Abstract
Continued range expansion into physiologically challenging environments requires invasive species to maintain adaptive phenotypic performance. The adrenocortical stress response, governed in part by glucocorticoid hormones, influences physiological and behavioural responses of vertebrates to environmental stressors. However, any adaptive role of this response in invasive populations that are expanding into extreme environments is currently unclear. We experimentally manipulated the adrenocortical stress response of invasive cane toads (Rhinella marina) to investigate its effect on phenotypic performance and fitness at the species' range front in the Tanami Desert, Australia. Here, toads are vulnerable to overheating and dehydration during the annual hot–dry season and display elevated plasma corticosterone levels indicative of severe environmental stress. By comparing unmanipulated control toads with toads whose adrenocortical stress response was manipulated to increase acute physiological stress responsiveness, we found that control toads had significantly reduced daily evaporative water loss and higher survival relative to the experimental animals. The adrenocortical stress response hence appears essential in facilitating complex phenotypic performance and setting fitness trajectories of individuals from invasive species during range expansion.
Keywords: extreme environments, survival, glucocorticoid hormones, acute stress response, adaptive phenotypic performance, fitness
1. Introduction
Rapid range expansion by invasive species indicates these species' striking capacity for ongoing adaptation to novel environments [1,2]. At the level of the individual, rapid evolution and phenotypic plasticity are key mechanisms that enable invasive species to expand their ranges [2,3]. What makes invasive species' extremely impressive is their ability to produce integrated phenotypes to reconcile distinct, and often competing, pressures from multiple selective agents as they colonize novel environments [4]. For example, behaviours such as dispersal proclivity, boldness and aggression are increasingly recognized as key traits of successful invasive vertebrates [5–8]. Similarly, traits that govern the physiological tolerances to local biophysical conditions (e.g. temperature, rainfall) are instrumental in ensuring the survival of individual invaders, and by extrapolation, further range expansion by the species into novel environments [9–11].
Invasive species hence present excellent models for investigating how organisms reconcile diverse and rapid environmental changes. To understand how invasive species adapt to environmental change during range expansion, it is necessary to identify physiological mechanisms that both regulate and integrate multi-trait phenotypic performance and thus optimize the fitness of individuals [9,12–14]. The hypothalamic pituitary interrenal/adrenal (HPI/A) axis, via production of glucocorticoid (GC) hormones acting on hormone-specific receptors, produces general physiological and behavioural responses that augment phenotypic performance and fitness during exposure to stressors [12–18]. In particular, the rate and duration of physiological or behavioural responsiveness, triggered by an individual's acute adrenocortical stress response phenotype, is expected to have important fitness outcomes via producing adaptive or non-adaptive phenotypic performance under environmental selection [18,19]. However, a clear demonstration that the acute adrenocortical stress response can directly mediate adaptive phenotypic performance and higher fitness under strong natural selection in free-living populations is currently lacking [19–22]. Here, we make use of an invasive vertebrate, the cane toad (Rhinella marinus), to investigate the role of the acute adrenocortical stress response in determining phenotypic performance and fitness under extreme environmental selection [12,23].
Cane toads (R. marinus), a terrestrial amphibian native to Central and South America, were deliberately introduced into north eastern Australia in 1935 [24]. Since their release, toads have had exceptional invasion success and now occupy diverse environments ranging from tropical forests and savannahs to deserts [25]. Toad range expansion into the Australian desert has taken them well outside their native realized niche into a climatically extreme environment with prolonged periods of dry weather and extreme heat [26,27].
In hot–dry environments, toads evade potentially life threatening body temperatures and water loss by seeking refuges in the shade that are near or in the water [26–28]. However, even when using habitat shelters, toads in arid landscapes experience hot temperatures that cause significant thermal and desiccation stress [26,28]. Without access to refuges, toads rapidly overheat, dehydrate and die [26]. Because dehydration is such an important factor affecting the survival of individual toads, we would expect toads that have invaded arid areas to possess adaptations in physiological traits that influence water loss and underpin thermal and water homeostasis. Such adaptations could arise through phenotypic plasticity or natural selection [3]. Intuitively, the acute adrenocortical stress response (i.e. actions of GC hormones acting on receptors) could mediate holistic phenotypic function in toads exposed to thermal or hydric stressors by altering concurrent physiological processes [16–18]. These include vascular blood flow influencing evaporative cooling and water loss, glucose availability for activity and regulation of other hormones directly involved in water or thermal homeostasis [29,30].
Using mensurative and manipulative field experiments, we measured the interaction between environmental conditions and the effects of the acute adrenocortical stress response on phenotypic performance and survival in cane toads at their arid range front. By sampling toads in situ living under extreme conditions at the edge of their range during the hot–dry season, we first measured the relationships between environmental conditions, toad plasma corticosterone (a hormone-based marker of adrenocortical stress responsiveness) and hydric state to infer the degree of environmental stress experienced. Next, using experimental manipulations (i.e. phenotypic engineering [31,32]) of the acute adrenocortical stress response, we increased rates of corticosterone synthesis and GC-receptor activity to produce increased acute stress responsiveness in range front toads. We then determined whether these more responsive acute adrenocortical stress responses affected rates of evaporative water loss (i.e. organismal performance) and survival (i.e. fitness) relative to unmanipulated control toads following exposure to hot and dry environmental conditions in experimental shelters. If the acute adrenocortical stress response regulates organismal performance in a way that produces locally adapted populations, we predicted that unmanipulated control toads should have better survival than more responsive acute adrenocortical stress phenotypes under the extreme environmental conditions at our hot and arid study site.
2. Material and methods
(a). Study sites
Research was conducted at Camfield (17°04′ S, 131°39′ E) and Dungowan Stations (17°17′ S, 131°43′ E) on the northern edge of the Tanami Desert (17°17′ S, 131°43′ E) in Australia's Northern Territory during late September (seasonally hot and dry) in 2010 and 2011 (figure 1a,b). This semi-arid region experiences a bi-phasic wet–dry season with most rainfall occurring from December to April. Other months have low rainfall and low humidity, with average monthly humidity lowest in September (20%). The mean annual rainfall at the nearest weather station to the study site (Wave Hill) is 580 mm. Monthly air temperatures peak during the mid-late dry season (September–November). Maximal mean monthly shade air temperatures for September, October and November are 35.1°C (30.8–38.5; 90% CI), 37.9°C (34.3–40.7; 90% CI) and 38.6°C (35.1–41.9; 90% CI), respectively (sourced from Australian Bureau of Meteorology). Importantly, this area experiences on average 39 days per year in which shade temperatures exceed 40°C. These are potentially lethal temperatures for toads in the absence of shelters that buffer them from thermal exposure [33]. Cane toads first invaded the northern part of the study area in 2007–2008 and are expanding their range westward and southwards into increasingly arid regions [26]. During the dry season, toad populations are concentrated around natural and man-made (e.g. dams used for cattle ranching) permanent water sources [26].
