Abstract
Since 1954, when the first tropical tephritid fruit fly was detected in California, a total of 17 species in four genera and 11 386 individuals (adults/larvae) have been detected in the state at more than 3348 locations in 330 cities. We conclude from spatial mapping analyses of historical capture patterns and modelling that, despite the 250+ emergency eradication projects that have been directed against these pests by state and federal agencies, a minimum of five and as many as nine or more tephritid species are established and widespread, including the Mediterranean, Mexican and oriental fruit flies, and possibly the peach, guava and melon fruit flies. We outline and discuss the evidence for our conclusions, with particular attention to the incremental, chronic and insidious nature of the invasion, which involves ultra-small, barely detectable populations. We finish by considering the implications of our results for invasion biology and for science-based invasion policy.
Keywords: Tephritidae, invasion biology, subdetectable populations, eradication
1. Introduction
Tropical fruit flies (Tephritidae), such as the Mediterranean fruit fly (Ceratitis capitata) from Africa, the oriental fruit fly (Bactrocera dorsalis) from Asia and the Mexican fruit fly (Anastrepha ludens) from the Americas, are recognized by entomologists as among the most destructive agricultural insect pests in the world [1,2]. Because of tephritids' economic importance, US states such as California—considered by both the US Department of Agriculture (USDA) and the California Department of Food and Agriculture (CDFA) to be free of these pests, but with climates favourable to their establishment—invest heavily in measures to keep tephritids from becoming established. These steps include restricting importation of commodities that originate in regions with ongoing tephritid outbreaks, requiring post-harvest treatments for imported fruits and vegetables grown in areas where the pests are endemic or established, maintaining large-scale monitoring programmes for early detection, supporting preventive release programmes of sterile flies to pre-empt establishment, and launching eradication campaigns to eliminate pest populations once discovered. Indeed, 90% of the eradication projects (243 of 274) initiated in California between 1982 and 2007 were directed against tropical fruit flies (see electronic supplementary material, table S1).
The historical challenges posed by the fruit fly threat to California are similar to those posed by many other invasive insect species [3]. For example, the propagule pressure of fruit flies resulting from the ever-increasing movement of people and products [4–6] is an ongoing challenge posed by all invasive species. Similarly, global warming has resulted in the expansion of pest ranges worldwide [7]. Fewer frost days, longer growing seasons, more heat waves and greater frequency of warm nights in California [8,9], combined with an abundance of suitable hosts [2] in both urban and commercial environments, create ideal conditions for a wide range of species, particularly tropical tephritids, to successfully invade.
Two aspects of California's fruit fly invasions are unique, however. First, in most years and locations, fruit fly detections are extremely rare because of a combination of the slow population growth of newly introduced species and of population suppression from intervention programmes. This combination of elements makes it difficult to decipher patterns in detections, because there are few ‘dots’ to connect, and small numbers of captures separated in both time and space may give the illusion that previously detected populations have been eliminated. Second, an unprecedented number of pest tephritids have been detected in California in recent decades, including a more than eightfold increase in the number of species (i.e. n = 2 in 1954; n = 17 in 2012), and thousands more flies have been captured in California than in all other US mainland states combined. We are unaware of any other single taxonomic group (family) that consists of such a large number of economically important invasive species that are continually reappearing in the same region.
Our broad goal in this paper is to bring principles of invasion biology [3,10,11], mapping techniques and quantitative methods to bear on detection and interception data to answer questions about the residency status of tropical fruit flies captured in California. We show that, despite the due diligence, quick responses and massive expenditures of government agencies to prevent entry and establishment of these pests, virtually all of the species against which eradication projects were directed have been reappearing; several species reappear annually, and several others every 2–5 years. The preponderance of evidence supports the hypothesis that at least five and as many as nine species are established in the state. We discuss both scientific and practical implications of these findings.
2. Methods
(a). Data sources
We obtained, from the California Department of Food and Agriculture (CDFA), historical detection data (1950–2011, in Excel spreadsheets) for all 17 tropical fruit fly species (see electronic supplementary material, figure S1 and table S1a,b) that have been trapped in California [12,13]. We also used the California Plant Pest and Disease Reports available online, medfly detection data in the database of J.R.C. (1975–1994) and the CDFA webpage for recent (2012) detections. Separate records were created for each tephritid adult, including its species, sex, date, mating status (females only) and precise location (latitude and longitude). We also examined information on larval finds obtained by ground crews searching for infested hosts in the 24–48 h window between capture of an adult fly and intervention. Although no tropical tephritid species was detected in the state until 1954, California fruit growers had been on high alert for tephritid introductions ever since the first medfly detection in Hawaii in 1910 [14,15].
(b). Mapping
All detection data were entered into ArcGIS Map 10 (Esri, New York, NY) and transformed to WGS 84 coordinates. They were then re-projected to UTM coordinates of the appropriate zone. Finally, historical detections for each species were mapped at local, regional and state-wide scales.
(c). Tephritid propagule pressure and climatically favourable regions
We used information on both domestic and international interceptions at ports of entry (see electronic supplementary material, tables S3–S6) to estimate relative propagule pressure in fruit-fly-friendly regions of California (all areas except far northern and alpine regions) relative to other regions of North America (southern states) and the Mediterranean Basin (all countries) that have climates favourable to tephritid establishment [12,16–20]. Information on tephritid interception pathways and field detections in fruit-fly-friendly regions other than California served as controls; that is, similar rates of tephritid interception at ports of entry for different regions do not ‘explain’ why there are ongoing field detections in one region (California) but not in the others. The relationship between port of entry and field detections must be considered to avoid misrepresenting correlation as causation (the false-cause fallacy), as has been carried out in the past (see citations in [21,22]).
(d). Modelling
A natural estimate of the probability of capture in a region within n years of an initial detection is the fraction of detection years in that region in which the species is detected again within n years of the most recent detection. We computed this fraction for each species at two spatial scales: the county scale and a local scale based on the size of the exclusion area surrounding a detection. To define localities, we subdivided the area into a lattice of square cells of side length 14 km, a dimension that approximates nearly the same area (196 km2) as the federally mandated treatment area, a circle of radius 8 km (201 km2) around a fruit fly discovery. We considered each lattice cell to be a separate region. We analysed southern California and the Bay Area separately. There were sufficient data for a detailed analysis of two species, B. dorsalis and C. capitata, in the Bay Area, and of these two species plus A. ludens in southern California. Results of the analysis of these three species are reported in detail at all scales. Results for the other species are summarized graphically at the county level.
