Abstract
Digit length ratios, especially the second-to-fourth digit ratio (2D : 4D), are associated with various pathological and behavioural conditions in many species including humans and are dependent upon prenatal androgen to oestrogen balance. It is unknown whether digit ratios are modified by environmental exposure to ubiquitous endocrine disruptors. We studied the effect on adult male Wistar rat digit ratios of a gestational exposure to the oestrogenic and antiandrogenic compounds bisphenol A (BPA), genistein and vinclozolin, in low doses, and in combination with investigating in parallel a possible sexual dimorphism of this trait. We also investigated the effects on the male progeny not exposed during gestation. X-rays were taken of the left and right forepaws, and 2D–5D proximal to distal phalanx distances were measured by a standardized procedure based on semi-automatic image analysis. We provide evidence that there is a sexual dimorphism of digit ratios in the Wistar rat, and we found that BPA alone or in combination with genistein and vinclozolin significantly feminized digit ratios in male rats. Intriguingly, significant feminization of digit ratios was also found in the unexposed male progeny of males that had been exposed to compound mixtures. In conclusion, prenatal environmental levels of endocrine-active substances permanently disrupt digit ratios. Digit ratio measurement in adults is thus a promising biomarker of prenatal exposure to low-dose endocrine disruptors in rodents, with potential implications for future studies in humans.
Keywords: 2D : 4D, bisphenol A, genistein, vinclozolin, endocrine-active substance, endocrine disruptor
1. Introduction
In humans and several other mammalian species, digit length and digit length ratio are sexually dimorphic [1] and thought to be correlated with prenatal levels of testosterone (PT) and oestrogen (PE) [2]. In humans, second-to-fourth digit length ratio (2D : 4D) is associated with many sexually dimorphic traits and various health conditions [3,4] including susceptibility to prostate cancer [5] and male reproductive health [6,7]. Males have a longer 4D and a significantly lower 2D : 4D on the right hand than females and this difference persists into adulthood (see [8] for review). The 2D : 4D is also lower in males than in females in several other mammals and non-mammalian species [9,10]. The developmental links between 2D : 4D, and PT and PE remained unclear until Zheng & Cohn [11] elegantly demonstrated the hormonal and molecular mechanisms linking PT and PE with mouse digit development. They showed that the developing digits, including the fourth digit in particular, contained abundant androgen receptors (ARs) and oestrogen receptors (ERs). Either by activating AR and ER or using pharmacological doses of the receptor agonists dihydrotestosterone and oestradiol, they showed that 2D : 4D was determined by the balance of PT and PE in the fourth digit (AR increases and ER decreases chondrocyte proliferation in the fourth digit). They also showed that postnatal exposure to receptor antagonists and hormones does not influence 2D : 4D. Thus, 2D : 4D is determined not by PT alone, but also by the balance of PT to PE signalling in a narrow time window of fetal digit development.
In this study on male rats, we tested the hypothesis that low (environmental)-dose exposure to oestrogenic ‘feminizing’ and/or antiandrogenic ‘demasculinizing’ endocrine-active substances (EASs) in utero affects digit lengths and ratios. Exposure to oestrogenic and antiandrogenic EASs, even in low doses, during the prenatal period, causes a wide spectrum of developmental, pathological and behavioural effects in the male [12,13]. If our hypothesis is correct, digit length measurement, as the anogenital distance (AGD) or anogenital index [14], is a potentially useful indicator of prenatal endocrine disruption. We used a previously described rat model [15] to investigate the possible impact on adult digit lengths of three EASs that may be associated in humans. This work is part of a larger study with the general aim of assessing the effects of chronic exposure to low-dose EASs mixtures on various organs, tissues and behaviours, in the exposed males of the F1 generation and the unexposed F2 generation sired by these rats. We previously reported the impact of low doses of the phytoestrogen genistein and the antiandrogenic fungicide vinclozolin and the additive effects of their combination on the male genital tract and fertility [15].