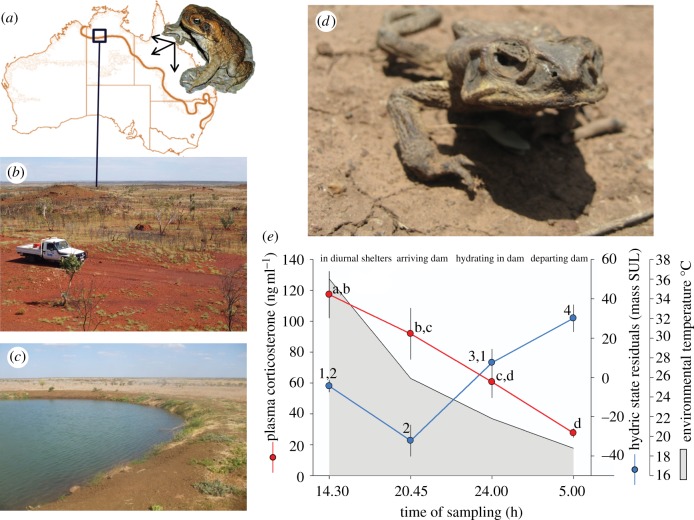
Figure 1.
(a) Cane toads were introduced into north eastern Australia in 1935 and promptly underwent range expansion (arrows). Their current range front is depicted by the wavy boundary line. (b) Part of the current range front extends into the northern Tanami desert in Australia's Northern Territory where this study was conducted. (c) Here, during the dry season, toads aggregate around permanent natural and artificial water sources (e.g. dams) for survival. (d) The hot–dry season exposes the toad to strong selection from thermal and desiccation stress, and at this study site, it is possible to encounter toads that have died in nocturnal transit from their diurnal shelters to water sources. (e) During the hot–dry season toads experienced significant daily variation in their plasma corticosterone levels (red line) and hydric state (blue line, mass/snout-urostyle length (SUL)), both responses to hot ambient environmental temperatures. Each point represents the mean value and standard error of 10 individuals sampled at each time period. Post hoc differences are indicated by letters and numerals associated with sampling periods. Captions above graph depict the activities of toads at each sampling period.
(b). Assessment of environmental stress on toads
To evaluate the physiological effects of seasonally hot and dry weather (i.e. environmental stress) on toads, we measured their hydric state and collected blood samples at four time periods (14.30, 20.45, 24.00 and 05.00 h) in late September 2010. These times were chosen to coincide with common time-appropriate daily behaviours. At 14.30, toads were sampled while inactive in below-ground refugia (soil cracks) used to reduce overheating and desiccation during the day. We captured toads by hand after locating them in soil cracks. As shelters differ in microhabitat quality and hence environmental buffering, we standardized our sampling to sample toads from similar quality shelters (i.e. flat-ground, without shrub cover and orientated perpendicular to the sun's trajectory and that extended to at least 60 cm below-ground). We then selected toads that were positioned 30–40 cm below ground to further standardize effects of shelter environment on physiological metrics. Being nocturnal, toads emerged from shelters at night (after 19.00) and moved to artificial water sources (dams) to rehydrate or commence foraging activities. At 20.45, we sampled toads as they arrived at the water's edge to rehydrate. At 24.00, we sampled toads that were partially immersed in water rehydrating. At 05.00, we sampled toads as they departed from water to return to refugia at dawn.
To obtain physiological measures of environmental stress, we first euthanized toads using rapid cervical dislocation to ensure toads did not urinate during handling and confound measures of hydric state. Body mass and body length were measured to estimate each individual's hydric state using the residual scores derived from a log mass against log length linear regression model. Because toads can store more than 50% of their mass as water in their bladder, evaporative water loss leads to large changes in daily body mass and is highly indicative of toad hydric state and hence desiccation stress [25–28,33]. We collected 2 ml of blood following decapitation (less than 1 min). Blood samples were placed into lithium heparin vials and stored on ice for up to 4 h before they were centrifuged at 5037g for 5 min and the plasma separated. Samples were stored in a freezer at −20°C until they were assayed. Environmental temperatures to which toads were exposed at each sampling time were measured with a digital thermometer.
(c). Effect of hormone manipulations on acute adrenocortical stress response phenotypes, dehydration and survival rates
We conducted two separate hormone manipulation experiments over 8 and 48 h periods with a single day's break between experiments in late September 2011. Both experiments commenced at 08.00 to measure the interactions among local weather conditions and hormone treatments on toad adrenocortical stress responses, evaporative water loss and survival.
(i). Experiment 1
To ensure that hormone manipulations increased responsiveness of the acute adrenocortical stress response, we monitored corticosterone levels to ensure that we obtained stress phenotypes different from the controls. We then measured the influence of hormone manipulations on rates of evaporative water loss in toads placed into artificial shelters and exposed to prevailing weather over an 8 h period. Artificial shelters were used to standardize micro-environmental conditions and to facilitate rapid blood sampling, measurements of evaporative water loss and survival after hormone injection. Three artificial shelters (1 m wide × 1 m long × 0.50 m high) were made from three overlapping layers of shade cloth (90% UV block), which provided toads access to full shade and reduced air movement to simulate conditions in natural shelters. A previous toad population-level water exclusion experiment conducted under similar weather conditions demonstrated that substantial mortality (80%) occurred within the first 12 h and that no toad survived 72 h after water exclusion [26]. This evidence provided the range for how much environmental buffering the artificial shelters should maintain to be considered ecologically plausible. Peak daily shelter air temperature was 36.8°C and lowest relative humidity of 15%.
