Abstract
Foraging skills of young individuals are assumed to be inferior to those of adults. The reduced efficiency of naive individuals may be the primary cause of the high juvenile mortality and explain the deferment of maturity in long-lived species. However, the study of juvenile and immature foraging behaviour has been limited so far. We used satellite telemetry to compare the foraging movements of juveniles, immatures and breeding adult wandering albatrosses Diomedea exulans, a species where foraging success is positively influenced by the distance covered daily. We showed that juveniles are able to use favourable winds as soon as the first month of independence, but cover shorter distances daily and spend more time sitting on water than adults during the first two months after fledging. These reduced movement capacities do not seem to be the cause of higher juvenile mortality. Moreover, juveniles almost never restrict their movement to specific areas, as adults and immatures frequently do over shelf edges or oceanic zones, which suggest that the location of appropriate areas is learned through experience. Immatures and adults have equivalent movement capacities, but when they are central place foragers, i.e. when adults breed or immatures come to the colony to display and pair, immatures make shorter trips than adults. The long duration of immaturity in this species seems to be related to a long period of learning to integrate the foraging constraints associated with reproduction and central place foraging. Our results indicate that foraging behaviour of young albatrosses is partly innate and partly learned progressively over immaturity. The first months of learning appear critical in terms of survival, whereas the long period of immaturity is necessary for young birds to attain the skills necessary for efficient breeding without fitness costs.
Keywords: albatross, learning, movement, immaturity, telemetry
1. Introduction
Explaining the mortality of juveniles and immature individuals is fundamental to the study of population demography and persistence of endangered populations, since the mortality of young individuals can be a limiting factor for the dynamics of age-structured populations [1]. Overall, the mortality of young individuals is much higher than that of adults in animal species (e.g. for fishes [2], for large herbivores [1], and for seabirds [3]). After independence, young naive individuals often disperse over long distances and are difficult to track and locate and can be more vulnerable than adults to some threats (for example, juveniles can be more susceptible to mortality in fisheries [4,5]). Therefore, improving our knowledge of the factors influencing young individuals’ mortality appears to be of major interest to life-history theory, population ecology and conservation biology in general. One of the most recognized hypotheses invoked to explain higher levels of juvenile and immature mortalities compared with adult mortalities was proposed by Lack, and then developed by Ashmole [6,7]. They suggested that young individuals, combining lack of experience and physical immaturity, had inferior foraging skills compared to adults. A period of learning of foraging skills would thus occur during the immaturity phase, possibly associated with an improvement in physical condition [7,8]. By ‘learning’, we here mean a change in behaviour resulting from experience [9]. In particular, learning of foraging techniques during immaturity has been studied in primates [10], bears [11], dolphins [12], seabirds [3,13,14], and insects (e.g. [15]). The study of learning behaviours during immaturity raises important questions about innate and learned behaviours, and the evolutionary [16], social [17] and memorization [9] processes involved. Learning processes can result from individual experience [3,14,15] or can also imply a transfer of information between conspecifics: this is generally considered as ‘social learning’ [10–12].
The occurrence of a learning period during the immaturity phase can have implications other than higher levels of mortality in young individuals: for many long-lived animals, the age at first reproduction is delayed well after the age at which individuals are physiologically mature [7,18]. ‘Deferred breeding’ has been observed in mammals and seabirds in particular [19–21], and Ashmole [7] suggested that the age at first breeding would depend on the rate of improvement of foraging behaviours during immaturity, and thus on the complexity of the foraging skills required for successful breeding. Thus, early breeding would be too costly to be advantageous in terms of fitness because individuals have to hone their foraging skills. MacLean [13] showed in three species of gulls that immature individuals foraged less efficiently than adults, and that age at first reproduction was related to performances during the immaturity phase.
Very few studies have focused on juvenile foraging behaviour (but see [3]) because of the difficulty of tracking young individuals for long periods. This is particularly true for pelagic species. Wandering albatrosses Diomedea exulans (L.) are large pelagic seabirds for which the foraging behaviour of adults is now well documented [22–24], but still little is known for individuals during their first years of independence [5]. It is a long-lived species [25] with an extended period of immaturity (mean duration: 11 years [26]). During the first year of independence, juveniles disperse alone over very long distances [5]. Since wandering albatrosses rely on widely dispersed resources whose distribution is mostly unpredictable in space and time (they mainly scavenge for dead squid floating on the surface [27,28]), the foraging success of individuals depends on a maximization of daily distances covered [22]. To do so, the foraging strategy of wandering albatrosses relies on very low flight costs, made possible by the dynamic soaring flight whereby individuals optimize the orientation of their movement with wind direction, birds strongly favouring tail and side winds [29].
In this study, we compare for the first time to our knowledge, the movements of a long-lived pelagic animal at the successive stages of its life by tracking with satellite transmitters naive juveniles (first year at sea after fledging and being independent of parents), immatures (age 3–10 years) and adult wandering albatrosses. By comparing the foraging behaviour of young, inexperienced individuals with that of mature, experienced adults, we addressed two main questions: (i) are there differences in movement performances between age classes, especially daily distance covered, and use of wind, and can we detect progressive change suggesting that the juvenile and immature phases involve learning? and (ii) do individuals of different age classes use similar habitats?
2. Material and methods
(a). Bird locations and environmental data
Field studies were carried out on Crozet (46° S, 52° E) and Kerguelen islands (49° S, 70° E). Between 2001 and 2010, 77 wandering albatrosses (23 juveniles fledging from the colony, 21 immatures aged 4–8 years, which have not yet been recruited, and 33 adults older than 7 years with at least one breeding attempt) were fitted with Argos satellite transmitters (Platform Terminal Transmitter, PTT 100, Microwave Telemetry, Columbia, USA) powered with a battery and working in continuous mode, or powered with solar panels and working with duty-cycles. Transmitters were fixed on back feathers using adhesive tape, and the mass of devices (18–52 g) represented less than 1% of the mass of the birds. Immature and adult individuals were sexed from plumage characteristics, breeding duties and morphometric measurements, and juveniles using a molecular sexing method [30]. Survival of juvenile individuals from their departure to their first return to the colony is known for Possession Island individuals, since the entire population is monitored annually [31], and 95% of juvenile wandering albatrosses return to their birth colony to breed [25]. Since juveniles from Crozet were equipped in 2001, they are likely to have returned to Crozet at least once if they are still alive, as immatures first come back to the colony on average at the age of six [32].
