend beyond assessment of mitonuclear compatibility. Males with better cellular functionality, whether due to genetic or environmental effects, will be healthier and thus able to provide more resource benefits such as a greater contribution towards production of offspring or better territories. OXPHOS efficiency signalled by ornamentation could also correlate with nuclear genes that encode for proteins outside of OXPHOS complexes, such as genes for parasite resistance [11] or with sperm with less DNA damage from inefficient OXPHOS processes [42]. By rejecting poorly ornamented males, females reject not only genetically incompatible males but also males with poor phenotypes and unfit genes in other capacities [14].
It has long been recognized that females should seek to know the fundamental nature of the males with whom they sire offspring [86,87]. We propose that the most fundamental aspect of a prospective mate lies in its ability to generate ATP from OXPHOS, which hinges critically on compatibility of mitochondrial and nuclear genes.
Acknowledgements
We thank W. Hood, N. Lane, S. Edwards, C. Bonneaud, S. F. Dobson and members of the Hood and Hill labs for comments.
Funding statement
Work supported by National Science Foundation grant nos. IOS-0923600 and IOS-1243207.
References
- 1.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray [Google Scholar]
- 2.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 3.Hill GE. 2006. Female choice for ornamental coloration. In Bird coloration: function and evolution, vol. 3 (eds Hill GE, McGraw KJ.), pp. 137–200 Cambridge, MA: Harvard University Press [Google Scholar]
- 4.Hill GE. In press. The evolution of ornaments and armaments. In Animal behavior: how and why animals do the things they do. Vol. 3: Function and evolution of animal behavior New York, NY: Praeger/ABC-CLIO [Google Scholar]
- 5.Kirkpatrick M, Ryan MJ. 1991. The evolution of mating preferences and the paradox of the lek. Nature 350, 33–38 (doi:10.1038/350033a0) [Google Scholar]
- 6.Lande R. 1981. Models of speciation by sexual selection on polygenic traits. Proc. Natl Acad. Sci. USA 78, 3721–3725 (doi:10.1073/pnas.78.6.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkpatrick M. 1982. Sexual selection and the evolution of female choice. Evolution 36, 1–12 (doi:10.2307/2407961) [DOI] [PubMed] [Google Scholar]
- 8.Prum RO. 2010. The Lande–Kirkpatrick mechanism is the null model of evolution by intersexual selection: implications for meaning, honesty, and design in intersexual signals. Evolution 64, 3085– 3100 (doi:10.1111/j.1558-5646.2010.01054.x) [DOI] [PubMed] [Google Scholar]
- 9.Svensson EI, Gosden TP. 2007. Contemporary evolution of secondary sexual traits in the wild. Funct. Ecol. 21, 422–433 (doi:10.1111/j.1365-2435.2007.01265.x) [Google Scholar]
- 10.Kodric-Brown A, Brown JH. 1984. Truth in advertising: the kinds of traits favored by sexual selection. Am. Nat. 124, 309–323 (doi:10.1086/284275) [Google Scholar]
- 11.Hamilton WD, Zuk M. 1982. Heritable true fitness and bright birds: a role for parasites? Science 218, 384–386 (doi:10.1126/science.7123238) [DOI] [PubMed] [Google Scholar]
- 12.Grafen A. 1990. Sexual selection unhandicapped by the Fisher process. J. Theor. Biol. 144, 473–516 (doi:10.1016/S0022-5193(05)80087-6) [DOI] [PubMed] [Google Scholar]
- 13.Hoelzer GA. 1989. The good parent process of sexual selection. Anim. Behav. 38, 1067–1078 (doi:10.1016/S0003-3472(89)80146-0) [Google Scholar]
- 14.Hill GE. 2011. Condition-dependent traits as signals of the functionality of vital cellular processes. Ecol. Lett. 14, 625–634 (doi:10.1111/j.1461-0248.2011.01622.x) [DOI] [PubMed] [Google Scholar]