Although the recapture model was developed for forecasting recurrence, we also used it in a randomization trial to test a null hypothesis of random introduction against an alternative hypothesis of reoccurrence in a currently infested region. We separated the Bay Area and the Los Angeles area as above. For a given species in each area, we selected only lattice cells in which an infestation had been detected at least once. For example, for B. dorsalis, there are 30 and 77 such cells in the Bay Area and the Los Angeles region, respectively. We numbered from 1 to n the years in which a member of the species was captured, skipping years with no capture. For B. dorsalis, n = 27 and n = 45 for the Bay Area and Los Angeles region, respectively. Let U be a vector of length n − 1, and let Uj = 1 if there is at least one cell in which a member of the species was captured in that cell in both years j and j + 1, and let Uj = 0 if there is no such cell in year j. Let N equal the sum of the elements of U. Then the statistic N is a measure of the persistence of the infestation in the same cells. This model was used in a randomization test by comparing the actual observed value of N with values obtained under the null hypothesis of random introduction into the same set of cells each year. The analysis was carried out only for those species with sufficient data (at least 50 unique records) for a lattice-level recurrence analysis, as described in the previous paragraph.
3. Results
(a). Historical overview of detections
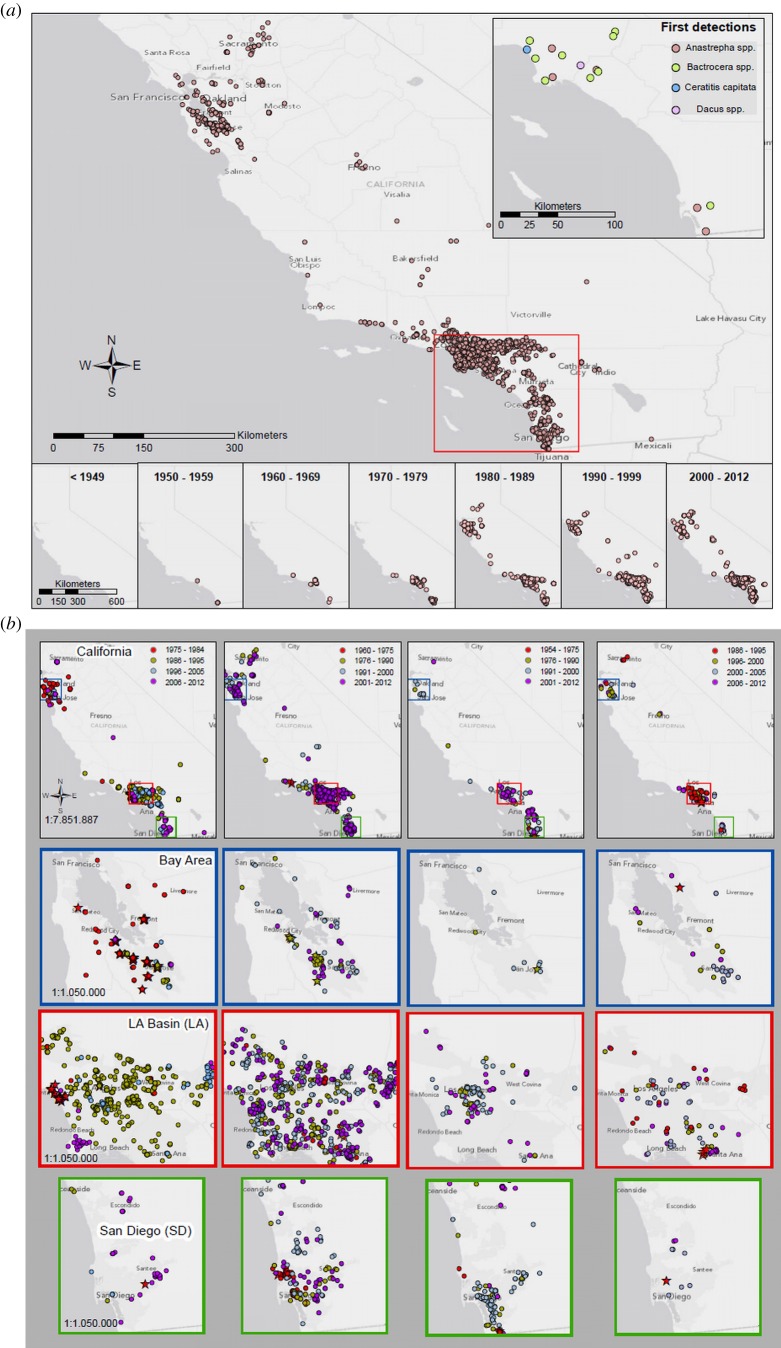
Tephritids have been detected in nearly all regions of California where conditions are favourable for fruit fly establishment (table 1 and figure 1a; see also electronic supplementary material, figures S2–S18). Although the largest numbers of detections by far have been in the greater metropolitan areas of southern California, including the Los Angeles Basin and San Diego, a substantial number of flies were also detected in northern California, in the San Francisco Bay Area. Tephritids also began appearing in the state's main agricultural growing region, the Central Valley, which includes the Sacramento and San Joaquin Valleys, and the Imperial Valley. The non-random pattern of the invasions is reflected in the fact that 100% of first records for all species were in southern California (figure 1a inset), all but one of which were found in two regions: Los Angeles and San Diego. These regions contain only around one-third of the state's population, yet account for 100% of the tephritid first records.
Table 1.
Tephritid fruit fly species in four genera (Anastrepha, Bactrocera, Ceratitis and Dacus) that have been detected in California to end of 2012, including scientific and common names, city, county, year of first detection, year last detected, and basic metrics of capture, including numbers of individuals, detections and cities infested. LA, Los Angeles; OR, Orange; SD, San Diego. Numbers of olive fly detections and cities are not included because CDFA decided that this species was too widespread and deeply entrenched to attempt eradication, and thus considered it established a few years after its discovery.