So that data can be comparable, data obtained from transmitters working in continuous mode were re-sampled to obtain the same cycle period as for duty-cycled transmitters, i.e. 10 h ON and 24 h OFF. To check whether the resampling interval could impact the results, we also performed analyses with a resampling cycle of 5 h ON and 24 h OFF, and found that it did not change the results (see the electronic supplementary material, table S2 for details). All Argos locations (classes A, B, 0, 1 to 3) were used, but unrealistic positions were filtered out by removing locations obtained at less than 10 min intervals, because the distance a bird could travel during this short time was that of the inherent error of the locations, and by removing those with an estimated speed above 90 km h−1, following a filtering procedure described in the study of McConnell et al. [33]. Locations were then related to local conditions (bathymetry, sea surface temperatures (SSTs), wind and position of sun, see the electronic supplementary material).
(b). Movement types
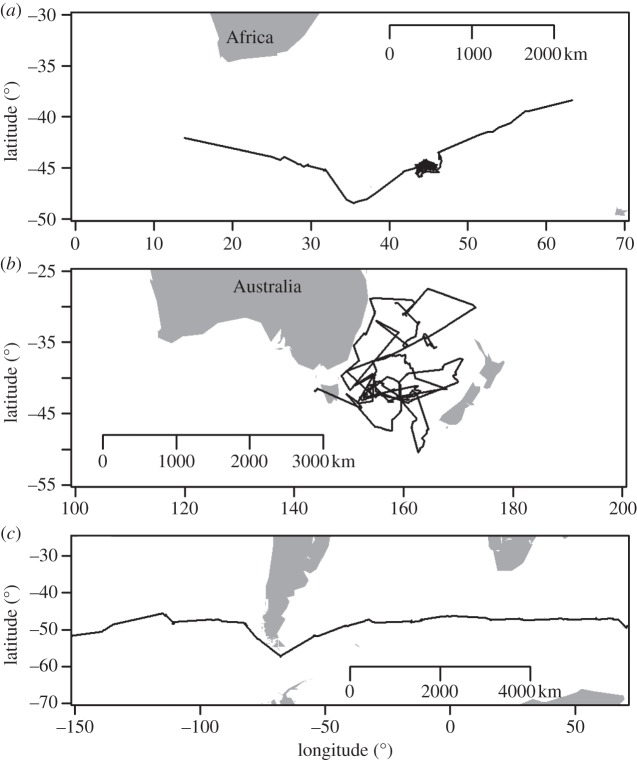
We consider two states: (i) when birds are central place foragers from colonies (adult breeding birds and immatures), and (ii) when birds (adults, juveniles and immatures) are not foraging from a central place. When they are breeding, wandering albatrosses are central place foragers: in January–February, during the incubation period, birds use typical long foraging trips, either looping or return trips [29]. Immature birds, when they return to land prior to recruitment in January–February, also use this central place foraging behaviour at sea between periods spent on land for display. This behaviour, here called ‘central place foraging’, is used during a few months in January–March. After this period, immature individuals disperse without returning to land, as do juveniles when they fledge and adults during their sabbatical years. At this time, birds engage in large-scale movements not connected to the breeding sites, and three types of behaviour can be distinguished. To be able to compare these movements between adults, immatures and juveniles, we had to separate for each individual the different types of behaviour, as was suggested by Turchin [34], and we used a specific rule of thumb to do so (e.g. figure 1). The ‘restricted foraging’ behaviour is a small-scale movement taking place over a restricted area, smaller than 100 km in diameter, performed for at least three successive days. During restricted foraging, the individual continuously changes flight direction, with angles between global directions separating consecutive days smaller than 90° (figure 1a). The ‘large-scale loop’ is a behaviour where individuals regularly change flight direction (as for ‘restricted foraging’ with change in direction being determined by an angle smaller than 90° separating the directions between three consecutive days), but birds operate over a zone larger than 100 km, which generally extends over several thousands of kilometres in diameter (figure 1b). Finally, the ‘transit’ behaviour is a typical large-scale and linear movement; we considered that the angle separating the direction of an individual between consecutive days should not be less than 90°, and this for at least three consecutive days (figure 1c).
Figure 1.
Examples of three movement types (black lines). (a) Track of an immature male between performing a ‘restricted foraging’ behaviour between two ‘transit’ phases. (b) Track of an immature male performing a ‘large-scale loops’ behaviour. (c) Track of a juvenile male performing a ‘transit’ behaviour.
(c). Movement description parameters
Using scripts developed with R v. 2.15.1 [35], several parameters were calculated at the individual level, for each month spent at sea since departure from the colony, and per movement type. In the analyses concerning movement description parameters, only the ‘large-scale loop’ behaviour was used, because this type of movement was used by all the stages of the life cycle, and by most individuals, whereas the other two behaviours were limited to some individuals. Daily distances covered (kilometres) were calculated as the total distance covered during the month while performing ‘large-scale loop’ behaviour, divided by the duration in days. To differentiate periods when the individual is flying or sitting on the water, a threshold of 18 km h−1 was used, considering higher speeds as flying birds [36]. Only periods when the transmitter was working continuously were included in the calculation of the percentage of time spent sitting on the water, using this speed criteria. All along the route, the angle between the flight track and wind direction was calculated, excluding locations where the individual was considered to be sitting on water. For ‘central place foraging’ movements, several standard parameters were calculated to be compared between adults and immatures; total duration of the trip to sea, total distance covered, maximum distance from the colony and mean daily distance covered. All statistical analyses were performed using R v. 2.15.1 [35], and in particular the ‘nlme’ package [37] and following procedures described in Zuur et al. [38]. See the electronic supplementary material for details.
3. Results
(a). Habitats and movement types of juveniles, immatures and adults outside the breeding season
Distributions of locations for bathymetry and SSTs encountered at sea were significantly different between all stages (figure 2; Kolmogorov–Smirnov tests, p < 0.0001, except for the comparison of bathymetries between immatures and adults: D = 0.049, p = 0.010). Juveniles preferentially used oceanic zones more than 2000 m deep and were restricted to sub-tropical waters, characterized by SSTs of 17°C on average. By contrast, adults were found over shelf edges, and sub-Antarctic as well as sub-tropical deep waters. Immature birds were intermediate, using more shelf edges and sub-Antarctic waters than juveniles (figure 2).