Artificial shelters were constructed adjacent to a man-made dam with permanent water that harboured a large resident population of toads and enabled rapid collection of fully hydrated individuals. All individuals were injected within 10 min of each other to produce similar experimental starting times commencing at 08.00. Following capture, adult toads (mean mass: 161 ± 2.4 g; mean length: 110 ± 0.4 mm) were allocated to three treatments. One group was injected intraperitoneally (IP) with dexamethasone (DEX), a potent synthetic GC that binds with extremely high affinity to GC receptors, causing rapid activation of cellular processes and ensuing physiological responses throughout the organism, while also inducing negative feedback onto the HPI/A and suppressing synthesis of endogenous corticosterone. Prior to injection, DEX (Sigma Chemical) was dissolved in 100% ethanol and injected in doses of 100 μg kg−1 (suspended in 100 μl ethanol).
The second group was injected IP with porcine adrenocorticotrophic hormone (ACTH) to again stimulate a more responsive acute stress phenotype. Exogenous ACTH induces increased synthesis of corticosterone that causes GC-receptor activation. This produces a more responsive acute stress phenotype relative to controls. ACTH (Sigma Chemical) was dissolved in distilled water diluted with amphibian Ringer's solution, and injected in doses of 50 international units (IU) kg−1. All doses were injected in a volume of 100 μl. Prior to each trial, fresh frozen ACTH was dissolved and immediately used in daily experiments. The third group comprised unmanipulated controls that were injected IP with 100 μl ethanol.
The DEX and ACTH manipulations enabled us to produce more responsive acute adrenocortical stress phenotypes with more rapid and larger magnitude activation of receptors, which resulted in more responsive but shorter duration responses relative to unmanipulated controls. ACTH and DEX dosage protocols were based on other studies that investigated their effects on the acute corticosterone stress response in ectothermic vertebrates [34].
Each hormone treatment had 40 toads. All toads were marked with a unique number using a non-toxic permanent marker and measured for mass and length before being injected and allocated to artificial shelters. Immediately after injection, 10 toads per treatment were killed via cervical dislocation and decapitated to collect 2 ml of blood to measure plasma corticosterone at the start of the experiment. The remaining 30 toads per hormone treatment were then placed into shelters. Toads within each treatment were divided into two subgroups. One subgroup was used to provide blood samples (20 toads per treatment) at 4 and 8 h after injection using the above blood sampling techniques. The second subgroup comprised 10 toads from each treatment that were sequentially weighed at 1, 2, 3, 4, 5, 6, 7 and 8 h to measure the effect of manipulated corticosterone stress phenotypes on rates of evaporative water loss across the hottest part of the day (see the electronic supplementary material). Rates of evaporative water loss were measured by the changes in body mass at each time period relative to a starting mass (time zero).
(ii). Experiment 2
To determine the effect of manipulated acute adrenocortical stress response phenotypes on toad survival, 80 toads were allocated per hormone treatment. As per experiment 1, toads were individually marked, measured for mass and length and injected with one hormone or control solution and placed into artificial shelters. Sampling for survival was conducted at 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 21, 23, 25, 27, 29, 31, 33 and 35 h after hormone injection (see the electronic supplementary material). Sampling was more intense during the day when shelter temperatures were hotter (peak daily shelter air temperatures were 35.7°C and 36.4°C and lowest daily relative humidities were 18% and 16%) and less intense during the night when conditions were cooler. Survival was defined as the ability of toads to right themselves within 10 s of being gently placed on to their backs. Toads that were able to right themselves were scored as alive, whereas those that were too moribund to do so were scored as dead. All moribund toads were immediately euthanased by rapid cervical dislocation. The duration of this experiment lasted until the last individual was recorded as moribund.
(d). Corticosterone assay
Total plasma concentrations of corticosterone were measured using a commercially available ELISA kit (Cayman Chemical, MI, USA). Preliminary assays determined that 10 µl of plasma was sufficient for assay use. Plasma samples were twice extracted in 3 ml of diethyl ether. The efficiency of extraction was measured by adding 20 µl of 3H-corticosterone (approx. 2000 CPM) (MP Biomedicals, Solon, OH, USA). To estimate steroid extraction efficiency, 50 µl of each extracted sample was placed into a scintillation vial containing 2 ml of scintillation fluid (Ultima Gold). Sample radioactivity was estimated using a Beckman 2100R Liquid Scintillation Counter. We followed the Cayman Chemical corticosterone enzyme immunoassay (EIA) procedures without modification to measure plasma corticosterone concentrations. During each assay, samples were run in duplicate alongside a standard curve of eight known concentrations of corticosterone (5000, 2000, 800, 320, 128, 51.2, 20.5, 8.2 pg ml−1). We calculated final steroid concentrations from standard curves and then corrected for individual sample recovery and addition of 3H-corticosterone. Average extraction efficiency for corticosterone over the four assays was 86.2 ± 1.0%. For corticosterone assays, intra- and inter-assay coefficients of variance were estimated at 4.9% and 11.0%, respectively. To validate the use of the corticosterone EIA kit with toad plasma, we established parallelism between the standard curve and serial dilutions of pooled plasma samples.
(e). Data analysis
Generalized linear models (GLM) with distribution appropriate errors and canonical links, and if necessary covariates and random effects, were used to analyse the data. To evaluate whether corticosterone levels of toads varied at different time periods across the day, we used GLM with a Gaussian error distribution and identity link. We considered time of sampling for assessing variation in toad plasma corticosterone levels across the day. As individual toad hydric state was a continuous variable, it was treated as a covariate in the factorial model and hence also acted to standardize corticosterone values to a uniform hydration state to eliminate plasma volume variation in toads. This was necessary to control for individual differences in evaporative water loss that could lead to differences in corticosterone owing to plasma volume differences. To analyse variation in toad hydric state, we used a GLM with sampling time as the main effect. The effects of hormone manipulations on toad plasma corticosterone levels over time after injection were analysed with a GLM. Here, we considered hormone treatment, time after injection and a hormone treatment by time after injection interaction as effects for analysis.
To analyse the effects of hormone treatments on evaporative water loss, we considered individual changes in body mass as the dependent variable. As individuals were repeatedly measured, we used a GLM with individual toad identity as a random effect to account for autocorrelation. We evaluated whether toad water loss varied due to the effects of hormone treatment, time after injection and an interaction between the terms. We also included body length as a covariate in the model to account for size-dependent differences in individual starting mass.