In this study, we investigated the effects on the digit ratios of exposed male rats and their unexposed progeny of prenatal exposure to low environmental doses of bisphenol A (BPA) alone or in combination with low-dose genistein and vinclozolin. These three compounds are known for oestrogenic and/or antiandrogenic activities and may be found in human diets. We selected BPA because it is a well-known ubiquitous oestrogenic chemical [16] used in the manufacture of polycarbonate plastics and epoxy resins for use in plastic food containers and can linings, for example [17]. We found that BPA alone or in combination with genistein and vinclozolin significantly feminized digit length ratios, particularly for right forepaw 2D : 4D. Intriguingly, significant feminization of 2D : 4D was also found in the unexposed progeny. We also comparatively assessed the AGD—a confirmed sexually dimorphic marker in rodents that is disrupted during the perinatal period by sex steroids as well as endocrine disruptor exposure (at non-environmental levels) [18]—because it represents the reference endpoint routinely measured in reproductive toxicology as well as in tests for regulatory purposes, and we found neither differences in AGD when comparing exposed and control rats or an association between AGD and digit ratios.
2. Material and methods
(a). Chemicals
BPA (greater than or equal to 99% purity) was purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France). Genistein (greater than or equal to 99% purity) and vinclozolin (greater than or equal to 95% purity) were obtained as previously described [15].
(b). Choice of the doses used
One aim of the study is to assess the impact of low doses of the compounds selected, similar to those actually present in the environment. The dose of BPA selected, 5 µg kg−1 d−1 (i.e. an order of magnitude below the no observed adverse effect level (NOAEL)), approached human environmental exposure levels, generally thought to be less than 1 µg kg−1 d−1 [19]. We used a dose of 1 mg (1000 µg) kg−1 d−1 for genistein as this level is similar to that present in soya-based diets [20] and has been reported to cause reproductive disorders in male rats [15]. We selected two doses of vinclozolin, 1 mg (1000 µg) kg−1 d−1, corresponding to the reference low dose in our previous fertility study [15] and considered as the ‘high’ dose here, and a second notably lower dose, 10 µg kg−1 d−1, representing an intermediary dose between the United States Environmental Protection Agency NOAEL (1.2 mg kg−1 d−1, based on a combination of chronic toxicity, carcinogenicity and toxicity reproduction in rats [21]) and the level of human food contamination with vinclozolin residues (an estimated daily intake of 3.3 µg kg−1 in France [22]), twice the acceptable daily intake of 0.005 mg kg−1 d−1 according to the European Food Safety Authority [23]. The mixtures studied here were selected for the objectives of the main experimental multi-disciplinary programme and not for our particular study, taking into account the results of a previous study with the combined compounds genistein and vinclozolin, from a descriptive point of view rather than from a mechanistic point of view: to date, the assumption of additivity or synergy can only be made for non-environmental doses of EASs having the same (or univocal) mode of action [24] which was not the case of our experimental conditions. We thus investigated six exposure groups: BPA alone (5 µg kg−1 d−1), a genistein/vinclozolin mixture (1000 µg kg−1 d−1 + 1000 µg kg−1 d−1) in accordance with our previous fertility study [15], a second more realistic genistein/vinclozolin mixture with the ‘low’ dose of vinclozolin (1000 µg kg−1 d−1 genistein + 10 µg kg−1 d−1 vinclozolin) and the following mixtures incorporating BPA: 5 µg kg−1 d−1 BPA + 1000 µg kg−1 d−1 genistein, 5 µg kg−1 d−1 BPA + 10 µg kg−1 d−1 vinclozolin and 5 µg kg−1 d−1 BPA + 1000 µg kg−1 d−1 genistein + 10 µg kg−1 d−1 vinclozolin. The control group was exposed to vehicle alone (see below).