Figure 2.
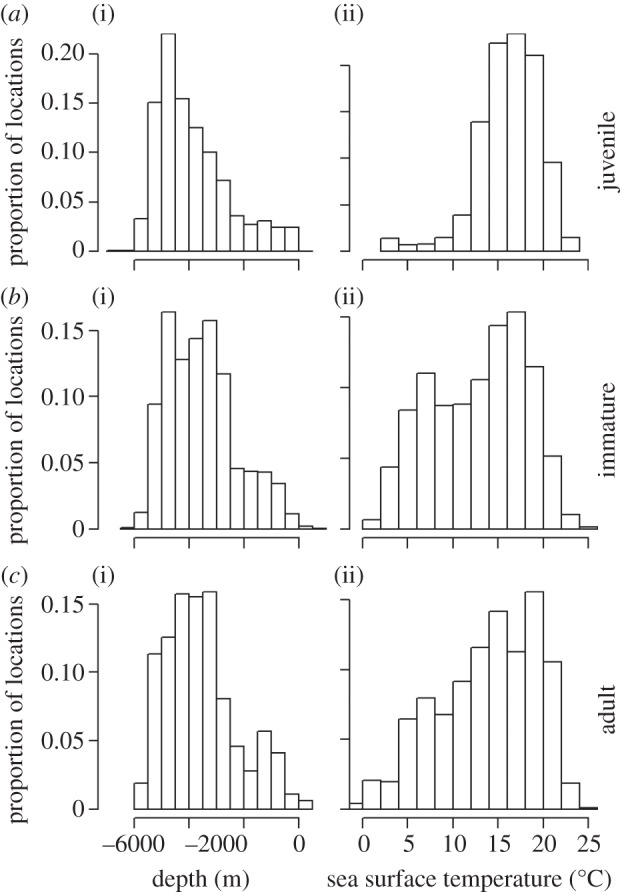
Distributions of (i) bathymetries and (ii) SSTs of locations for (a) juveniles (n = 20 individuals), (b) immatures (n = 13) and (c) adults (n = 7).
Juveniles almost never used the ‘restricted foraging’ behaviour; they spent 5% of their time using this type of behaviour (figure 3), but it was a more frequent behaviour for immatures and adults (31% and 24% of their time respectively): the effect of the stage on the proportion of time spent performing this type of behaviour was significant (Kruskal–Wallis test, H2 = 10.9, p = 0.0043). All stages spent the same proportion of time doing ‘transit’ behaviours (H2 = 2.6, p > 0.1). There was a significant effect of the stage on the proportion of time spent doing ‘large-scale loops’ (H2 = 12.8, p = 0.0016); juveniles spent more time doing this type of behaviour than adults and immatures (77%, 40%, 47% of their time, respectively).
Figure 3.
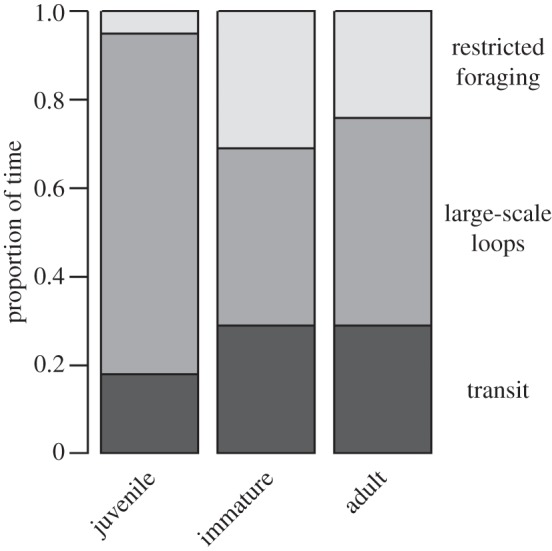
Mean proportions of time spent by individuals of each stage performing each type of movement behaviour, per month, during sabbatical phases (juveniles, n = 23; immatures, n = 19; adults, n = 8).
In cold waters (with SST less than 12°C), juveniles still undertook the least amount of restricted foraging (juveniles, 0%; immatures, 14%; adults, 22%), as in warm waters (juveniles, 5%; immatures, 14%; adults, 25%). It was also the case in deep waters (more than 2000 m deep; juveniles, 4%; immatures, 29%; adults, 16%) and in shallow waters (juveniles, 8%; immatures, 41%; adults, 31%). The effect of the stage on the proportion of time spent doing restricted foraging behaviour was significant in cold waters (Kruskal–Wallis test; H2 = 6.1, p = 0.047), in deep waters (H2 = 12.5, p = 0.0019) and in shallow waters (H2 = 7.1, p = 0.028), but not in warm waters (H2 = 0.4, p > 0.1).
(b). Movement description parameters
For these analyses, parameters were calculated per month spent since the departure from the colony, and only for ‘large-scale loops’ behaviours. Over the six first months spent at sea since the departure from the colony, juveniles significantly increased daily distances covered, from 169 ± 40 to 254 ± 68 km d−1 (mean ± s.d., likelihood ratio test on the ‘month’ effect: L1 = 26.12, p < 0.0001, see the electronic supplementary material, table S1, linear mixed model (LMM) 1), and decreased the proportion of time spent sitting on water, from 76 ± 21% to 49 ± 28% (mean ± s.d., L1 = 41.0, p < 0.0001; electronic supplementary material, table S1, LMM 2). Juvenile females covered greater distances per day and spent a smaller proportion of time sitting on water than juvenile males (mean ± s.d. daily distance covered by juvenile females: 235 ± 70 km d−1, for males: 200 ± 61 km d−1, likelihood ratio test on the ‘sex’ effect: L1 = 7.8, p = 0.0053; mean proportion of time spent on water by juvenile females: 58 ± 28%, by males: 69 ± 25%, L1 = 10.9, p = 0.0009; see the electronic supplementary material, table S1, LMM 1 and LMM 2, respectively). Juveniles from Crozet covered greater distances per day and spent less time on the water than juveniles from Kerguelen (mean daily distance covered by Crozet individuals: 240 ± 67 km d−1, by Kerguelen individuals: 191 ± 61 km d−1, LMM, likelihood ratio test on the ‘site’ effect: L1 = 9.1, p = 0.0026; mean proportion of time spent sitting on water by Crozet individuals: 58 ± 28%, by Kerguelen individuals: 70 ± 24%, L1 = 10.2, p = 0.0014; see the electronic supplementary material, table S1, LMM 1 and LMM 2 respectively).