| city (county) | year |
number |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| species | common name | first record | first | last | span | detection years | adults (larvae) | detections | cities |
| 1. A. ludens | Mexican fruit fly | San Ysidro (SD) | 1954 | 2012 | 58 | 41 | 889 (295) | 465 | 77 |
| 2. B. cucurbitae | melon fly | Westwood (LA) | 1956 | 2011 | 55 | 15 | 34 (0) | 28 | 17 |
| 3. B. dorsalis | oriental fruit fly | Anaheim (LA) | 1960 | 2012 | 52 | 44 | 2266 (1755) | 1558 | 244 |
| 4. A. striata | guava fruit fly (A) | San Ysidro (SD) | 1963 | 2012 | 48 | 9 | 11 (0) | 10 | 11 |
| 5. A. obliqua | West Indian fruit fly | Wilmington (LA) | 1967 | 2005 | 42 | 6 | 7 (0) | 7 | 8 |
| 6. C. capitata | Mediterranean fruit fly | Santa Monica (LA) | 1975 | 2012 | 37 | 26 | 2068 (3884) | 1417 | 169 |
| 7. A. suspensa | Caribbean fruit fly | San Diego (SD) | 1983 | 2004 | 21 | 4 | 6 (0) | 3 | 3 |
| 8. B. zonata | peach fruit fly | El Segundo (LA) | 1984 | 2010 | 26 | 12 | 61 (0) | 57 | 23 |
| 9. B. tryoni | Queensland fruit fly | La Mesa (SD) | 1985 | 1991 | 6 | 2 | 2 (0) | 1 | 2 |
| 10. B. correcta | guava fruit fly (B) | Westminster (OR) | 1986 | 2012 | 26 | 23 | 131 (0) | 126 | 59 |
| 11. B. scutellata | striped fruit fly | Los Angeles (LA) | 1987 | 2010 | 33 | 6 | 15 (0) | 15 | 8 |
| 12. D. bivitattus | African pumpkin fly | Cerritos (LA) | 1987 | 1987 | 1 | 1 | 1 (0) | 1 | 1 |
| 13. A. serpentine | Sapote fruit fly | Los Angeles (LA) | 1989 | 1989 | 1 | 1 | 1 (0) | 1 | 1 |
| 14. B. latifrons | Malaysian fruit fly | South Gate (LA) | 1998 | 1998 | 1 | 1 | 1 (0) | 1 | 1 |
| 15. B. facialis | no common name | La Verne (LA) | 1998 | 1998 | 1 | 1 | 1 (0) | 1 | 1 |
| 16. B. oleae | olive fly | Los Angeles (LA) | 1998 | 2012 | 14 | 14 | NA | NA | NA |
| 17. B. albistrigata | white striped fruit fly | Pomona (LA) | 2008 | 2009 | 2 | 2 | 9 (0) | 9 | 4 |
| Σ 5503 (5934) | 3700 | ||||||||
Figure 1.
Locations of tropical fruit fly detections in California (i.e. each point represents one or more individuals detected at a single location and date). (a) Cumulative captures of all 17 tephritid species from the first detections in the 1950s to the most recent. Inset shows the locations of first detections for all species, and the mini-maps at the bottom depict the detections (non-cumulative) by decade. (b) Detection patterns of the four most frequently captured fruit fly species at the state level (top row) as well as in three regions: the San Francisco Bay Area, the Los Angeles Basin and the San Diego greater metropolitan area. Stars indicate location of initial regional captures. Maps show detection locations for (left to right) C. capitata, B. dorsalis, A. ludens and B. correcta.
California was free of any tropical fruit fly species before the mid-1950s (figure 1a mini-maps), despite the rapid growth of the fruit industry in the late nineteenth century and first half of the twentieth century, as well as relatively lax regulatory protocols at ports of entry [23,24]. Two species were detected during the 1950s (A. ludens in the greater San Diego area and B. cucurbitae in the Los Angeles Basin), followed by four more in the 1960s and 1970s. The tephritid situation in the state changed drastically in the 1980s because of: (i) continued reappearances and spread of previously detected species in metropolitan Los Angeles and San Diego; (ii) seven new species detected, raising the total in the state from six to 13 species (table 1); and (iii) first tephritid detections in northern California (figure 1a,b), including a massive, widespread medfly outbreak in the Bay Area [21,25,26].
Three new tephritid species captured in the 1990s raised the total in the state to 16. The economic stakes were elevated to a new level when one of these, the olive fly (B. oleae), was declared established, and several previously detected species appeared in the Central Valley growing region. Even though only one new species has been captured during the past 12 years, nine previously detected species have been recaptured repeatedly over ever-expanding areas (table 1 and figure 1a), including seven that have been captured multiple times during the past 3 years (excluding B. oleae). The magnitude and geographical scope of the recurrent detections are evident in maps in figure 1b, showing the historical records of the hundreds of state-wide, regional and local detections of the four most frequently captured species.
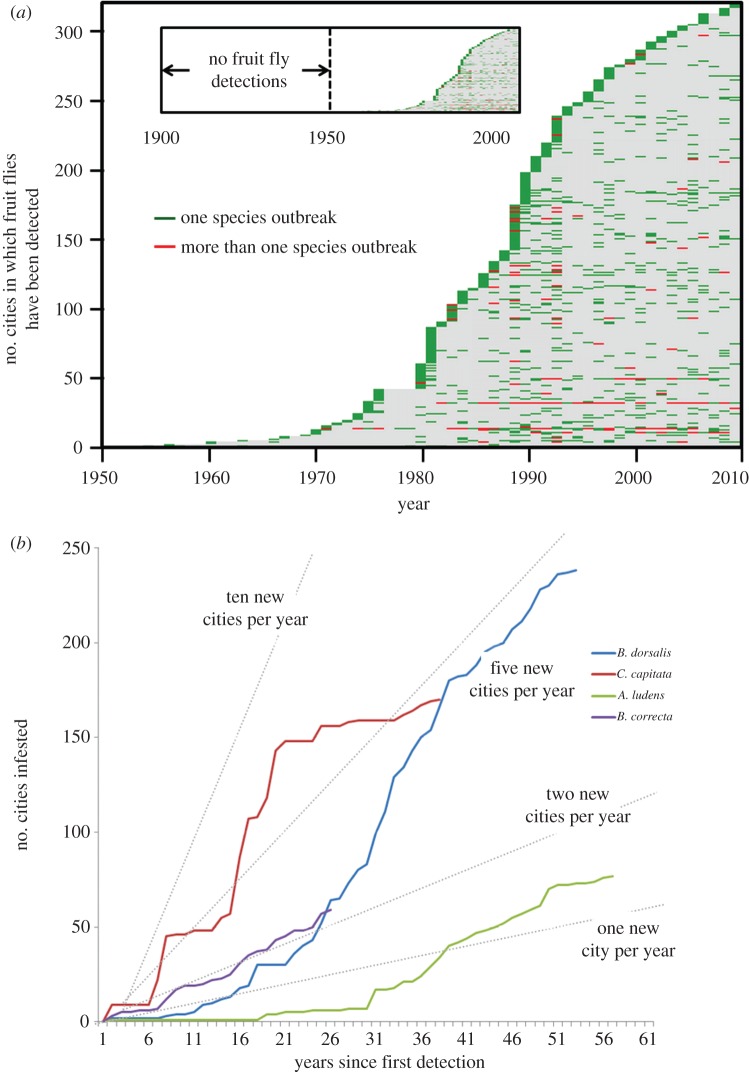
Using the number of cities in which a tephritid species has been detected as a proxy for area infested, figure 2a shows that in 1960 there were only two California cities in which tephritids had been detected (one in the San Diego region and one in the Los Angeles region). However, by 1970 the number of cities with a tephritid detection had increased to 13, by 1990 to a remarkable 200 cities, and by 2010 to more than 300 cities. Although 10 different species (excluding the olive fly) contributed to these totals, A. ludens, C. capitata and B. dorsalis contributed the most, appearing in 77, 168 and 245 new cities, respectively, by 2012 (figure 2b; see also electronic supplementary material, video S1).