For toad survival data, we considered a bivariate outcome of an individual's righting response to score toads alive or dead across sequential time periods. Toad survival was evaluated for variation due to the effects of hormone treatment, time after injection and an interaction between these terms. Individual identity was included as a random effect and as data were drawn from a binomial distribution, the GLM was fitted with a binomial error structure and logit link. We used a Kaplan–Meier survival analysis to estimate mean survival times for each hormone treatment over the experiment.
Post hoc pairwise comparisons using Fisher's protected least significant difference (LSD) tests were used to infer significant differences within effects in all GLM analyses. We performed all analyses using the software IBM SPSS v. 20.
3. Results
(a). Daily patterns in plasma corticosterone and hydric state
Toads exhibited significant daily variation in plasma corticosterone levels at different time periods (GLM χ2 = 23.3; p < 0.001) coinciding with changes in environmental temperature and hydric state (figure 1e). Highest daily mean plasma corticosterone levels (117.2 ± 15.1 ng ml−1) were recorded at 14.30, when toads were inactive in sub-ground shelters and exposed to hot environmental temperatures. As toads departed shelters at night and arrived at the dams, corticosterone levels had begun to decline (20.45; 91.9 ± 16.6 ng ml−1) and continued to do so as toads hydrated in dams throughout the night (24.00; 60.7 ± 10.2 ng ml−1) as environmental temperatures decreased. Plasma corticosterone levels were lowest at dawn prior to toads departing from dams (05.00; 27.6 ± 2.7 ng ml−1) and coincided with cool environmental temperatures.
Toads also exhibited significant and large daily variation in their hydric state indicative of evaporative water loss and desiccation stress coinciding with differences in environmental temperatures encountered at each sampling period (GLM χ2 = 54.1; p < 0.001; figure 1e). Toads had their lowest hydric state upon nocturnal arrival at dams (20.45; −30.1 ± 6.8 residuals of mass/snout-urostyle length) after spending the day in shelters. Thus, water loss continued well beyond the heat of the day (14.30: −4.4 ± 2.9) as shelter temperatures cooled slowly from peak temperatures. By midnight, toads had rehydrated (7.5 ± 6.9) and they continued to rehydrate during the night. Toads had the highest hydration state at dawn (05.00; 30.1 ± 6.8) prior to migrating back to diurnal shelters.
(b). Effects of adrenocorticotrophic hormone and dexamethasone on plasma corticosterone profiles
Toads injected with DEX, ACTH or control treatments exhibited significant hormone treatment (GLM χ2 = 38.4; p < 0.001) and time after injection effects (GLM χ2 = 72.9; p < 0.001) on plasma corticosterone levels (figure 2). These results confirmed successful experimental manipulation of acute adrenocortical stress response phenotypes. For the hormone treatment effect, post hoc analyses indicated that ACTH-injected toads (182.4 ± 12.3 ng ml−1) produced significantly more corticosterone relative to DEX (78.2 ± 12.1 ng ml−1; p < 0.001) and unmanipulated control treated toads (110.5 ± 11.2 ng ml−1; p < 0.001). DEX-injected toads produced significantly lower corticosterone levels when compared with unmanipulated control toads (p < 0.05), but the overall corticosterone difference relative to control levels was less than that achieved by the ACTH treatment across sampling periods. In addition, there was a significant hormone treatment by time after injection interaction effect (GLM χ2 = 23.6; p < 0.001) on plasma corticosterone levels. This interaction indicated that toad corticosterone levels in all treatments started at similar values, but then increased at different rates as the treatments responded differently to the experimental duration.
Figure 2.
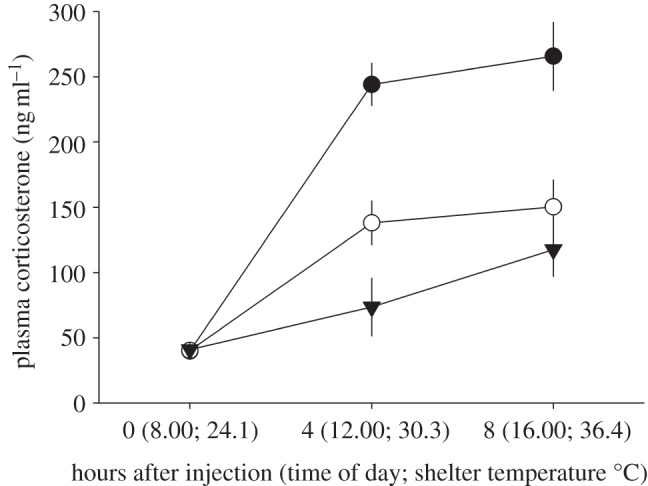
Hormone treatments experimetypes, as measured from changes to plasma corticosterone levels, in cane toads housed in experimental shelters and exposed to ambient environmental temperatures—between 08.00 and 16.00. The x-axis presents the time and air temperature within artificial shelters at the time of blood sampling. Each point represents the mean value and standard error for 10 individuals sampled within that time period. Filled circles denote ACTH treatment (50 IU kg−1); open circles denote control; inverted triangles denote DEX (100 IU kg–1).
(c). Acute adrenocortical stress phenotype effects on water loss rates
Measurement of evaporative water loss in toads injected with DEX, ACTH or unmanipulated control hormone treatments indicated significant hormone treatment (GLM χ2 = 8.01; p < 0.018) and a time after injection (GLM χ2 = 715.5; p < 0.001) effect (figure 3). Mean water loss was lowest in unmanipulated control toads (17.9 ± 1.6 g water per toad) compared with ACTH (24.9 ± 1.7 g water per toad) and DEX-treated (20.3 ± 1.4 g water per toad) toads. Post hoc testing indicated significant pairwise differences between ACTH and unmanipulated control and DEX toad treatments. DEX-treated toads had marginally lower (p = 0.09) mean evaporative water loss than unmanipulated control toads. In addition, there was a significant hormone treatment by time after injection interaction effect on rates of water loss (GLM χ2 = 71.5; p < 0.001; figure 2). This interaction indicated that toad water reserves in all treatments started at similar values, but then increased at different rates as the treatments responded differently to the experimental duration.