For all months spent at sea, including the first month following the departure from the colony, juveniles used preferentially tail and side winds (Kolmogorov–Smirnov tests, comparison of monthly distributions with the uniform distribution between 0 and π, all p < 0.0001 except for the first month, D = 0.11, p = 0.014). There was a significant difference in the use of wind between the first and the second months (Kolmogorov–Smirnov test, D = 0.14, p = 0.014; figure 4), as juveniles seemed to increase the use of tail winds after the first month, but no significant change occurred after (Kolmogorov–Smirnov tests, all p > 0.1). This change in the orientation of juveniles regarding wind direction did not seem to be due to a change in the characteristics of wind field: a linear mixed model on the wind strengths encountered did not retain the ‘month’ effect (likelihood ratio test on the ‘month’ effect; L1 = 3.0, p > 0.1), and there was no difference in the distributions of wind orientations between the two first months spent at sea (Kolmogorov–Smirnov test, D = 0.11, p > 0.1).
Figure 4.
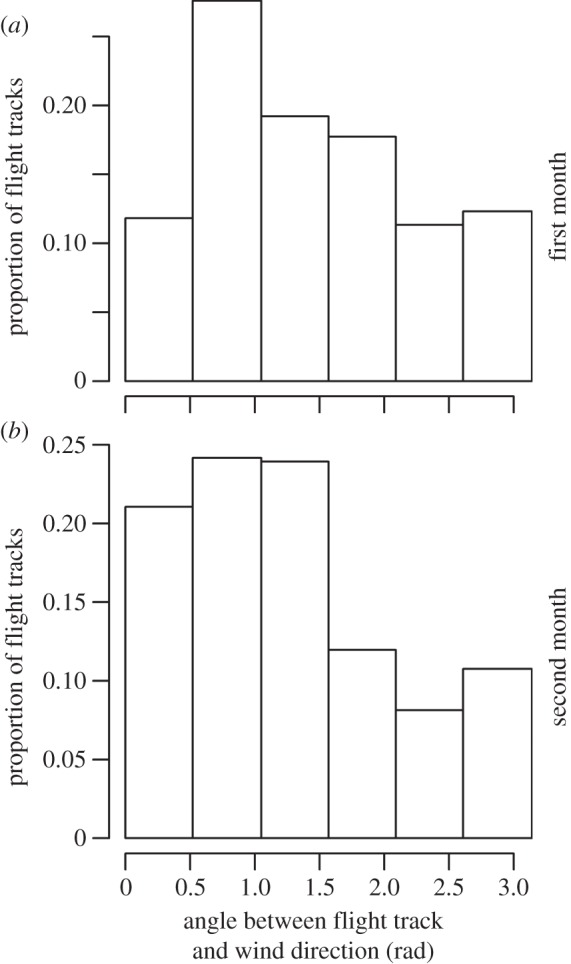
Proportions of flight tracks where juvenile albatrosses were flying with various wind orientations; from tail winds (left, 0 rad), to head winds (right, 3 rad), during ‘pelagic loops’ behaviour, during (a) the first (n = 15 individuals) and (b) the second (n = 20 individuals) months spent at sea.
When taking into account all three stages (juveniles, immatures and adults), there was a very significant interaction between the variables ‘month’ and ‘stage’, when explaining daily distances covered (likelihood ratio test on the interaction term ‘stage : month’; L2 = 52.8, p < 0.0001, see the electronic supplementary material, table S1, LMM 3) and proportions of time spent on water (L2 = 15.0, p = 0.0006), suggesting that the difference between juveniles on the one hand, and immatures and adults on the other hand, depended on the number of months spent at sea since departure from the colony (figure 5). Juveniles reached levels of distances covered daily and proportions of time spent on water similar to that of adults as quickly as the third month since fledging (figure 5). All stages taken together, males spent significantly more time sitting on water than females (mean proportion of time for females: 56 ± 29%, for males: 63 ± 26%; likelihood ratio test on the ‘sex’ effect; L1 = 6.4, p = 0.012; electronic supplementary material, table S1, LMM 4), but there was no significant effect of the sex on daily distance covered (mean daily distance covered by females: 273 ± 127 km, by males: 252 ± 116 km; L1 = 1.5, p > 0.1; electronic supplementary material, table S1, LMM 3). To check whether the interaction terms ‘stage : month’ were still significant if we controlled for latitudes, we performed additional LMM retaining only points north of 42°S, which showed that the interaction term ‘stage : month’ was still significant (for daily distance covered: L2 = 28.42, p < 0.0001, for proportion of time spent on water: L2 = 15.49, p = 0.0004).
Figure 5.