(c). Animals and regimens
All animals were treated humanely and suffering was minimized. They were maintained in accordance with the French Ministry of Agriculture guidelines for the care and use of laboratory animals. We purchased 100 specific pathogen-free (SPF) female and 100 SPF male Wistar Han rats, at the age of five weeks, from Harlan France Sarl (Gannat, France). They were acclimatized to the animal facility conditions (22°C with constant humidity and a 12 L : 12 D period) for nine weeks before mating. Polypropylene cages and water bottles were used to prevent BPA or phthalate contamination. Aspen sawdust was used as bedding, to prevent exposure to endocrine-like chemical residues. During the acclimatization period, animals were fed a phytoestrogen-free diet (INRA, Jouy-en-Josas, France) and supplied with charcoal-filtered water ad libitum.
(d). Animal husbandry and exposure conditions
At the age of 14 weeks, females and males were allowed to mate (one female and one male per cage) for a maximum of 5 days. Females were examined daily. The presence of spermatozoa in vaginal smears or a plug was used to identify the first day of gestation. Groups of 12–14 gravid females were caged separately and randomized to seven groups corresponding to control and exposure groups (see below). At parturition, the litters were sexed and standardized to 10 offspring (five males and five females per litter). Animals were randomized, the experimental allocation of each animal depending on the general experimental design of the main study which included earlier time points (pre-pubertal and post-pubertal sacrifices) and study of different organs and tissues or behavioural traits on other males than those used for digit ratio measurements. The sample size of 20 adult males per exposure group (1–2 per litter, see below, analysis section) was selected especially according to previous experiments (e.g. [15]) owing to the variability associated with different endpoints studied in the main study (digit length ratio is an endpoint among others) and the chance to detect effects, also considering that the study was based on low-exposure doses whose phenotypic effects are not predictable/obvious a priori. At weaning (postnatal day 21 (PND 21)), 20 male offspring per group were caged separately (four per cage), fed the same diet and water supplied ad libitum until PND 100. From PND 100, exposed F1 males were mated with 140 unexposed females (one female and one male per cage) purchased from Harlan France Sarl (Gannat, France) and acclimatized to the animal facility conditions for nine weeks before mating. The F2 generation was produced in a similar manner and the same randomization method was used. Every 4 days, all animals were weighed and inspected for anomalies. Dams, F1 and F2 adult rats (both at PND110) were anaesthetized with isoflurane (2.5%) and killed by exsanguination. The right and left forepaws of the F1 and F2 male rats were sectioned, frozen and stored at −20°C until measurement (see below). In addition, right and left forepaws of 20 adult control (unexposed) females and 20 adult control (unexposed) males with the same husbandry conditions were also sectioned and stored at −20°C with the aim of assessing a possible sexual dimorphism of digit length ratios in the Wistar rat. In the main protocol of the study, dams (F0) received BPA or mixtures orally from the first day of gestation until weaning. The F1 rats were treated every 2 days until death. BPA or the selected chemical mixtures were dissolved in corn oil (Lesieur, Asnières, France) and administered (in 0.4 ml kg−1 solutions) via a micropipette. Photolysis and oxidation were prevented by storing BPA and mixture solutions at 4°C, in aluminium foil-wrapped vials. Control animals received vehicle alone. The F2 rats were not exposed to the test compounds.