Figure 2.
Appearance of tephritid fruit flies in Californian cities. (a) Event-history chart depicting the cumulative number of Californian cities that experienced one or more fruit fly infestations from 1950 to the present. Each horizontal line represents a Californian city, and coloured ticks depict the year in which one or more species was detected. Inset situates this schematic in a 100+ year context (California became a state in 1850). (b) Cumulative number of new Californian cities from which a detection of one of four different fruit fly species was reported. The abrupt levelling of the trajectory for C. capitata at 150 cities infested occurred at the same time that the California medfly preventive release programme was implemented in 1996.
(b). Reintroductions versus established populations
A long-standing explanation for recurring fruit fly detections is that flies are continually being reintroduced, either in cargo shipments or by people carrying infested fruit from fruit-fly-infested regions of the world [27–29]. We test this hypothesis and an alternative one to account for the recurring detections; both hypotheses were originally framed by Carey for the medfly [25,30] as (i) reintroduction hypothesis—recurring tephritid detections are due to repeated introductions—and (ii) established population hypotheses—recurring detections are due to resident fly populations. We assess the strength of these two hypotheses by comparing and identifying inconsistencies in relative numbers, diversity and frequency (or lack) of detections in California and in other fruit-fly-friendly regions.
(i). Absence of tephritid detections in most at-risk US states
Tephritids are intercepted at all airports across the USA (see electronic supplementary material, table S2) including all airports located in the southern states considered at risk for tephritid introductions. California ports of entry accounted for less than 20% of all insects intercepted in at-risk states (see electronic supplementary material, tables S3 and S4).
Assuming that insect interceptions (and specifically tephritid interceptions) can serve as proxies for the relative propagule pressure, if reintroductions were the primary source of detections, then the number of fruit fly detections in fruit-fly-friendly regions of the USA outside of California, compared with detections in California, should be roughly five to one, because California contributes about 20% of detections. Yet no tephritids were detected in the majority of states (i.e. all southern states) that are deemed at risk for fruit fly introduction [12,19,31] and maintain robust monitoring programmes (i.e. Arizona, Florida and Texas had relatively few detections).
(ii). High tephritid interception rate in European Union but near-absence of new species
Although the medfly and the olive fly are the only two tropical tephritid species that are long-term residents of fruitfly-friendly regions (southern countries) in the European Union (EU), interception rates of other tephritid species at ports of entry throughout the EU are quite high. One source of evidence for this is EUROPHYT, the European notification system for plant health interceptions. This system's database revealed that, of the total number of interceptions of harmful organisms in plants and plant products imported into the EU in 2011 (n = 1600), fully one-third (n = 534) were tephritids (see electronic supplementary material, table S6; see also [6]), and showed that, from 2007 to 2009, more than 700 individual tephritids in three genera (Anastrepha, Bactrocera, Ceratitis) and nine species not established in Europe were intercepted at Paris's International Airport (see electronic supplementary material, table S5). If the diversity and number of tephritid interceptions at the scores of international airports located in fruit-fly-friendly southern Europe, northern Africa and the Middle East are similar to those at the Paris airport, then the tephritid propagule pressure throughout this world region is far greater than in California. Yet, despite this pressure, with the exception of the peach fruit fly (Bactrocera zonata), discovered in 1998 in Egypt [32], no other tropical tephritids have been detected throughout the Mediterranean Basin for a century.
(iii). Evidence of breeding populations
Evidence of breeding populations in California is indicated by larval collections (table 1) for three species: (i) oriental fruit fly (B. dorsalis)—a total of 1755 larvae were collected in 169 locations over several periods totalling 21 years between 1974 and 2002; (ii) Mexican fruit fly (A. ludens)—a total of 295 larvae were collected at 15 locations in 4 years between 1995 and 2002; and (iii) medfly (C. capitata)—a total of 3884 larvae were collected in 572 locations over periods totalling 17 years between 1975 and 2009. Additional evidence for continuous populations of the medfly in California is in the papers by Meixner et al. [33] and Bonizzoni et al. [34], both of which showed that genetic analysis of captured flies over many years was consistent with continuous populations (see also electronic supplementary material, figure S21).
(iv). Repeat finds
At the state level from 1980 through 2012, one to two different tephritid species were captured every year (i.e. during 100% of this 33-year period), and the following numbers of species were captured during the percentages of this period indicated for each: three species, 97; four species, 78; five species, 72; six species, 62; and seven species, 25. In one of these years (1998), a remarkable 11 different species were captured, eight of which were repeats. At the county level (figure 3), depending upon species, the estimated probability of recapture after 1 year ranged from 0.1 to nearly 0.9, after 5 years from 0.5 to nearly 0.95, and after 10 years from 0.7 to near 1.0. At the city level, the frequency of repeat detections was high for many species, but extraordinarily so for three: 49 cities experienced repeat detections of C. capitata from two to 11 times, and 25 and 92 cities experienced respective repeat detections of A. ludens and B. dorsalis from two to 19 times. At the lattice cell level (14 × 14 km), the estimated probability of recapture in the Los Angeles area for A. ludens was 0.41 after 1 year, 0.70 after 5 years and 0.92 after 10 years. For B. dorsalis, these probabilities were 0.45, 0.79 and 0.94, respectively, and for C. capitata, they were 0.32, 0.55 and 0.64, respectively. In the Bay Area, the corresponding 1-, 5- and 10-year recapture probabilities were 0.12, 0.39 and 0.68, respectively, for B. dorsalis and 0.56, 0.59 and 0.88, respectively, for C. capitata.
Figure 3.
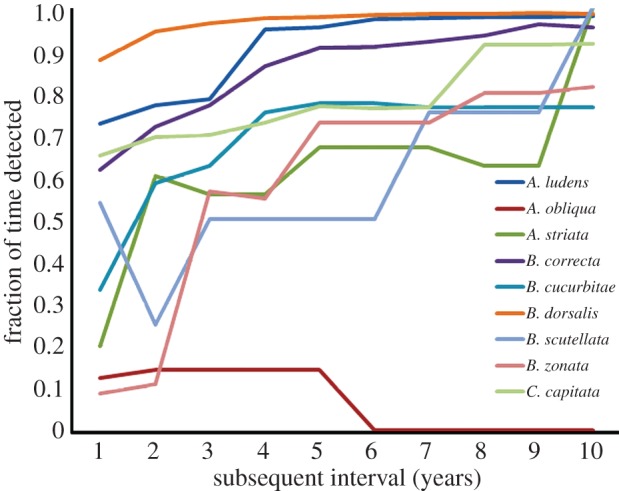
Estimated fruit fly recapture probabilities at the county level for nine fruit fly species in California in subsequent years (1–10 years) following a detection. For example, the probabilities of a repeat outbreak occurring for A. striata, C. capitata and B. dorsalis the first year following a detection are around 0.20, 0.65 and 0.88, respectively; the fifth year after a detection they are around 0.65, 0.75 and 0.98, respectively; and the 10th year after a detection they are around 1.00, 0.91 and 0.98, respectively.