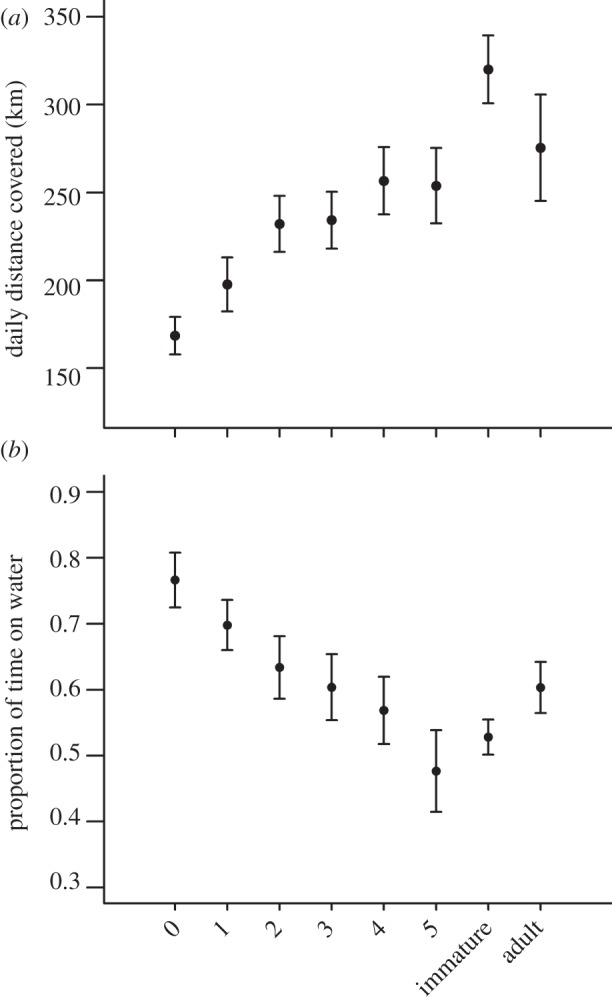
(a) Mean daily distances covered and (b) proportions of time spent on water during ‘pelagic loops’ behaviour, for every month since departure of the colony for juveniles, and all months taken together for immatures and adults. Error bars represent s.e.
Juveniles and immatures flew with similar wind orientation, but significantly differently than adults (Kolmogorov–Smirnov tests, juveniles versus immatures: D = 0.053, p > 0.1, juveniles versus adults: D = 0.18, p < 0.001, immatures versus adults: D = 0.16, p < 0.001). Juveniles and immatures favoured tail and side winds, whereas adults favoured mostly side winds (figure 6). However, when controlling for latitudes (performing the comparisons for locations with latitudes north of 42°S), there was no difference in wind orientations between the different stages (Kolmogorov–Smirnov tests, all p > 0.1).
Figure 6.
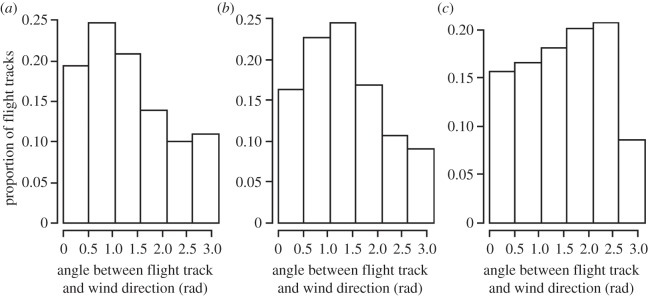
Proportions of flight tracks where (a) juvenile (n = 15 individuals), (b) immature (n = 11 individuals) and (c) adult (n = 5 individuals) albatrosses were flying with various wind orientations; from tail winds to head winds, during ‘pelagic loops’ behaviour.
(c). Central place foraging loops for adults and immatures
There were significant differences between immatures and adults during central place foraging trips. Adults made longer trips, covered longer total distances and had a tendency to reach greater distances from the colony (table 1). However, there was no significant difference between immatures and adults in daily distances covered (table 1).
Table 1.
Wilcoxon rank-sum tests on the ‘stage’ (immatures versus adults) effect for description parameters of central place foraging loops, mean values for immatures and adults (only one loop was considered per individual).
| mean parameter for immatures | mean parameter for adults | Wilcoxon test | |
|---|---|---|---|
| trip duration | 9 ± 13 days | 11 ± 5 days | W = 112, p = 0.0059 |
| total distance covered | 3287 ± 5298 km | 4022 ± 2977 km | W = 139, p = 0.039 |
| maximum distance from the colony | 838 ± 1220 km | 1178 ± 911 km | W = 145, p = 0.060 |
| mean daily distance covered | 257 ± 173 km | 321 ± 121 km | W = 168, p > 0.1 |
(d). Juvenile survival and movement description parameters
Out of the 13 juveniles equipped on Crozet, 10 survived until their first return on land and their transmitters worked for 110.35–360.05 days, three did not return and their transmitters stopped after 61.02–83.53 days, suggesting that the birds died during the first three months at sea. Considering large-scale looping movements used by all birds, no significant difference was detected between juveniles surviving or for daily distances covered (surviving, 246 ± 68 km d−1; dying, 203 ± 47 km d−1; on the first six months at sea: likelihood ratio test on the ‘survival’ effect: L1 = 0.00006, p = 0.099) and proportion of time spent sitting on water (surviving, 56 ± 28%; or not, 71 ± 26%; on the first six months at sea: likelihood ratio test on the ‘survival’ effect; L1 = 0.0066, p > 0.1). There was no significant difference in the use of wind between juveniles surviving or not (comparison of the distributions of angles between flight track and wind direction, Kolmogorov–Smirnov test, D = 0.063, p > 0.1).
4. Discussion
(a). The juvenile phase
This study is one of the first to compare foraging movements of a marine predator during the different stages of its life cycle. We showed that during the first two months spent at sea, juvenile individuals cover shorter daily distances, and spend a greater proportion of their time sitting on water, than later and older individuals. However, they reach values similar to those of adults as early as the third month since independence, suggesting a progressive improvement of movement performances during the first two months since fledging. Given the strong relationship between daily covered distances and foraging success in this species [22,27,28], juvenile individuals are therefore very likely to have inferior foraging skills to adults during their first two months spent at sea. These inferior movement performances could be explained by a lack of experience of optimal behaviours and/or physical immaturity. This result is consistent with a study by Yoda et al. [14], which showed that juvenile brown boobies improved their flight abilities during their first month after fledging. Similarly, juvenile European shags compensate for poor foraging success by foraging for a higher proportion of the day in the four first months after independence, compared with adults [3].
However, immediately after fledging, juvenile wandering albatrosses favour tail and side winds, i.e. they are able to orientate themselves regarding wind direction similar to adults, in a way that minimizes the instantaneous energy expenditure [29], even if there seems to be a slight difference between the first month spent at sea and the following months. This is an important result, since wandering albatrosses rely on this orientation strategy to minimize the energy cost of flight [29]. Therefore, the preferred orientation of movement regarding wind direction does not seem to be the result of a long learning process but is rather an innate component of the flight behaviour of albatrosses.