- 15.Zahavi A. 1975. Mate selection–a selection for a handicap. J. Theor. Biol. 53, 205–214 (doi:10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 16.Cotton S, Fowler K, Pomiankowski A. 2004. Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proc. R. Soc. Lond. B 271, 771–783 (doi:10.1098/rspb.2004.2688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotton S, Small J, Pomiankowski A. 2006. Sexual selection and condition-dependent mate preferences. Curr. Biol. 16, R755–R765 (doi:10.1016/j.cub.2006.08.022) [DOI] [PubMed] [Google Scholar]
- 18.Hill GE. 2006. Environmental regulation of ornamental coloration. In Bird coloration, volume 1: mechanisms and measurements (eds Hill GE, McGraw KJ.), pp. 507–560 Cambridge, MA: Harvard University Press [Google Scholar]
- 19.Neff BD, Pitcher TE. 2005. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 14, 19–38 (doi:10.1111/j.1365-294X.2004.02395.x) [DOI] [PubMed] [Google Scholar]
- 20.Prokop ZM, Michalczyk L, Drobniak SM, Herdegen M, Radwan J. 2012. Meta-analysis suggests choosy females get sexy sons more than ‘good genes’. Evolution 66, 2665–2673 (doi:10.1111j.1558-5646.2012.01654.x) [DOI] [PubMed] [Google Scholar]
- 21.Calvo SE, Mootha VK. 2010. The mitochondrial proteome and human disease. Annu. Rev. Genomics Hum. Genet. 11, 25–44 (doi:10.1146/annurev-genom-082509-141720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rand DM, Haney RA, Fry AJ. 2004. Cytonuclear coevolution: the genomics of cooperation. Trends Ecol. Evol. 19, 645–653 (doi:10.1016/j.tree.2004.10.003) [DOI] [PubMed] [Google Scholar]
- 23.Bar-Yaacov D, Blumberg A, Mishmar D. 2012. Mitochondrial–nuclear co-evolution and its effects on OXPHOS activity and regulation. BBA-Gene Regul. Mech. 1819, 1107–1111 (doi:10.1016/j.bbagrm.2011.10.008) [DOI] [PubMed] [Google Scholar]
- 24.Lane N. 2011. Mitonuclear match: optimizing fitness and fertility over generations drives ageing within generations. Bioessays 33, 860–869 (doi:10.1002/bies.201100051) [DOI] [PubMed] [Google Scholar]
- 25.Blier PU, Dufresne F, Burton RS. 2001. Natural selection and the evolution of mtDNA-encoded peptides: evidence for intergenomic co-adaptation. Trends Genet. 17, 400–406 (doi:10.1016/s0168-9525(01)02338-1) [DOI] [PubMed] [Google Scholar]
- 26.Lane N, Martin W. 2010. The energetics of genome complexity. Nature 467, 929–934 (doi:10.1038/nature09486) [DOI] [PubMed] [Google Scholar]
- 27.Dey R, Barrientos A, Moraes CT. 2000. Functional constraints of nuclear–mitochondrial DNA interactions in xenomitochondrial rodent cell lines. J. Biol. Chem. 275, 31 520–31 527 (doi:10.1074/jbc.M004053200) [DOI] [PubMed] [Google Scholar]
- 28.Lane N. 2011. The costs of breathing. Science 334, 184–185 (doi:10.1126/science.1214012) [DOI] [PubMed] [Google Scholar]
- 29.Burton RS, Ellison CK, Harrison JS. 2006. The sorry state of F-2 hybrids: consequences of rapid mitochondrial DNA evolution in allopatric populations. Am. Nat. 168, S14–S24 (doi:10.1086/509046) [DOI] [PubMed] [Google Scholar]
- 30.Moreno-Loshuertos R, Acin-Perez R, Fernandez-Silva P, Movilla N, Perez-Martos A, de Cordoba SR, Gallardo ME, Enriquez JA. 2006. Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nat. Genet. 38, 1261–1268 (doi:10.1038/ng1897) [DOI] [PubMed] [Google Scholar]
- 31.Pichaud N, Ballard JWO, Tanguay RM, Blier PU. 2012. Naturally occurring mitochondrial DNA haplotypes exhibit metabolic differences: insight into functional properties of mitochondria. Evolution 66, 3189–3197 (doi:10.1111/j.1558-5646.2012.01683.x) [DOI] [PubMed] [Google Scholar]