Figure 3.
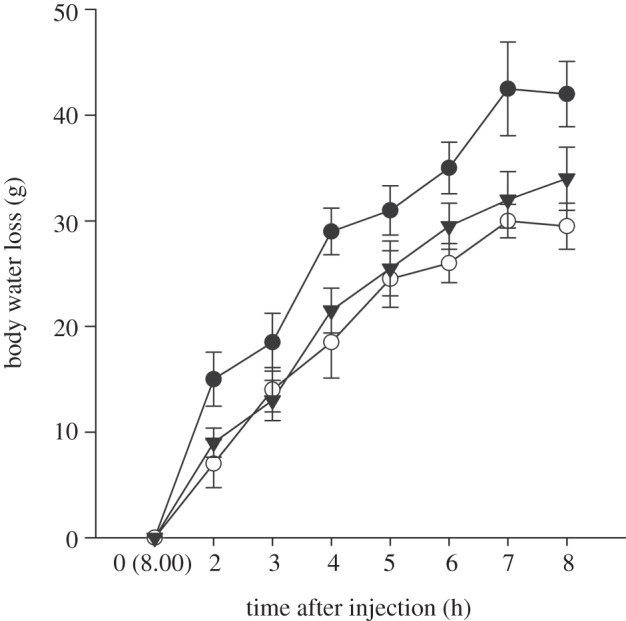
Effects of hormone treatments on rates of evaporative water loss in cane toads housed in experimental shelters and exposed to ambient environmental temperatures between 08.00 and 16.00. Injection with ACTH (filled circles) or DEX (inverted triangles) causes toads to produce a more responsive acute adrenocortical stress response relative to controls (open circles).
(d). Acute adrenocortical stress phenotype effects on survival rates
Significant differences in toad survival were evident due to hormone treatment (GLM χ2 = 443.1; p < 0.001) and time after injection effects (GLM χ2 = 6008.9; p < 0.001), but there was no hormone treatment by time after injection interaction effect (GLM χ2 = 14.7; p = 0.39; figure 4). Unmanipulated control toads had the highest mean survival duration (30.1 ± 0.18 h) compared with both ACTH- (27.6 ± 0.23 h) and DEX-treated (28.9 ± 0.21 h) toads. Post hoc testing indicated significant pairwise (p < 0.05) differences among all treatments.
Figure 4.
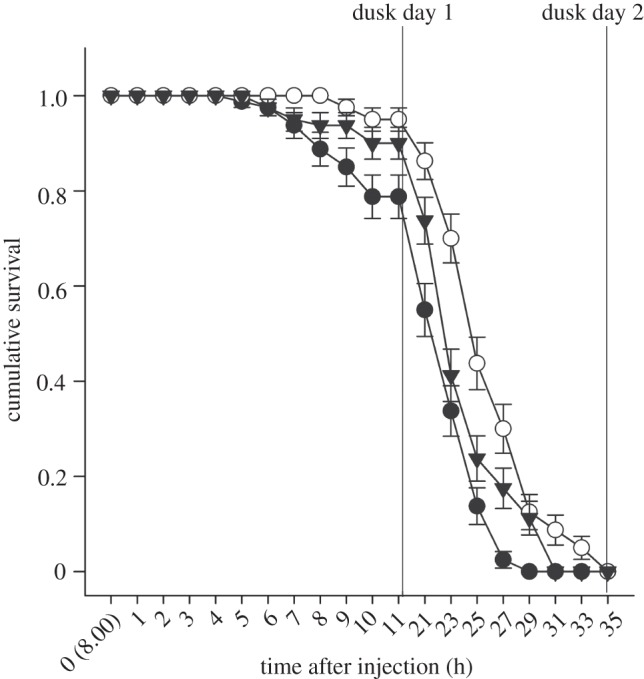
Effects of hormone treatments on survival in toads housed in experimental shelters and exposed to ambient environmental temperatures over a 36 h period commencing at 08.00. Each treatment commenced with 80 individuals, and survival was followed until no toad was able to maintain a righting response indicative of being moribund. The vertical lines at 11 and 35 h indicate the onset of dusk and the earliest time at which toads could transit to water sources to commence hydration had they had access to water. Injection with ACTH (filled circles) or DEX (inverted triangles) causes toads to produce a more responsive acute adrenocortical stress response relative to controls (open circles).
4. Discussion
We investigated whether response differences in acute adrenocortical stress phenotype affected phenotypic performance and fitness in an invasive vertebrate living under extreme environmental conditions. Invasive species that colonize novel environments require ongoing phenotypic plasticity, or rapid evolution, in the adaptive traits of individuals to permit further range expansion [23]. Until now, evidence for an adaptive role of the acute adrenocortical stress response in linking phenotypic performance and fitness was lacking in vertebrates that experienced extreme environmental stress, despite the logical connection [19–22]. By manipulating the acute adrenocortical stress phenotype, we have now demonstrated an important role for the adrenocortical stress response in regulating evaporative water loss, a key performance trait that facilitates the survival of individual cane toads under the extreme environmental conditions they experience at their arid range front. Regulation of water loss in toads is crucial to maintaining water and thermal homeostasis, and as revealed here, is linked to individual fitness through survival.
Throughout their range in Australia, cane toads use diurnal habitat shelters that buffer them from daily and seasonal environmental extremes [28]. It is plausible to suggest that because shelters differ spatially in their buffering capacity (i.e. quality and distance from water), toads encounter varying selection during the hot–dry season, which causes differential fitness. During late September, toads exhibited substantial daily variation in plasma corticosterone and hydric state commensurate with exposure to hot environmental temperatures and low humidity. Large-magnitude changes in both physiological measures are consistent with local climatic conditions being physiologically stressful to toads relative to populations in other environments [35]. As yet, we have not measured changes in toad abundance that directly indicates increased mortality across the hot–dry season. However, regular dry season population censuses at permanent water sources has revealed that toads can perish on transit from diurnal shelters to water (approx. 400 m), or while in refugia, indicating natural selection in action during the dry season [26]. Thus, cane toads living in arid environments face strong seasonal selection from temperature and desiccation stressors. Their ability to regulate physiological traits that balance thermal and water homeostasis is essential for survival and range occupancy [25].