The main difference between juvenile and adult wandering albatrosses in their movement abilities is not in their use of wind, but in the higher proportions of time spent sitting on water. Juveniles progressively reduce the time spent on water and increase the time spent in flight, thus searching for food. The reason for this increase is not clear, but may be related to a progressive acquisition of flight competence or to the increasing necessity of birds to find food, since when they fledge, they have a certain amount of fat reserves that can sustain them for a few weeks [39].
A second major difference between age classes, and especially between naive juvenile birds and older birds, is in the habitats foraged and the proportions of time spent performing different types of behaviour. Juvenile individuals almost never do ‘restricted foraging’ behaviours, whereas it is a frequent behaviour for immatures and adults. This is still true when we control for the temperature conditions, when we consider behaviour only in warm waters or cold waters. This could suggest that the localization of specific zones, favourable to this kind of behaviour, is learned by experience. Indeed, adults perform ‘restricted foraging’ mainly over shelf edges and oceanic mounts, which are characterized by higher levels of productivity and predictability than oceanic waters [28]. This was confirmed by our data: 77% of the time spent carrying out ‘restricted foraging’ behaviours by adults occurred over waters less than 2000 m deep, and juveniles spent less than 10% of their time over shallow waters, compared with 17% for immatures and 25% for adults. Moreover, Weimerskirch et al. [23] showed that this type of behaviour is probably triggered in adults by the recognition of favourable foraging areas rather than by a prey finding event, which suggests that individual experience and environment knowledge have to be gained to be efficient. Our data support this hypothesis: when juvenile individuals performed ‘restricted foraging’ behaviours, only for 38% of time was it over favourable areas, i.e. over waters less than 2000 m deep. Moreover, juveniles spent less time over shallow waters than adults and immatures. This accords with the idea that young individuals have to learn to recognize areas of high predictability to perform this kind of behaviour.
Juvenile individuals are mainly found in warmer areas than adults [5,26], in sub-tropical zones, less windy, therefore less favourable to flight [29,40], and less productive [41], suggesting the use of separate areas by different age classes selected to reduce intraspecific competition [5]. Resource partitioning in marine vertebrates between age classes has been shown for emperor penguins [42] and southern elephant seals [43]. This phenomenon, also referred to as ‘ontogenetic niche shift’, plays an important role in defining the total niche width for some species [44]. One important factor is that when they leave the colony, juvenile wandering albatrosses are heavier than adults [39]. This higher mass is generally considered an advantage since it represents energy stores for the first months of life. However, it might also reduce the flight capabilities of birds by increasing wing loading. Juvenile wandering albatrosses are not likely to experience this disadvantage, because it is compensated for by longer wings than adults [39]. As a result, juveniles experience lower wing loading than adults, and this could be an adaptation to flight in sub-tropical zones, much less windy than sub-Antarctic zones favoured by adults [39,40].
Those juvenile birds that did not survive until recruitment died during the first three months spent at sea. Surprisingly, we detected no statistically significant difference in movement performances between juvenile individuals surviving and those dying. This suggests that the increase in daily distances covered and the decrease in the proportion of time spent sitting on water over the first three months spent at sea are due to individual improvement, and not to the death of least efficient individuals. Juvenile mortality could be explained by other factors, such as body condition at fledging [39], but our limited present data do not support this hypothesis (H. Weimerskirch 2013, unpublished data). Moreover, we acknowledge that our results do not directly measure foraging success (by measuring daily food intake, for example), but use movement performance as an indicator. Other factors, such as ability to detect prey in flight, since a large majority of the prey caught is detected when in flight by wandering albatrosses individuals [23], and different prey handling, could have implications on foraging success, explain juvenile mortality and involve longer learning periods than what is apparently necessary to acquire efficient movement foraging skills. For example, MacLean [13] showed that immature gulls are less efficient than adults at prey detection and prey capture.
(b). The immature phase
Whereas we detected several differences in the foraging zones and movements between juveniles and older birds, the differences between immature birds and mature adults were more subtle.
No statistically significant difference in movement performances, as measured by daily distances covered and proportion of time spent sitting on water during ‘large-looping’ behaviours, was detected between immatures and adults. This result raises the question of whether the long period of immaturity in this species is a period of learning, as generally considered [7,20]. In the waters surrounding the colony, high levels of competition are likely to occur, and the level of dominance between adults and young individuals could play an important role. Indeed, in a situation of direct competition, immature individuals may be rejected by adults, and the level of dominance has been shown to impact on deferred breeding for other species [21]. Moreover, we showed that in a situation of central place foraging, immature individuals make shorter trips, cover smaller total distances and tend to attain shorter maximum distances from the colony than adults. This could be explained by the fact that immature individuals do not yet have to deal with the constraints of reproduction. Indeed, reproductive adults have to acquire and store sufficient energy reserves to stay on the nest without food supply for long periods, when their partner is at sea [45]. Therefore, even if immature individuals have similar movement performances to adults’, the fact that they return to the colony and perform incubation-type loops expose them to higher competition with congeners than far from colonies. Thus, immatures might have to acquire sufficient experience of the specific constraints of central place foraging before their first reproductive attempt [20]. In particular, they have also to acquire resources not only for themselves, but for reproduction, i.e. to provision the offspring and store reserves for incubation fast.
5. Conclusion
Our results indicate that learning processes occur during the immaturity phase; juvenile individuals reach adults' movement performances within the first three months of independence, and almost never perform ‘restricted foraging’ behaviour, which is a frequent behaviour for immatures and adults performed over specific areas whose localization seems to be learned by experience. Inferior movement performances and the non-occurrence of ‘restricted foraging’ behaviour suggest inferior foraging skills of juvenile individuals. However, lower levels of movement performances do not explain higher levels of juvenile mortality. Similarly, movement performances alone do not explain the duration of the immaturity period in this species, but the acquisition of experience for central place foraging, specific to the reproductive phase, is likely to be a major factor, as well as the learning of the marine environment around the breeding grounds. However, some aspects of albatrosses' behaviour seem to be innate, as during the initial dispersal phase (which we did not study here), juveniles followed inherited preferred routes to the north [5]. Moreover, it is worth noting that a crucial aspect of wandering albatrosses’ movement behaviour, the orientation towards wind [29], seems to be at least in part innate, as juvenile individuals master it as soon as their first month of independence, without parental teaching phase and without post-fledging social learning, as juveniles leave the colony alone. Since wandering albatrosses are probably using olfaction for prey detection [46], it would also be interesting to examine with fine-scale studies of movements whether they use this sense during the early phase of their life. Further studies should compare these results with other species, especially other seabirds, to better understand the evolution of innate and learned parts of foraging behaviour and its ontogeny. These questions are particularly pertinent to be able to explain how late breeding, in terms of lifetime benefit, outweigh the fitness costs.