- 32.Ellison CK, Burton RS. 2008. Interpopulation hybrid breakdown maps to the mitochondrial genome. Evolution 62, 631–638 (doi:10.1111/j.1558-5646.2007.00305.x) [DOI] [PubMed] [Google Scholar]
- 33.Gershoni M, Templeton AR, Mishmar D. 2009. Mitochondrial bioenergetics as a major motive force of speciation. Bioessays 31, 642–650 (doi:10.1002/bies.200800139) [DOI] [PubMed] [Google Scholar]
- 34.Chou JY, Leu JY. 2010. Speciation through cytonuclear incompatibility: insights from yeast and implications for higher eukaryotes. Bioessays 32, 401–411 (doi:10.1002/bies.200900162) [DOI] [PubMed] [Google Scholar]
- 35.Maynard Smith J. 1991. Theories of sexual selection. Trends Ecol. Evol. 6, 146–151 (doi:10.1016/0169-5347(91)90055-3) [DOI] [PubMed] [Google Scholar]
- 36.Cogliati KM, Corkum LD, Doucet SM. 2010. Bluegill coloration as a sexual ornament: evidence from ontogeny, sexual dichromatism, and condition dependence. Ethology 116, 416–428 (doi:10.1111/j.1439-0310.2010.01755.x) [Google Scholar]
- 37.Sullivan BK, Walsberg GE. 1985. Call rate and aerobic capacity in Woodhouse's toad (Bufo woodhousei). Herpetologica 41, 404–407 [Google Scholar]
- 38.Alonso-Alvarez C, Bertrand S, Faivre B, Chastel O, Sorci G. 2007. Testosterone and oxidative stress: the oxidation handicap hypothesis. Proc. R. Soc. B 274, 819–825 (doi:10.1098/rspb.2006.3764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill GE, Montgomerie R. 1994. Plumage colour signals nutritional condition in the house finch. Proc. R. Soc. Lond. B 258, 47–52 (doi:10.1098/rspb.1994.0140) [Google Scholar]
- 40.McGraw KJ, Ardia DR. 2003. Carotenoids, immunocompetence, and the information content of sexual colors: an experimental test. Am. Nat. 162, 704–712 (doi:10.1086/378904) [DOI] [PubMed] [Google Scholar]
- 41.Buchanan KL, Grindstaff JL, Pravosudov VV. 2013. Condition dependence, developmental plasticity, and cognition: implications for ecology and evolution. Trends Ecol. Evol. 28, 290–296 (doi:10.1016/j.tree.2013.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Velando A, Torres R, Alonso-Alvarez C. 2008. Avoiding bad genes: oxidatively damaged DNA in germ line and mate choice. Bioessays 30, 1212–1219 (doi:10.1002/bies.20838) [DOI] [PubMed] [Google Scholar]
- 43.von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H. 1999. Good genes, oxidative stress and condition-dependent sexual signals. Proc. R. Soc. Lond. B 266, 1–12 (doi:10.1098/rspb.1999.0597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brand MD. 2005. The efficiency and plasticity of mitochondrial energy transduction. Biochem. Soc. Trans. 33, 897–904 (doi:10.1042/BST20050897) [DOI] [PubMed] [Google Scholar]
- 45.Calvo S, et al. 2006. Systematic identification of human mitochondrial disease genes through integrative genomics. Nat. Genet. 38, 576–582 (doi:10.1038/ng1776) [DOI] [PubMed] [Google Scholar]
- 46.Hill GE, Johnson JD. 2012. The vitamin A–redox hypothesis: a biochemical basis for honest signaling via carotenoid pigmentation. Am. Nat. 180, E127–E150 (doi:10.1086/667861) [DOI] [PubMed] [Google Scholar]
- 47.Johnson JD, Hill GE. 2013. Is carotenoid ornamentation linked to the inner mitochondria membrane potential? A hypothesis for the maintenance of signal honesty. Biochimie 95, 436–444 (doi:10.1016/j.biochi.2012.10.021) [DOI] [PubMed] [Google Scholar]
- 48.Hill GE. 1991. Plumage coloration is a sexually selected indicator of male quality. Nature 350, 337–339 (doi:10.1038/350337a0) [Google Scholar]
- 49.Kelly RJ, Murphy TG, Tarvin KA, Burness G. 2012. Carotenoid-based ornaments of female and male American goldfinches (Spinus tristis) show sex-specific correlations with immune function and metabolic rate. Physiol. Biochem. Zool. 85, 348–363 (doi:10.1086/666059) [DOI] [PubMed] [Google Scholar]