Corticosterone, the major GC hormone of amphibians [36], had a role in regulating rates of water loss, and ultimately survival, of cane toads under the hot and dry climatic conditions at their range front. Toads with the least responsive acute adrenocortical stress phenotype exhibited reduced rates of water loss and longer survival than those with more responsive phenotypes induced by manipulations with ACTH and to a lesser extent, DEX. Thus, we suggest that the toad acute adrenocortical stress response was under either directional or stabilizing selection [37,38]. However, we argue that the most logical explanation for the type of selection acting on the existing toad acute stress phenotype is stabilizing selection [32,37,38]. This is because range front cane toads are exposed to two daily and potentially competing lethal stressors: desiccation and heat. If the acute stress response influences water loss (as our results indicate), it must also produce a fitness trade-off between optimal physiological strategies for coping with desiccation (best avoided by retaining water) and heat stress (best avoided by losing water to induce evaporative cooling to lower body temperature). Therefore, if toads with the more responsive adrenocortical stress phenotypes produce higher rates of water loss, it must benefit evaporative cooling and reduce body temperature below sub-lethal or even lethal daily limits [33]. However, rapid water loss inevitably exposes toads to increased risk of lethal desiccation later in the day. Conversely, if toads greatly reduced their acute stress response (e.g. from intense directional selection due to a single stressor) below the current range phenotype, then toads would conserve water. Reduced evaporative cooling (i.e. through cutaneous water loss) exposes toads to a higher risk of sub-lethal or lethal heat stress during the day [39]. Hence, an intermediate adrenocortical stress response seems necessary to ensure water loss rates are optimal to counter opposing, and hence stabilizing, selection induced by thermal and desiccation stressors co-acting in this environment.
So how could we better estimate how close is the existing acute stress response of range front cane toads to their fitness peak in this environment [38]? All we can say is that the unmanipulated, control stress phenotype toads used in our experiments had higher survival and hence closer to the local fitness peak than more responsive acute stress phenotypes we manipulated by endocrine manipulations. Ideally, we would have liked to experimentally suppress the acute adrenocortical stress response below the levels of the unmanipulated controls to produce a less responsive acute adrenocortical stress phenotype. This would enable us to directly determine the type of selection and to better evaluate the fitness value of the existing acute stress response to extreme environmental stress. However, experimental protocols for pharmacological suppression of the adrenocortical stress response in amphibians appear ineffective (e.g. use of mitotane), or produce toxic side effects that could confound acute stress-influenced performance and survival responses to environmental stressors (e.g. use of metyropone) [40]. Additionally, measuring individual variation in the acute stress response (e.g. plasma corticosterone levels) to a standardized stressor protocol, and its relationship to subsequent survival in this population would also provide a challenging natural experiment to better understand how this hormone influences cane toad fitness. Such approaches have been successfully used elsewhere in natural bird populations where individuals can be easily marked and followed through time to determine their survival or reproductive effort [41,42].
Because we expect selection by environmental pressures to vary in strength, direction, form, space and time across the current cane toad range, it is plausible to suggest that the most adaptive, and hence responsive, of the acute adrenocortical stress phenotypes could vary geographically to maximize local fitness [38]. At our Tanami Desert study site, the unmanipulated adrenocortical stress response permitted better phenotypic performance and higher survival relative to two more responsive acute phenotypes. This may explain why correlations between individual- or population-level GC stress responsiveness and fitness in other systems have rarely appeared adaptive in natural populations exposed to extreme environmental selection [20]. Experimental stress hormone-phenotypic performance-fitness frameworks such as ours may well require careful consideration of the type and strength of selection and hence timing of the study. Having a priori predictions based on careful consideration (or measurement) of the selective environment and ensuing phenotypic and fitness measures would help standardize inference for future studies that explore GC stress-phenotypic performance-fitness dynamics in vertebrates.
Our results raise several questions. First, we do not know the mechanism, be it phenotypic plasticity, natural selection or both, that underpins the phenotypic variation of the adrenocortical stress responses on cane toad water loss and survival [9,25]. To distinguish between these alternative mechanisms, we would need to use common garden or reciprocal transplant experiments to compare whether differences in adaptation were the product of genetic change or due to phenotypic plasticity alone. Nor can we explain how differences in the acute adrenocortical stress response influenced toad physiology to produce differences in water loss and survival responses. Based on existing knowledge, it is plausible to suggest that more responsive experimental adrenocortical stress phenotypes induced rapid activation of GC and mineralocorticoid receptors via crosstalk feedback mechanisms [43], increasing water loss and thus reducing survival. GC-receptor activation could result in lower upregulation of genes and proteins necessary for increases in Na+ absorption in the kidneys and urinary bladder, which are necessary to maintain osmotic balance [29,30]. In addition, acute experimental elevation of corticosterone in ectothermic vertebrates can cause increased metabolism [44,45], suggesting another mechanism that could increase evaporative water loss in cane toads. However, from our results, we cannot specifically identify how corticosterone affects the many potential regulatory processes (i.e. from genes, cellular mechanisms through to physiological networks) that influence water or thermal homeostasis in cane toads. If we are to better understand the adaptive role of steroid hormones and their capacity for mediating complex organismal function, testing the recently developed concept of physiological integration networks, may be a useful, but challenging, starting point [14].
Our results suggest that GC hormones have contributed to the ability of cane toads to colonize novel environments by regulating performance of general trait complexes that influence the survival of individuals. In cane toads, the incumbent adrenocortical stress response increases survival during the dry season relative to experimentally increased stress phenotypes. Clearly, survival through the dry season is essential for adult recruitment, reproduction and dispersal during the wet season. These key demographic parameters maintain propagule pressure and drive the ongoing invasion of cane toads into arid Australia [25,26]. Had we observed that the experimental stress phenotypes outperformed unmanipulated controls, we might have concluded that mean population fitness was distant from the adaptive landscape peak and that further invasion into arid landscapes would be difficult.