Acknowledgements
We thank all the people involved in the deployment of tags at the Crozet and Kerguelen Islands, and in the long-term monitoring program. We thank Karine Delord for the database management, David Pinaud for advices in analyses and Cédric Cotté for wind data extraction. We thank two anonymous referees for their constructive comments on an early version of this manuscript.
The field studies were approved by the Ethic Committee of IPEV, and by the Comité de l'Environnement Polaire.
Funding statement
This study is part of the program EARLYLIFE funded by an Advanced Grant from European Research Council (ERC-2012-ADG_20120314) to H.W. This study received logistical and financial support from Institut Polaire Paul Emile Victor (IPEV, program no. 109 to H.W.). It was also funded by Fondation Prince Albert 2 de Monaco, and some of the loggers were kindly provided by Susanne Åkesson.
References
- 1.Gaillard JM, Festa-Bianchet M, Yoccoz NG. 1998. Population dynamics of large herbivores: variable recruitment with constant adult survival. Trends Ecol. Evol. 13, 58–63 (doi:10.1016/S0169-5347(97)01237-8) [DOI] [PubMed] [Google Scholar]
- 2.Victor BC. 1986. Larval settlement and juvenile mortality in a recruitment-limited coral reef fish population. Ecol. Monogr. 56, 145–160 (doi:10.2307/1942506) [Google Scholar]
- 3.Daunt F, Afanasyev V, Adam A, Croxall JP, Wanless S. 2007. From cradle to early grave: juvenile mortality in European shags Phalacrocorax aristotelis results from inadequate development of foraging proficiency. Biol. Lett. 3, 371–374 (doi:10.1098/rsbl.2007.0157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson JW, Epperly SP, Shah AK, Foster DG. 2005. Fishing methods to reduce sea turtle mortality associated with pelagic longlines. Can. J. Fish. Aquat. Sci. 62, 965–981 (doi:10.1139/f05-004) [Google Scholar]
- 5.Weimerskirch H, Åkesson S, Pinaud D. 2006. Postnatal dispersal of wandering albatrosses Diomedea exulans: implications for the conservation of the species. J. Avian Biol. 37, 23–28 [Google Scholar]
- 6.Lack D. 1954. The natural regulation of animal numbers. Oxford, UK: Clarendon [Google Scholar]
- 7.Ashmole NP. 1963. The regulation of numbers of tropical oceanic birds. Ibis 103, 458–473 [Google Scholar]
- 8.Clutton-Brock TH, Russell AF, Sharpe LL, Brotherton PNM, McIlrath GM, White S, Cameron EZ. 2001. Effects of helpers on juvenile development and survival in meerkats. Science 293, 2446–2449 (doi:10.1126/science.1061274) [DOI] [PubMed] [Google Scholar]
- 9.Dukas R. 1998. Evolutionary ecology of learning. In Cognitive ecology: the evolutionary ecology of information processing and decision making (ed. Dukas R.), pp. 129–174 Chicago, IL: University of Chicago Press [Google Scholar]
- 10.Lefebvre L. 1995. Culturally-transmitted feeding behaviour in primates: evidence for accelerating learning rates. Primates 36, 227–239 (doi:10.1007/BF02381348) [Google Scholar]
- 11.Mazur R, Seher V. 2008. Socially learned foraging behaviour in wild black bears, Ursus americanus. Anim. Behav. 75, 1503–1508 (doi:10.1016/j.anbehav.2007.10.027) [Google Scholar]
- 12.Mann J, Sargeant B. 2003. Like mother, like calf: the ontogeny of foraging traditions in wild Indian Ocean bottlenose dolphins (Tursiops sp.). In The biology of traditions: models and evidence (eds Fragaszy DM, Perry S.), pp. 236–266 Cambridge, UK: Cambridge University Press [Google Scholar]
- 13.MacLean AA. 1986. Age-specific foraging ability and the evolution of deferred breeding in three species of gulls. Wilson Bull. 98, 267–279 [Google Scholar]
- 14.Yoda K, Kohno H, Naito Y. 2004. Development of flight performance in the brown booby. Proc. R. Soc. Lond. B 271, S240–S242 (doi:10.1098/rsbl.2003.0157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boivin G, Roger C, Coderre D, Wajnberg E. 2010. Learning affects prey selection in larvae of a generalist coccinellid predator. Entomol. Exp. Appl. 135, 48–55 (doi:10.1111/j.1570-7458.2009.00964.x) [Google Scholar]
- 16.Clobert J, Danchin E, Dhondt A, Nichols J. 2001. Dispersal. Oxford, UK: Oxford University Press [Google Scholar]
- 17.Galef B, Jr, Giraldeau L. 2001. Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim. Behav. 61, 3–15 (doi:10.1006/anbe.2000.1557) [DOI] [PubMed] [Google Scholar]
- 18.Tavecchia G, Pradel R, Boy V, Johnson AR, Cézilly F. 2001. Sex-and age-related variation in survival and cost of first reproduction in greater flamingos. Ecology 82, 165–174 (doi:10.1890/0012-9658(2001)082[0165:SAARVI]2.0.CO;2) [Google Scholar]
- 19.Fisher HI. 1975. The relationship between deferred breeding and mortality in the Laysan albatross. Auk 92, 433–441 (doi:10.2307/4084599) [Google Scholar]
- 20.Forslund P, Pärt T. 1995. Age and reproduction in birds: hypotheses and tests. Trends Ecol. Evol. 10, 374–378 (doi:10.1016/S0169-5347(00)89141-7) [DOI] [PubMed] [Google Scholar]
- 21.Komers PE, Pélabon C, Stenström D. 1997. Age at first reproduction in male fallow deer: age-specific versus dominance-specific behaviors. Behav. Ecol. 8, 456–462 (doi:10.1093/beheco/8.4.456) [Google Scholar]