- 50.Watt WB, Carter PA, Donohue K. 1986. Females’ choice of ‘good genotypes’ as mates is promoted by an insect mating system. Science 233, 1187–1190 (doi:10.1126/science.3738528) [DOI] [PubMed] [Google Scholar]
- 51.Hedenstrom A, Alerstam T. 1996. Skylark optimal flight speeds for flying nowhere and somewhere. Behav. Ecol. 7, 121–126 (doi:10.1093/beheco/7.2.121) [Google Scholar]
- 52.Hoback WW, Wagner WE. 1997. The energetic cost of calling in the variable field cricket, Gryllus lineaticeps. Physiol. Entomol. 22, 286–290 (doi:10.1111/j.1365-3032.1997.tb01170.x) [Google Scholar]
- 53.Nicoletto PF. 1993. Female sexual-response to condition-dependent ornaments in the guppy, Poecilia reticulata. Anim. Behav. 46, 441–450 (doi:10.1006/anbe.1993.1213) [Google Scholar]
- 54.Stoddard PK, Salazar VL. 2011. Energetic cost of communication. J. Exp. Biol. 214, 200–205 (doi:10.1242/jeb.047910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nowicki S, Searcy WA. 2004. Song function and the evolution of female preferences—why birds sing, why brains matter. Behav. Neurobiol. Birdsong 1016, 704–723 (doi:10.1196/annals.1298.012) [DOI] [PubMed] [Google Scholar]
- 56.Dowling DK, Friberg U, Lindell J. 2008. Evolutionary implications of non-neutral mitochondrial genetic variation. Trends Ecol. Evol. 23, 546–554 (doi:10.1016/j.tree.2008.05.011) [DOI] [PubMed] [Google Scholar]
- 57.Bazin E, Glemin S, Galtier N. 2006. Population size does not influence mitochondrial genetic diversity in animals. Science 312, 570–572 (doi:10.1126/science.1122033) [DOI] [PubMed] [Google Scholar]
- 58.Wallace AR. 1889. Darwinism. London, UK: Macmillian [Google Scholar]
- 59.Mayr E. 1963. Animal species and evolution. Cambridge, MA: Harvard University Press [Google Scholar]
- 60.Qvarnstrom A, Bailey RI. 2009. Speciation through evolution of sex-linked genes. Heredity 102, 4–15 (doi:10.1038/hdy.2008.93) [DOI] [PubMed] [Google Scholar]
- 61.Lane N. 2009. On the origin of bar codes. Nature 462, 272–274 (doi:10.1038/462272a) [DOI] [PubMed] [Google Scholar]
- 62.Petit RJ, Excoffier L. 2009. Gene flow and species delimitation. Trends Ecol. Evol. 24, 386–393 (doi:10.1016/j.tree.2009.02.011) [DOI] [PubMed] [Google Scholar]
- 63.Hill GE, McGraw KJ. 2004. Correlated changes in male plumage coloration and female mate choice in cardueline finches. Anim. Behav. 67, 27–35 (doi:10.1016/j.anbehav.2003.02.002) [Google Scholar]
- 64.Drown DM, Preuss KM, Wade MJ. 2012. Evidence of a paucity of genes that interact with the mitochondrion on the X in mammals. Genome Biol. Evol. 4, 875–880 (doi:10.1093/gbe/evs064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hastings IM. 1994. Manifestations of sexual selection may depend on the genetic-basis of sex determination. Proc. R. Soc. Lond. B 258, 83–87 (doi:10.1098/rspb.1994.0146) [DOI] [PubMed] [Google Scholar]
- 66.Reeve HK, Pfennig DW. 2003. Genetic biases for showy males: are some genetic systems especially conducive to sexual selection? Proc. Natl Acad. Sci. USA 100, 1089–1094 (doi:10.1073/pnas.0337427100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kirkpatrick M, Hall DW. 2004. Sexual selection and sex linkage. Evolution 58, 683–691 (doi:10.1554/03-332) [DOI] [PubMed] [Google Scholar]
- 68.Kirkpatrick M, Hall DW. 2004. Male-biased mutation, sex linkage, and the rate of adaptive evolution. Evolution 58, 437–440 (doi:10.1554/03-333) [PubMed] [Google Scholar]