5. Conclusions
Because GC hormones affect multiple components of the phenotype, they can either result in adaptive outcomes due to past selection regimes; or constrain future evolution if they cause sub-optimal trade-offs among correlated traits under current selection [32,46]. Hence, depending how these hormones influence organismal performance, survival and ultimately local adaptation, is key to understanding range expansion by invasive vertebrates. By manipulating the acute adrenocortical stress response of cane toads, we have demonstrated its value for: (i) phenotypic integration of complex selection by environmental stressors, and (ii) better performance of general trait complexes that facilitate the survival of individuals in a novel and physiologically stressful environment. Our study suggests that the adrenocortical stress response is essential in setting fitness trajectories of invasive species during range expansion.
Acknowledgements
We thank the managers of Camfield and Dungowan Stations for permission to access their land. Statement of authorship. T.S.J. conceived and designed the study. T.S.J., M.L., J.K.W. and T.D. performed experiments. T.S.J. conducted data analysis. T.S.J., M.L., J.K.W. and T.D. wrote the manuscript.
Experimentation was performed in accordance to University of Melbourne Animal Ethics Permit (0911328.2).
Funding statement
Funding for this research was provided by an ECR grant to T.S.J. and a Hermon Slade Foundation grant to M.L., J.K.W. and T.D.
References
- 1.Sax DF, et al. 2007. Ecological and evolutionary insights from species invasions. Trends Ecol. Evol. 22, 465–471 (doi:10.1016/j.tree.2007.06.009) [DOI] [PubMed] [Google Scholar]
- 2.Reznick DN, Ghalambor CK. 2001. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica 112, 183–198 (doi:10.1023/A:1013352109042) [PubMed] [Google Scholar]
- 3.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407 (doi:10.1111/j.1365-2435.2007.01283.x) [Google Scholar]
- 4.Lee CE. 2002. Evolutionary genetics of invasive species. Trends Ecol. Evol. 17, 386–391 (doi:10.1016/S0169-5347(02)02554-5) [Google Scholar]
- 5.Cote J, Fogarty S, Weinersmith K, Brodin T, Sih A. 2010. Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc. R. Soc. B 277, 1571–1579 (doi:10.1098/rspb.2009.2128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duckworth RA, Badyaev AV. 2007. Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl Acad. Sci. USA 104, 15 017–15 022 (doi:10.1073/pnas.0706174104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fogarty S, Cote J, Sih A. 2011. Social personality polymorphism and the spread of invasive species: a model. Am. Nat. 177, 273–287 (doi:10.1086/658174) [DOI] [PubMed] [Google Scholar]
- 8.Lockwood JL, Cassey P, Blackburn T. 2005. The role of propagule pressure in explaining species invasions. Trends Ecol. Evol. 20, 223–228 (doi:10.1016/j.tree.2005.02.004) [DOI] [PubMed] [Google Scholar]
- 9.Chown SL, Hoffmann AA, Kristensen TN, Angilletta MJ, Jr, Stenseth NC, Pertoldi C. 2010. Adapting to climate change: a perspective from evolutionary physiology. Clim. Res. 43, 3–15 (doi:10.3354/cr00879) [Google Scholar]
- 10.Kearney MR, Simpson SJ, Raubenheimer D, Kooijman SALM. 2012. Balancing heat, water and nutrients under environmental change: a thermodynamic niche framework. Funct. Ecol. 27, 950–966 (doi:10.1111/1365-2435.12020) [Google Scholar]
- 11.Seebacher F, Franklin CE. 2011. Physiology of invasion: cane toads are constrained by thermal effects on physiological mechanisms that support locomotor performance. J. Exp. Biol. 214, 1437–1443 (doi:10.1242/jeb.053124) [DOI] [PubMed] [Google Scholar]
- 12.Wingfield JC. 2008. Comparative endocrinology, environment and global change. Gen. Comp. Endocrinol. 157, 207–216 (doi:10.1016/j.ygcen.2008.04.017) [DOI] [PubMed] [Google Scholar]
- 13.Martin LB, Liebl AL, Trotter JH, Richards CL, McCoy K, McCoy MW. 2011. Integrator networks: illuminating the black box linking genotype and phenotype. Integr. Comp. Biol. 51, 514–527 (doi:10.1093/icb/icr049) [DOI] [PubMed] [Google Scholar]
- 14.Cohen AA, Martin LB, Wingfield JC, McWilliams SR, Dunne JA. 2012. Physiological regulatory networks: ecological roles and evolutionary constraints. Trends Ecol. Evol. 27, 428–434 (doi:10.1016/j.tree.2012.04.008) [DOI] [PubMed] [Google Scholar]
- 15.Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. 1998. Ecological bases of hormone–behavior interactions: the emergency life history stage. Am. Zool. 38, 191–206. (10.1093/icb/38.1.191) [Google Scholar]
- 16.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 17.Romero LM. 2004. Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 19, 249–255 (doi:10.1016/j.tree.2004.03.008) [DOI] [PubMed] [Google Scholar]
- 18.Wingfield JC. 2013. Ecological processes and the ecology of stress: the impacts of abiotic environmental factors. Funct. Ecol. 27, 37–44 (doi:10.1111/1365-2435.12039) [Google Scholar]
- 19.Breuner CW, Patterson SH, Hahn TP. 2008. In search of relationships between the acute adrenocortical response and fitness. Gen. Comp. Endocrinol. 157, 288–295 (doi:10.1016/j.ygcen.2008.05.017) [DOI] [PubMed] [Google Scholar]
- 20.Romero LM, Wikelski M. 2001. Corticosterone levels predict survival probabilities of Galapagos marine iguanas during El Nino events. Proc. Natl Acad. Sci. USA 98, 7366–7370 (doi:10.1073/pnas.131091498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cote J, Clobert J, Meylan S, Fitze PS. 2006. Experimental enhancement of corticosterone levels positively affects subsequent male survival. Horm. Behav. 49, 320–327 (doi:10.1016/j.yhbeh.2005.08.004) [DOI] [PubMed] [Google Scholar]