- 22.Salamolard M, Weimerskirch H. 1993. Relationship between foraging effort and energy requirement throughout the breeding season in the wandering albatross. Funct. Ecol. 7, 643–652 (doi:10.2307/2390184) [Google Scholar]
- 23.Weimerskirch H, Pinaud D, Pawlowski F, Bost CA. 2007. Does prey capture induce area-restricted search? A fine-scale study using GPS in a marine predator, the wandering albatross. Am. Nat. 170, 734–743 (doi:10.1086/522059) [DOI] [PubMed] [Google Scholar]
- 24.Weimerskirch H, Louzao M, De Grissac S, Delord K. 2012. Changes in wind pattern alter albatross distribution and life-history traits. Science 335, 211–214 (doi:10.1126/science.1210270) [DOI] [PubMed] [Google Scholar]
- 25.Inchausti P, Weimerskirch H. 2002. Dispersal and metapopulation dynamics of an oceanic seabird, the wandering albatross, and its consequences for its response to long-line fisheries. J. Anim. Ecol. 71, 765–770 (doi:10.1046/j.1365-2656.2002.00638.x) [Google Scholar]
- 26.Weimerskirch H, Jouventin P. 1987. Population dynamics of the wandering albatross, Diomedea exulans, of the Crozet Islands: causes and consequences of the population decline. Oikos 49, 315–322 (doi:10.2307/3565767) [Google Scholar]
- 27.Weimerskirch H, Gault A, Cherel Y. 2005. Prey distribution and patchiness: factors in foraging success and efficiency of wandering albatrosses. Ecology 86, 2611–2622 (doi:10.1890/04-1866) [Google Scholar]
- 28.Weimerskirch H. 2007. Are seabirds foraging for unpredictable resources? Deep Sea. Res. Part 2 Top. Stud. Oceanogr. 54, 211–223 (doi:10.1016/j.dsr2.2006.11.013) [Google Scholar]
- 29.Weimerskirch H, Guionnet T, Martin J, Shaffer SA, Costa DP. 2000. Fast and fuel efficient? Optimal use of wind by flying albatrosses. Proc. R. Soc. Lond. B 267, 1869–1874 (doi:10.1098/rspb.2000.1223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weimerskirch H, Lallemand J, Martin J. 2005. Population sex ratio variation in a monogamous long-lived bird, the wandering albatross. J. Anim. Ecol. 74, 285–291 (doi:10.1111/j.1365-2656.2005.00922.x) [Google Scholar]
- 31.Weimerskirch H, Brothers N, Jouventin P. 1997. Population dynamics of wandering albatross Diomedea exulans and Amsterdam albatross D. amsterdamensis in the Indian Ocean and their relationships with long-line fisheries: conservation implications. Biol. Conserv. 79, 257–270 (doi:10.1016/S0006-3207(96)00084-5) [Google Scholar]
- 32.Weimerskirch H. 1992. Reproductive effort in long-lived birds: age-specific patterns of condition, reproduction and survival in the wandering albatross. Oikos 64, 464–473 (doi:10.2307/3545162) [Google Scholar]
- 33.McConnell BJ, Chambers C, Fedak MA. 1992. Foraging ecology of southern elephant seals in relation to the bathymetry and productivity of the Southern Ocean. Antarct. Sci. 4, 393–398 (doi:10.1017/S0954102092000580) [Google Scholar]
- 34.Turchin P. 1998. Quantitative analysis of movement: measuring and modeling population redistribution in animals and plants. Sunderland, MA: Sinauer Associates [Google Scholar]
- 35.R Development Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 36.Weimerskirch H, Bonadonna F, Bailleul F, Mabille G, Dell'Omo G, Lipp HP. 2002. GPS tracking of foraging albatrosses. Science 295, 1259 (doi:10.1126/science.1068034) [DOI] [PubMed] [Google Scholar]
- 37.Pinheiro J, Bates D, DebRoy S, Sarkar D, The R Development Core Team 2013. nlme: linear and nonlinear mixed effects models. R package version 3.1–107. See: http://www.cran.r-project.org/web/packages/nlme/nlme.pdf
- 38.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. Berlin, Germany: Springer [Google Scholar]
- 39.Weimerskirch H, Barbraud C, Lys P. 2000. Sex differences in parental investment and chick growth in wandering albatrosses: fitness consequences. Ecology 81, 309–318 (doi:10.1890/0012-9658(2000)081[0309:SDIPIA]2.0.CO;2) [Google Scholar]
- 40.Shaffer SA, Weimerskirch H, Costa DP. 2001. Functional significance of sexual dimorphism in wandering albatrosses, Diomedea exulans. Funct. Ecol. 15, 203–210 (doi:10.1046/j.1365-2435.2001.00514.x) [Google Scholar]
- 41.Moore JK, Abbott MR. 2000. Phytoplankton chlorophyll distributions and primary production in the Southern Ocean. J. Geophys. Res. 105, 28 709–28 722 (doi:10.1029/1999JC000043) [Google Scholar]
- 42.Kooyman GL, Kooyman TG, Horning M, Kooyman CA. 1996. Penguin dispersal after fledging. Nature 383, 397 (doi:10.1038/383397a0) [Google Scholar]
- 43.Field IC, Bradshaw CJ, Burton HR, Sumner MD, Hindell MA. 2005. Resource partitioning through oceanic segregation of foraging juvenile southern elephant seals (Mirounga leonina). Oecologia 142, 127–135 (doi:10.1007/s00442-004-1704-2) [DOI] [PubMed] [Google Scholar]
- 44.Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML. 2003. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28 (doi:10.1086/343878) [DOI] [PubMed] [Google Scholar]
- 45.Weimerskirch H. 1995. Regulation of foraging trips and incubation routine in male and female wandering albatrosses. Oecologia 102, 37–43 [DOI] [PubMed] [Google Scholar]
- 46.Nevitt GA, Losekoot M, Weimerskirch H. 2008. Evidence for olfactory search in wandering albatross, Diomedea exulans. Proc. Natl Acad. Sci. USA 105, 4576–4581 (doi:10.1073/pnas.0709047105) [DOI] [PMC free article] [PubMed] [Google Scholar]