An argument often used to account for the repeat finds in the same local area is that the same persons repeatedly return to California with infested fruit, reintroducing the species. However, the data do not support this argument, because there were no between-year detections on the same properties; in addition, this ‘reinfestation behaviour’ is not seen in other states and should not be unique to California returnees only.
(v). Randomization test of the null hypothesis of random introduction
In each case, the value of the observed recapture statistic N was compared with 999 values of this statistic computed under the assumption of random introduction in each year. In the Bay Area, the probability of obtaining an N statistic at least as large as the observed value was estimated as p = 0.038 for B. dorsalis. In the Los Angeles area, the probability was estimated as p < 0.001 for B. dorsalis and A. ludens, and p = 0.007 for C. capitata. These tests are anti-conservative (i.e. the p-values may be too low) because they ignore differences in both habitat suitability and trapping intensity among the lattice cells. Such differences would tend to increase the probability of capture in certain cells even in the case of random release into these cells, which would reduce the significance of the N statistic. Nevertheless, the results provide further support for the idea that the recapture pattern is not one that would be observed if the insects were being reintroduced each year.
4. Discussion
Several lines of evidence support the hypothesis that from five to nine tephritid species have become self-sustaining (and therefore established [35]) populations in the state (see electronic supplementary material, table S7 for list, establishment probability categories and summary of invasion metrics): their abrupt first appearance in the mid-1950s followed by high incidence of repeat detections, their marked seasonality (see electronic supplementary material, figure S19) and northward spread (see electronic supplementary material, figure S20), the lack of new detections and/or introductions of new species in most other at-risk regions in the USA and Mediterranean Basin, and the high probabilities of repeatedly detecting many of the tephritid species in California while at the same time not detecting them in other at-risk areas. These findings do not rule out the possibility of multiple introductions into the state for tephritids such as the medfly [33,36–38]. However, the multiple detections of several species in nearly the same location anywhere from 10 to 30 years after they were first detected, without any captures during interim years, suggests that, as for many other invasive species [4,39,40], tephritids can be present in low numbers for decades [3,8,41–44]. Indeed, one of the important features of lags in invasion biology (i.e. the delayed onset or slow rate of an invasion event) that probably also applies to the tephritid invasion of California is that invasions are often not recognized until they are over [45,46].
Our findings that multiple species of tropical tephritids (including the Mediterranean, Mexican and oriental fruit flies, and possibly the peach, guava and melon fruit flies) have self-sustaining [41] and thus established populations in California have profound economic implications. For example, a 1995 study estimated that medfly establishment alone would result in $493 million to $875 million in annual direct costs, and the imposition of an embargo would cause an additional loss of $564 million. The state economy could lose $1.2 billion in gross revenue and more than 14 000 jobs [47].
However, two aspects of the invasions are advantageous for planners, programme directors and policy makers. The first is that local population sizes for all species are extremely small, and therefore likely to continue to be subdetectable. Therefore, based on phytosanitary standards of the International Plant Protection Convention [48,49], most regions of the state should continue to be classified as risk-free by trading partners. The second aspect of the invasions that can be exploited for the longer term involves the invasion lags, which imply that there can be relatively long windows of opportunity for developing new protocols and programmes. Commodity certification protocols can be developed for the creation of fly-free and low-prevalence zones [48], as can long-term research programmes on tephritid biology and management.
5. Implications for invasion science and policy
(a). Early detection: a misleading misnomer
Because the likelihood of slowing the spread of or eradicating an alien pest depends heavily upon its residency time [50–52], a basic invasion biology canon is that early detection is critical for rapid response (but see [52]). Our results reveal that, because the sources of repeat detections are captures from established populations rather from reintroduced ones, in most cases ‘early detection’ is a misnomer when applied to tephritid detections at all scales. Because this expression is often inaccurate, it is also misleading inasmuch as it implies that a policy primarily directed at preventing new introductions will solve the problem of recurrent detections or infestations.
(b). Rare-event detection problem
As is true for many alien insect populations [53], the majority of tephritid population growth and spread in the state is subdetectable because of the small size and cryptic habits of all life stages, the slow pace of naturalization processes, and suppression of populations by intervention programmes. In cancer diagnostics, this is referred to as the ‘rare-event detection problem’ [54]; in the context of fruit fly detections, the parallel concept is the difficulty in discovering exceedingly rare, scattered, ultra-small populations of tephritids that are mostly in pre-adult stages, hidden among millions of properties and tens of millions of micro-niches. The scores of examples of repeat tephritid finds within a small region of California, separated by decades, suggest that the efficiency of detecting small populations of fruit flies is grossly overestimated [55], and that the actual chances of discovering populations that are so tiny and scattered is vanishingly small.
(c). Cryptic invasion
Our findings are consistent with two interrelated invasion biology principles that underlie the ability of tephritid populations to establish and maintain residency at ultra-low, cryptic and insidious population levels. The first involves what Simberloff [4] refers to as the ‘mysterious lag phase’ in which new populations experience delayed growth [45]. It is unlikely that the magnitude of the lag period in tephritids would be similar to the 150+ years reported for some introduced plant species [39,56]. However, it is likely that tropical species of tephritids that are introduced to different climatic regions experience major population lags much like the multi-decade lags observed in the melon fly (B. cucurbitae) in Africa [57] and the cherry fruit fly (Rhagoletis cingulata) in Europe [43]. A second closely related principle is naturalization—genetic adaptation to local conditions [3]. Recent studies suggest that many species' invasion success may depend more heavily on their ability to respond to natural selection than on broad physiological tolerance or plasticity, and could also result from the need for multiple invasions to facilitate a sufficient evolutionary response [58].
(d). Natural rather than human-enhanced spread
Although it is widely believed that human movement of infested plant material plays a major role in spreading introduced pests [6,11], capture patterns for the Mexican fruit fly suggest that this is not the case for this species and, by extension, may not be the case for many of the other invasive tephritids. For example, in 2011, 43 million vehicles and 17 million pedestrians crossed the six ports of entry from Mexico (where A. ludens is endemic) to California [59,60]. Assuming that the direction of movement for roughly half of these vehicles and people was from Mexico to California, and if humans entering and dispersing around the state were responsible for the Mexico-to-California as well as the within-state movement of the Mexican fruit fly, then this species should have been detected more or less randomly throughout the state. But the vast majority of all A. ludens detections for nearly 60 years have been in the same areas in which this species continually reoccurs. At the same time, there have been virtually no discoveries of A. ludens in the regions of the state with extraordinarily high movement of Latino populations (including tens of thousands of migrant workers), such as the main agricultural areas in the Central, Salinas and Imperial Valleys (see detailed local distributions in the electronic supplementary material, figure S1).