(e). Image capture and digit length measurement
Digit length was determined by objective measurements of the phalanxes on forepaw X-rays. We obtained 560 digitized 2D X-rays (seven exposure groups × 20 animals per group × two forepaws × two generations). The X-ray source was a Kodak WCYA078 N° 2200 generator (Carestream Health, Inc. Croissy-Beaubourg, France) delivering a pulsed beam of 400 kHz (high-voltage current of 7 mA, high voltage of 60 kV, total filtration, 2.5 mm aluminium), these settings maximizing radiographic contrast. The tungsten focal spot was 0.7 × 0.7 mm. The optimal sensor–object distance was 380 mm. Each forepaw was firmly fixed to the sensor with adhesive tape across the digits, which were kept as elongated as possible. Consequently, the plane of the digits in contact with the sensor was parallel to the plane of the sensor, eliminating possible distortions that might bias the measurements. The X-ray sensor was a photostimulable phosphor (phosphor plate) 57 × 76 mm Dürr Dental sensor (Dürr Dental GmbH & Co. KG, Bietigheim-Bissingen, Germany). The associated reading device and software were a VistaScan Perio no. X003602 and the DBSWIN, v. 5.2.0 software (both from Dürr Dental GmbH & Co. KG, Bietigheim-Bissingen), respectively. This configuration made it possible to obtain 2473 × 3123 pixels—1069 pixels/inch final images with a 20 pl mm−1 resolution (for 5% contrast transfer), 12-bit-encoded in Tiff format. We checked that exposure was optimal with a Solidose 400 no. 4041 electronic dosimeter and an R100 Code no. 02465 8 detector (RTi Electronics AB, Mölndal, Sweden) suitable for X-ray energies. The optimal dose per image was 800 µGray. A set of lead numbers corresponding to the code numbers of the animals was used to facilitate identification. Finally, dimensional calibration was carried out for each image, by the simultaneous X-ray of the forepaws and a 0.03 mm-thick micrometre lead test pattern (Type 25 no. 72319, Nuclear Associates, Fluke Biomedical, Cleveland, OH, USA). Digit lengths were measured for all F1 and F2 male rats with ImageJ software (http://rsb.info.nih.gov.gate2.inist.fr/ij/) from the Tiff image files of their left and right forepaws projected onto a computer screen. We first manually measured the calibration test pattern on each X-ray to determine the pixel mm−1 ratio with ImageJ. This made it possible to express all measurements in millimetres. Digit length was defined as the sum of the lengths of the first, second and third phalanxes plus the interarticular distances, because complete extension of all digits for the measurement of a single segment per digit was not always possible. Broken lines were traced on the monitor and the broken line lengths, in pixels, were converted in mm to estimate the length of each digit, 2D, 3D, 4D and 5D. The broken line corresponding to the digit length consisted of three successive segments based on anatomical landmarks, giving standardized measurements along the axes of the third to the first phalanx of each digit. The first segment of the broken line was the distance from the apex of the third phalanx to the mid-interarticular point between the third and the second phalanxes. The second segment was the distance between the interarticular point of the third and second phalanxes and that of the second and first phalanxes. The third segment was the distance between the interarticular point of the second and first phalanxes and the mid-point of the segment joining the two promontories of the proximal phalanx. Measurement variation was minimized by having a single investigator making all measurements. We assessed intra-investigator reproducibility for the determination of digit lengths and related digit length ratios from two measurements at different periods. Digit length measurements did not differ significantly (−0.017 ± 0.20 mm for 56 pairs of right or left 2D or 4D in the various exposure groups; p = 0.37) and were highly correlated (Spearman r-coefficient = 0.91, p < 0.0001), whereas the corresponding digit ratios tended to differ (mean difference = 0.0099 ± 0.024; p = 0.06) despite strong correlation between paired values (Spearman r-coefficient = 0.65, p = 0.0002). All measurements were therefore made in duplicate, at different times, by the same person (J.A.) blind to the exposure conditions, and the mean value was used for finger length statistics and to determine 2D : 3D, 2D : 4D, 2D : 5D, 3D : 4D, 3D : 5D, 4D : 5D digit length ratios and the corresponding statistics.