- 69.Mank JE, Axelsson E, Ellegren H. 2007. Fast-X on the Z: rapid evolution of sex-linked genes in birds. Genome Res. 17, 618–624 (doi:10.1101/gr.6031907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nabholz B, Glemin S, Galtier N. 2009. The erratic mitochondrial clock: variations of mutation rate, not population size, affect mtDNA diversity across birds and mammals. BMC Evol. Biol. 9, 54 (doi:10.1186/1471-2148-9-54) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barja G. 2007. Mitochondrial oxygen consumption and reactive oxygen species production are independently modulated: implications for aging studies. Rejuv. Res. 10, 215–223 (doi:10.1089/rej.2006.0516) [DOI] [PubMed] [Google Scholar]
- 72.Johnson NA. 2010. Hybrid incompatibility genes: remnants of a genomic battlefield? Trends Genet. 26, 317–325 (doi:10.1016/j.tig.2010.04.005) [DOI] [PubMed] [Google Scholar]
- 73.Saether SA, et al. 2007. Sex chromosome-linked species recognition and evolution of reproductive isolation in flycatchers. Science 318, 95–97 (doi:10.1126/science.1141506) [DOI] [PubMed] [Google Scholar]
- 74.Backstrom N, Vali U. 2011. Sex- and species-biased gene flow in a spotted eagle hybrid zone. BMC Evol. Biol. 11, 9 (doi:10.1186/1471-2148-11-100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carling MD, Brumfield RT. 2008. Haldane's rule in an avian system: using cline theory and divergence population genetics to test for differential introgression of mitochondrial, autosomal, and sex-linked loci across the Passerina bunting hybrid zone. Evolution 62, 2600–2615 (doi:10.1111/j.1558-5646.2008.00477.x) [DOI] [PubMed] [Google Scholar]
- 76.Coyne JA. 1985. The genetic basis of Haldane's rule. Nature 314, 736–738 (doi:10.1038/314736a0) [DOI] [PubMed] [Google Scholar]
- 77.Wu CI, Johnson NA, Palopoli MF. 1996. Haldane's rule and its legacy: why are there so many sterile males? Trends Ecol. Evol. 11, 281–284 (doi:10.1016/0169-5347(96)10033-1) [DOI] [PubMed] [Google Scholar]
- 78.Borgia G. 1979. Sexual selection and the evolution of mating systems. In Sexual selection and reproductive competition in insects (eds Blum MS, Blum NA.), pp. 19–80 New York, NY: Academic Press [Google Scholar]
- 79.Taylor PD, Williams GC. 1982. The lek paradox is not resolved. Theor. Popul. Biol. 22, 392–409 (doi:10.1016/0040-5809(82)90052-1) [Google Scholar]
- 80.Charlesworth B. 1988. The evolution of mate choice in a fluctuating environment. J. Theor. Biol. 130, 191–204 (doi:10.1016/S0022-5193(88)80094-8) [DOI] [PubMed] [Google Scholar]
- 81.Charlesworth B. 1987. The heritability of fitness. In Sexual selection: testing the alternatives (eds Bradbury JW, Andersson MB.), pp. 21–40 Chichester, UK: Wiley [Google Scholar]
- 82.Maynard Smith J. 1978. The evolution of sex. Cambridge, UK: Cambridge University Press [Google Scholar]
- 83.Moller AP, Cuervo JJ. 2003. Sexual selection, germline mutation rate and sperm competition. BMC Evol. Biol. 3, 11 (doi:10.1186/1471-2148-3-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petrie M, Roberts G. 2007. Sexual selection and the evolution of evolvability. Heredity 98, 198–205 (doi:10.1038/sj.hdy.6800921) [DOI] [PubMed] [Google Scholar]
- 85.Rice WR. 1988. Heritable variation in fitness as a prerequisite for adaptive female choice: the effect of mutation-selection balance. Evolution 42, 817–820 (doi:10.2307/2408873) [DOI] [PubMed] [Google Scholar]
- 86.Williams GC. 1966. Adaptation and natural selection: a critique of some current evolutionary thought, 307 p Princeton, NJ: Princeton University Press [Google Scholar]
- 87.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man, 1871–1971 (ed. Campbell B.), pp. 136–179 Chicago, IL: Aldine [Google Scholar]