(e). Principles of invasion biology and population theory
We know of no historical precedent in the invasion biology literature similar to the tephritid situation in California, where not only are several insect species within a single family (Tephritidae) invading a region at the same time, but the group also contains species within multiple genera. The California tephritid invasion thus provides unique opportunities to compare the invasive properties of species across different genera with similar life histories [61], to explore reasons why 17 tephritid species have been detected in California but few to none in many other fruit-fly-friendly regions of the USA and the world, and to develop new population theory for ultra-low, cryptic populations.
(f). Conflation of criteria for eradication declaration
CDFA and USDA declared 100% success for each of the several hundred eradication programmes that were launched against fruit flies in California (see electronic supplementary material, table S1). These declarations were accurate according to legal criteria specified by the USDA [62] and the International Phytosanitary Commission [63]; that is, a region is declared (and thus certified) fruit-fly-free when no flies have been detected for a time period corresponding to three generations. Although these legal criteria are required for regulatory compliance to enable growers to ship their produce, our results reveal that the more stringent ecological requirements for eradication declaration were not met in the majority of cases. This underscores the continuing problem in the insect eradication literature of loosely and inaccurately applying a term (eradication) that has a clear definition. Those interested in insect eradication can learn much from the epidemiological literature on eradication programmes (e.g. malaria) regarding (i) frameworks for evaluating systematically the potential for eradication [64], (ii) clear definitions of concepts and terms [65], and (iii) perspectives on the preconditions, difficulties and challenges of successfully eradicating insects [66].
(g). Population establishment: categories of likelihood
Although some authors have characterized population establishment as self-sustaining populations [35,67], none has attempted to specify criteria. The likely reason is that, because of the uncertainty resulting from a combination of demographic stochasticity and detection constraints, it is virtually impossible to define a precise point at which a small population becomes self-sustaining. In the light of this problem, we propose that early-stage invasions can be categorized using methods similar to those we used for Californian tephritids (see electronic supplementary material, table S7). The establishment category for each species is necessarily subjective and can be based on a combination of detection metrics, including capture span (i.e. period between first and last year captured), total number of years captured, inter-year frequency of detections (e.g. annually; bi-annually), total numbers of individuals detected, within-state distribution and spatial patterns of apparent spread.
6. Conclusions
Our results highlight the enormous historical challenges in addressing the continuing problem of invasive tephritids in California.
One the one hand, the challenge of population suppression appears to have been met and a level of control achieved repeatedly for most tephritids (excepting olive fly) through various CDFA- and the USDA-supported intervention programmes. That is, the current responses to the presence of fruit flies have been extraordinarily successful in reducing invasive fruit fly populations to levels that satisfy legal and regulatory requirements for keeping commodity trade routes open.
On the other hand, the greater challenge of invasive tephritids in California, and the one we show has not been achieved for most tephritid species in the state, is true biological (as distinct from legal) eradication—the complete elimination of the last vestige of a population.
The long-term consequences of making short-term policy decisions based solely on the legal definition of eradication, while ignoring the biological reality (i.e. fruit fly establishment and spread), are potentially quite serious. Indeed, the call more than 20 years ago for decisive leadership to deal with medfly establishment in California because ‘the pest cannot be wished away or legislated out of existence’ [22, p. 516] now also applies to a number of the medfly's closest relatives.
Acknowledgements
We thank Kevin Hoffman, Martin Hauser, Stephen Gaimari and Peter Kerr at the California Department of Food and Agriculture for providing us with tephritid capture data in California, Marcia Kreith at the Agriculture Issues Center for economic information on invasive pests, and Balmès Valèrie at the Laboratoire National de la Protection des Végétaux in Montpellier for information on tephritid interceptions at Charles De Gaulle International Airport. The authors are grateful to Nan Wishner for editorial assistance. J.R.C. dedicates this paper to UC Davis Chancellor Emeritus Theodore Hullar for his unwavering support in the earlier days of fruit fly invasions in California.
Funding statement
N.T.P. research partially supported by the Organisation for Economic Co-operation and Development (OECD) Co-operative Research Program.
References
- 1.Bateman MA. 1972. The ecology of fruit flies. Annu. Rev. Entomol. 17, 493–518 (doi:10.1146/annurev.en.17.010172.002425) [Google Scholar]
- 2.Aluja M, Mangan RL. 2008. Fruit fly (Diptera: Tephritidae) host status determination: critical conceptual, methodological, and regulatory considerations. Annu. Rev. Entomol. 53, 473–502 (doi:10.1146/annurev.ento.53.103106.093350) [DOI] [PubMed] [Google Scholar]
- 3.Davis MA. 2009. Invasion biology. Oxford, UK: Oxford University Press [Google Scholar]
- 4.Simberloff D. 2009. The role of propagule pressure in biological invasions. Annu. Rev. Ecol. Syst. 40, 81–102 (doi:10.1146/annurev.ecolsys.110308.120304) [Google Scholar]
- 5.Perrings C, Dehnen-Schmutz K, Touza J, Williamson M. 2005. How to manage biological invasions under globalization? Trends Ecol. Evol. 20, 212–215 (doi:10.1016/j.tree.2005.02.011) [DOI] [PubMed] [Google Scholar]