- 22.Blas J, Bortolotti GR, Tella JL, Baos R, Marchant TA. 2007. Stress response during development predicts fitness in a wild, long lived vertebrate. Proc. Natl Acad. Sci. USA 104, 8880–8884 (doi:10.1073/pnas.0700232104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liebl AL, Martin LB. 2012. Exploratory behaviour and stressor hyper-responsiveness facilitate range expansion of an introduced songbird. Proc. R. Soc. B 279, 4375–4381 (doi:10.1098/rspb.2012.1606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shine R. 2010. The ecological impact of invasive cane toads (Bufo marinus) in Australia. Q. Rev. Biol. 85, 253–291 (doi:10.1086/655116) [DOI] [PubMed] [Google Scholar]
- 25.Kearney M, Phillips BL, Tracy CR, Christian KA, Betts G, Porter WP. 2008. Modelling species distributions without using species distributions: the cane toad in Australia under current and future climates. Ecography 31, 423–434 (doi:10.1111/j.0906-7590.2008.05457.x) [Google Scholar]
- 26.Florance D, Webb JK, Dempster T, Kearney MR, Worthing A, Letnic M. 2011. Excluding access to invasion hubs can contain the spread of an invasive vertebrate. Proc. R. Soc. B 278, 2900–2908 (doi:10.1098/rspb.2011.0032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tingley R, Greenlees MJ, Shine R. 2012. Hydric balance and locomotor performance of an anuran (Rhinella marina) invading the Australian arid zone. Oikos 121, 1959–1965 (doi:10.1111/j.1600-0706.2012.20422.x) [Google Scholar]
- 28.Schwarzkopf L, Alford RA. 1996. Desiccation and shelter-site use in a tropical amphibian: comparing toads with physical models. Funct. Ecol. 10, 193–200 (doi:10.2307/2389843) [Google Scholar]
- 29.Rogerson FM, Fuller PJ. 2000. Mineralocorticoid action. Steroids 65, 61–73 (doi:10.1016/S0039-128X(99)00087-2) [DOI] [PubMed] [Google Scholar]
- 30.McCormick SD, Bradshaw D. 2006. Hormonal control of salt and water balance in vertebrates. Gen. Comp. Endocrinol. 147, 3–8 (doi:10.1016/j.ygcen.2005.12.009) [DOI] [PubMed] [Google Scholar]
- 31.Sinervo B, Basalo AL. 1996. Testing adaptation using phenotypic manipulations. In Adaptation (eds Lauder G, Rose MR.), pp. 149–185 New York, NY: Academic Press [Google Scholar]
- 32.Ketterson ED, Nolan V., Jr 1999. Adaptation, exaptation, and constraint: a hormonal perspective. Am. Nat. 154, S4–S25 (doi:10.1086/303280) [DOI] [PubMed] [Google Scholar]
- 33.Tracy CR, Christian KA, Baldwin J, Phillips BL. 2012. Cane toads lack physiological enhancements for dispersal at the invasive front in northern Australia. Biol. ONE 1, 37–42 (doi:10.1242/bio.2011024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero LM, Wikelski M. 2006. Diurnal and nocturnal differences in hypothalamic–pituitary–adrenal axis function in Galapagos marine iguanas. Gen. Comp. Endocrinol. 145, 177–181 (doi:10.1016/j.ygcen.2005.09.011) [DOI] [PubMed] [Google Scholar]
- 35.Brown GP, Kelehear C, Shine R. 2011. Effects of seasonal aridity on the ecology and behaviour of invasive cane toads in the Australian wet-dry tropics. Funct. Ecol. 25, 1339–1347 (doi:10.1111/j.1365-2435.2011.01888.x) [Google Scholar]
- 36.Moore IT, Jessop TS. 2003. Stress, reproduction, and adrenocortical modulation in amphibians and reptiles. Horm. Behav. 43, 39–47 (doi:10.1016/S0018-506X(02)00038-7) [DOI] [PubMed] [Google Scholar]
- 37.Brodie ED, Moore AJ, Janzen FJ. 1995. Visualizing and quantifying natural selection. Trends Ecol. Evol. 10, 313–318 (doi:10.1016/S0169-5347(00)89117-X) [DOI] [PubMed] [Google Scholar]
- 38.Svensson E, Calsbeek R. 2012. The adaptive landscape in evolutionary biology. Oxford, UK: Oxford University Press [Google Scholar]
- 39.Jessop TS, Hamann M, Read MA, Limpus CJ. 2000. Evidence for a hormonal tactic maximizing green turtle reproduction in response to a pervasive ecological stressor. Gen. Comp. Endocrinol. 118, 407–417 (doi:10.1006/gcen.2000.7473) [DOI] [PubMed] [Google Scholar]
- 40.Breuner CW, Jennings DH, Moore MC, Orchinik M. 2000. Pharmacological adrenalectomy with mitotane. Gen. Comp. Endocrinol. 120, 27–34 (doi:10.1006/gcen.2000.7537) [DOI] [PubMed] [Google Scholar]
- 41.Brown CR, Brown MB, Raouf SA, Smith LC, Wingfield JC. 2005. Effects of endogenous steroid hormone levels on annual survival in cliff swallows. Ecology 86, 1034–1046 (doi:10.1890/04-0740) [Google Scholar]
- 42.McGlothlin JW, Jawor JM, Ketterson ED. 2007. Natural variation in a testosterone-mediated trade-off between mating effort and parental effort. Am. Nat. 170, 864–875 (doi:10.1086/522838) [DOI] [PubMed] [Google Scholar]
- 43.Stockand JD. 2002. New ideas about aldosterone signaling in epithelia. Am. J. Physiol. Renal Physiol. 282, F559–F576 (doi:10.1152/ajprenal.00320.2001) [DOI] [PubMed] [Google Scholar]
- 44.DuRant SE, Hopkins WA, Talent LG, Romero LM. 2008. Effect of exogenous corticosterone on respiration in a reptile. Gen. Comp. Endocrinol. 156, 126–133 (doi:10.1016/j.ygcen.2007.12.004) [DOI] [PubMed] [Google Scholar]
- 45.Wack CL, DuRant SE, Hopkins WA, Lovern MB, Feldhoff RC, Woodley SK. 2012. Elevated plasma corticosterone increases metabolic rate in a terrestrial salamander. Comp. Biochem. Physiol. A 161, 153–158 (doi:10.1016/j.cbpa.2011.10.017) [DOI] [PubMed] [Google Scholar]
- 46.McGlothlin JW, Ketterson ED. 2008. Hormone-mediated suites as adaptations and evolutionary constraints. Phil. Trans. R. Soc. B 363, 1611–1620 (doi:10.1098/rstb.2007.0002) [DOI] [PMC free article] [PubMed] [Google Scholar]