- 6.Bacon SJ, Bacher S, Aebi A. 2012. Gaps in border controls are related to quarantine alien insect invasions in Europe. PLoS ONE 7, e47689 (doi:10.1371/journal.pone.0047689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altermatt F. 2010. Climatic warming increases voltinism in European butterflies and moths. Proc. R. Soc. B 277, 1281–1287 (doi:10.1098/rspb.2009.1910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trumble JT, Butler CD. 2009. Climate change will exacerbate California's insect pest problems. Calif. Agric. 63, 73–78 (doi:10.3733/ca.v063n02p73) [Google Scholar]
- 9.Weare BC. 2009. How will changes in global climate influence California? Calif. Agric. 63, 59–66 (doi:10.3733/ca.v063n02p59) [Google Scholar]
- 10.Elton CS. 1958. The ecology of invasions by animals and plants. London, UK: Methuen [Google Scholar]
- 11.Davis M. 2011. Invasion biology. In Encyclopedia of biological invasions (eds Simberloff D, Rejmanek M.), pp. 364–369 Berkeley, CA: University of California Press [Google Scholar]
- 12.Burnett W, Enkerlin W, Gilmore JF, Rendon PA, Smith C, Zavala JL. 2006. International panel for review of the fruit fly surveillance programs in the United States. Riverdale, MD: United States Department of Agriculture/APHIS/PPQ/Fruit Fly Program [Google Scholar]
- 13.Gilbert AJ, Bingham RR, Nicolas MA, Clark RA. 2010. California insect trapping guide. Sacramento, CA: California Department of Food and Agriculture [Google Scholar]
- 14.Back EA, Pemberton CE. 1918. The Mediterranean fruit fly in Hawaii. U.S. Dept. Agric. Bull. 536, 1–120 [Google Scholar]
- 15.Woglum RS. 1929. The Mediterranean fruit fly. Los Angeles, CA: California Fruit Growers Exchange [Google Scholar]
- 16.Gevrey M, Worner SP. 2006. Prediction of global distribution of insect pest species in relation to climate by using an ecological informatics method. J. Econ. Entomol. 99, 979–986 (doi:10.1603/0022-0493-99.3.979) [DOI] [PubMed] [Google Scholar]
- 17.Vera MT, Rodriguez R, Segura DF, Cladera JL, Sutherst RW. 2002. Potential geographic distribution of the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae), with emphasis on Argentina and Australia. Environ. Entomol. 31, 1009–1022 (doi:10.1603/0046-225X-31.6.1009) [Google Scholar]
- 18.DeMeyer M, Robertson MP, Peterson AT, Mansell MW. 2008. Ecological niches and potential geographic distributions of Mediterranean fruit fly (Ceratitis capitata) and natal fruit fly (Ceratitis rosa). J. Biogeogr. 35, 270–281 [Google Scholar]
- 19.Margosian ML, Bertone CA, et al. 2007. Identification of areas susceptible to the establishment of fifty-three Bactrocera spp. (Diptera: Tephrididae: Dacinae) in the United States. See http://www.aphis.usda.gov/plant_health/plant_pest_info/fruit_flies/downloads/bactrocera-susceptibility-analysis.pdf .
- 20.Carey JR. 1996. The future of the Mediterranean fruit fly population in California: a predictive framework. Biol. Conserv. 78, 35–50 (doi:10.1016/0006-3207(96)00016-X) [Google Scholar]
- 21.Carey JR. 2010. The Mediterranean fruit fly (Ceratitis capitata) invasion of California deepens: response to an alternative explanation for recurring outbreaks. Am. Entomol. 56, 158–163 [Google Scholar]
- 22.Carey JR. 1992. The medfly in California: response to letters to the editor. Science 255, 514–516 (doi:10.1126/science.255.5044.515-a) [DOI] [PubMed] [Google Scholar]
- 23.Mathys G, Baker EA. 1980. An appraisal of the effectiveness of quarantines. Annu. Rev. Phytopathol. 18, 85–101 (doi:10.1146/annurev.py.18.090180.000505) [Google Scholar]
- 24.Anonymous 2009. The measure of California agriculture in University of California Agricultural Issues Center. Oakland, CA: University of California [Google Scholar]
- 25.Carey JR. 1991. Establishment of the Mediterranean fruit fly in California. Science 253, 1369–1373 (doi:10.1126/science.1896848) [DOI] [PubMed] [Google Scholar]
- 26.Jackson DS, Lee BG. 1985. Medfly in California 1980–1982. Bull. Entomol. Soc. Am. 31, 29–37 [Google Scholar]
- 27.Liebhold AM, Work TT, McCullough DG, Cavey JF. 2006. Airline baggage as a pathway for alien insect species invading the United States. Am. Entomol. 52, 48–54 [Google Scholar]
- 28.McCullough DG, Work TT, Cavey JF, Liebhold AM, Marshall D. 2006. Interceptions of nonindigenous plant pests at US ports of entry and border crossings over a 17-year period. Biol. Invas. 8, 611–630 (doi:10.1007/s10530-005-1798-4) [Google Scholar]
- 29.Voss HJ. 1992. The medfly in California: letter to the editor. Science 255, 514–515 (doi:10.1126/science.1736350) [DOI] [PubMed] [Google Scholar]
- 30.Carey JR. 1996. The incipient Mediterranean fruit fly population in California: implications for invasion biology. Ecology 77, 1690–1697 (doi:10.2307/2265775) [Google Scholar]
- 31.Sequeira R, Millar L, Bartels D. 2001. Identification of areas susceptible to the establishment of Anastrepha spp. fruit flies in the United States and analysis of selected pathways. See http://www.aphis.usda.gov/plant_health/plant_pest_info/fruit_flies/downloads/isa.pdf [Google Scholar]
- 32.Shehata NF, Younes MWF, Mahmoud YA. 2008. Biological studies on the peach fruit fly, Bactrocera zonata (Saunders) in Egypt. J. Appl. Sci. Res. 4, 1103–1106 [Google Scholar]
- 33.Meixner MD, McPheron BA, Silva JG, Gasparich GE, Sheppard WS. 2002. The Mediterranean fruit fly in California: evidence for multiple introductions and persistent populations based on microsatellite and mitochondrial DNA variability. Mol. Ecol. 11, 891–899 (doi:10.1046/j.1365-294X.2002.01488.x) [DOI] [PubMed] [Google Scholar]
- 34.Bonizzoni M, Katsoyannos BI, Marguerie R, Guglielmino CR, Gasperi G, Malacrida R, Chapman T. 2002. Microsatellite analysis reveals remating by wild Mediterranean fruit fly females, Ceratitis capitata. Mol. Ecol. 11, 1915–1921 (doi:10.1046/j.1365-294X.2002.01602.x) [DOI] [PubMed] [Google Scholar]
- 35.Williamson MH, Fitter A. 1996. The varying success of invaders. Ecology 77, 1661–1666 (doi:10.2307/2265769) [Google Scholar]
- 36.Bonizzoni M, Zheng L, Guglielmino CR, Haymer DS, Gasperi G, Homulski LM, Malacrida AR. 2001. Microsatellite analysis of medfly bioinfestations in California. Mol. Ecol. 10, 2515–2524 (doi:10.1046/j.0962-1083.2001.01376.x) [DOI] [PubMed] [Google Scholar]
- 37.Davies N, Villablanca FX, Roderick GK. 1999. Bioinvasions of the medfly Ceratitis capitata: source estimation using DNA sequences at multiple intron loci. Genetics 153, 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malacrida AR, Gomulski LM, Bonizzoni M, Bertin S, Gasperi G, Gulielmino CR. 2007. Globalization and fruitfly invasion and expansion: The medfly paradigm. Genetica 131, 1–9 (doi:10.1007/s10709-006-9117-2) [DOI] [PubMed] [Google Scholar]
- 39.Caley P, Groves RH, Barker R. 2008. Estimating the invasion success of introduced plants. Divers. Distrib. 14, 196–203 (doi:10.1111/j.1472-4642.2007.00440.x) [Google Scholar]
- 40.Crooks JA. 2005. Lag times and exotic species: the ecology and management of biological invasions in slow-motion. Ecoscience 12, 316–329 (doi:10.2980/i1195-6860-12-3-316.1) [Google Scholar]
- 41.Blackburn TM, Pysek P, Bacher S, Carlton JT, Duncan RP, Jarsik V, WIlson JRU, RIchardson DM. 2011. A proposed unified framework for biological invasions. Trends Ecol. Evol. 26, 333–339 (doi:10.1016/j.tree.2011.03.023) [DOI] [PubMed] [Google Scholar]
- 42.Hill JK, Griffiths HM, Thomas CD. 2011. Climate change and evolutionary adaptations at species’ range margins. Annu. Rev. Entomol. 56, 143–159 (doi:10.1146/annurev-ento-120709-144746) [DOI] [PubMed] [Google Scholar]
- 43.Johannesen J, Keyghobadi N, Schuler H, Stauffer C, Vogt H. 2013. Invasion genetics of American cherry fruit fly in Europe and signals of hybridization with the European cherry fruit fly. Entomol. Exp. Appl. 147, 61–72 (doi:10.1111/eea.12041) [Google Scholar]
- 44.Jacquard C, Virgilio M, David P, Quilici S, DeMeyer M, Delatte H. 2013. Population structure of the melon fly, Bactrocera cucurbitae, in Reunion Island. Biol. Invas. 15, 759–773 (doi:10.1007/s10530-012-0324-8) [Google Scholar]
- 45.Crooks JA. 2011. Lag times. In Encyclopedia of biological invasions (eds Simberloff D, Rejmanek M.), pp. 404–410 Berkeley, CA: University of California Press [Google Scholar]
- 46.Crooks JA, Soule ME. 1999. Lag times in population explosions of invasive species: Causes and implications. In Invasive species and biodiversity management (eds Sanderlund T, et al.), pp. 103–125 Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- 47.Siebert JB, Cooper T. 1995. Embargo on California produce would cause revenue, job loss. Calif. Agric. 49, 7–12 (doi:10.3733/ca.v049n04p7) [Google Scholar]
- 48.Devorshak C. 2007. Area-wide integrated pest management programmes and agricultural trade: Challenges and opportunities for regulatory plant protection. In Area-wide control of insect pests (eds Vreysen MJB, Robinson AS, Hendrichs J.), pp. 407–415 Dordrecht, The Netherlands: Springer [Google Scholar]
- 49.Follett PA, Neven LG. 2006. Current trends in quarantine entomology. Annu. Rev. Entomol. 51, 359–385 (doi:10.1146/annurev.ento.49.061802.123314) [DOI] [PubMed] [Google Scholar]
- 50.Westbrooks RG, Eplee RE. 2011. Early detection and rapid response. In Encyclopedia of biological invasions (eds Simberloff D, Rejmanek M.), pp. 169–177 Berkeley, CA: University of California Press [Google Scholar]
- 51.USDA/APHIS 2011. Exotic fruit fly strategic plan FY 2011–2015. Riverdale, MD: US Department of Agriculture [Google Scholar]
- 52.Pluess T, Cannon R, Jarosik V, Pergl J, Pysek P, Bacher S. 2012. When are eradication campaigns successful? A test of common assumptions. Biol. Invas. 14, 1365–1378 (doi:10.1007/s10530-011-0160-2) [Google Scholar]
- 53.Pysek P, Richardson DM. 2010. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Res. 35, 25–55 (doi:10.1146/annurev-environ-033009-095548) [Google Scholar]
- 54.Willyard C. 2012. Playing detective. Nature 491, S64–S65 (doi:10.1038/491S64a) [DOI] [PubMed] [Google Scholar]
- 55.Díaz-Fleischer F, Arredondo J, Flores S, Montoya P, Aluja M. 2009. There is no magic fruit fly trap: Multiple biological factors influence the response of adult Anastrepha ludens and Anastrepha obliqua (Diptera: Tephritidae) individuals to multilure traps baited with BioLure or NuLure. J. Econ. Entomol. 102, 86–94 (doi:10.1603/029.102.0113). [DOI] [PubMed] [Google Scholar]
- 56.Groves RH. 2006. Are some weeds sleeping? Some concepts and reasons. Euphytica 148, 111–120 (doi:10.1007/s10681-006-5945-5) [Google Scholar]
- 57.Virgilio M, DeLatte H, Backeljau T, DeMeyer M. 2010. Macrogeographic population structuring in the cosmopolitan agricultural pest Bactrocera cucurbitae (Diptera: Tephritidae). Mol. Ecol. 19, 2713–2724 (doi:10.1111/j.1365-294X.2010.04662.x) [DOI] [PubMed] [Google Scholar]
- 58.Lee CE. 2002. Evolutionary genetics of invasive species. Trends Ecol. Evol. 17, 386–391 (doi:10.1016/S0169-5347(02)02554-5) [Google Scholar]
- 59.Glenday C. 2009. Guinness world records. New York City, NY: Random House [Google Scholar]
- 60.Wikipedia. 2012. Mexico–United States border. See http://en.wikipedia.org/wiki/U.S.-Mexico_border .
- 61.Grosholz ED. 1996. Contrasting rates of spread for introduced species in terrestrial and marine systems. Ecology 77, 1680–1686 (doi:10.2307/2265773) [Google Scholar]
- 62.Floyd J, Lee BG, Schall R, Wade D.2002. Emergency programs manual. Washington, DC: United States Department of Agriculture, Surveillance and Emergency Programs, APHIS, PPQ.
- 63.IAEA 2003. Trapping guidelines for area-wide fruit fly programmes. Vienna, Austria: Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture [Google Scholar]
- 64.Aylward B, Hennessey KA, Zagaria N, Olive J-M, Chochi S. 2000. When is a disease eradicable? 100 years of lessons learned. Am. J. Public Health 90, 1515–1520 (doi:10.2105/AJPH.90.10.1515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Molyneux DH, Hopkins DR, Zagaria N. 2004. Disease eradication, elimination and control: the need for accurate and consistent usage. Trends. Parasitol. 20, 347–351 (doi:10.1016/j.pt.2004.06.004) [DOI] [PubMed] [Google Scholar]
- 66.Stepan NL. 2011. Eradication. Ithaca, NY: Cornell University Press [Google Scholar]
- 67.Williamson MH, Fitter A. 1996. The characteristics of successful invaders. Biol. Conserv. 78, 163–170 (doi:10.1016/0006-3207(96)00025-0) [Google Scholar]