(f). Anogenital distance assessment
We jointly assessed the AGD, a confirmed sexually dimorphic marker in rodents that is disrupted during the perinatal period by sex steroids as well as EAS exposure (at non-environmental levels) [18]. AGD was defined as the distance between the base of the genital papilla and the rostral end of the anal opening [25]. At PND 21, the anogenital area of the animals was photographed with a digital camera (Nikon Coolpix 990, Tokyo, Japan) fixed to a support, the rats being immobilized with a rodent restrainer (Harvard Apparatus, Les Ulis, France). AGD was measured by a person (R.B.) blind to the exposure conditions of the animals, with Optimas image processing software (Media Cybernetics, USA). A pilot study on 10 males and 10 females at PND21 indicated that measurements from photographs were significantly correlated with direct measurements with callipers (r = 0.85, p = 0.002). Results are expressed as relative AGD, calculated as AGD/body weight.
(g). Analysis
Because control and exposure groups were composed of balanced subsets of single or two animals per litter, all measurements from pairs of animals of the same litter were averaged and litter score rather than individual scores were subsequently used as an experimental unit allowing standard statistical methods to be used. All statistical comparisons of digit lengths and digit ratios between exposure groups were carried out by an analysis of variance (ANOVA). When the null hypothesis was rejected, post hoc Tukey tests were used for pairwise comparisons between different exposures (as shown in the tables). We show a box plot illustrating the distributions observed for each exposure group and, therefore, the true level of variability. For visual clarity, we present only the significant differences between exposures to compounds or mixtures and controls using the Mann–Whitney non-parametric test. Values of p < 0.05 were considered significant. However, where indicated, we used a threshold of 0.10, owing to the small size of the groups. Possible associations between digit length ratios and AGDs were assessed by Spearman's rank correlation test. All data analysis and statistics were performed using BMDP statistical software (Statistical Solutions, Cork, Ireland).
3. Results
There were sex differences for several digit ratios (see the electronic supplementary material, table S1), for example left and right 2D : 4D were significantly higher in female control rats compared with male control rats: 0.927 ± 0.025 versus 0.897 ± 0.026, p = 0.002 and, 0.918 ± 0.027 versus 0.879 ± 0.023, p = 0.0001, respectively. Examples of X-ray pictures of the right forepaw with projected anatomical landmarks and corresponding 2D : 4D digit length ratios for a typical control male, a typical control female and a typical male exposed to BPA are presented in figure 1.
Figure 1.
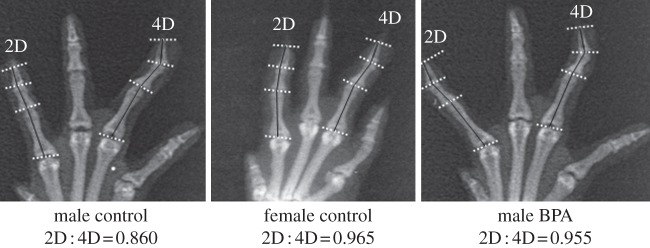
Examples of X-ray pictures of the right forepaw with projected anatomical landmarks and corresponding 2D : 4D digit length ratios (calculated from duplicates of digit length measurements using the image analysis software) for a typical control male, a typical control female and a typical male exposed to BPA.
Overall, F1 and F2 male adult digit lengths for the left forepaw did not differ significantly between the exposed and control groups. By contrast, for the right forepaw, 3D and 5D were significantly shorter in the male F2, and 4D was significantly shorter in both F1 and F2 exposed male rats than those in the controls (see the electronic supplementary material, table S2).
Exposure to the test compounds and their mixtures significantly modified left 2D : 3D and 3D : 4D, and significantly increased right 2D : 4D and 3D : 4D in the male F1 (see the electronic supplementary material, table S3). In the unexposed male F2 sired from exposed fathers, a number of digit ratios was significantly modified in comparison with controls (see the electronic supplementary material, table S4). Right 2D : 4D was significantly higher in all exposed groups except the ‘high’-dose vinclozolin/genistein in the male F1 (figure 2a). In the unexposed male F2, right 2D : 4D was also significantly increased when fathers were exposed to the vinclozolin/BPA, genistein/BPA, genistein/vinclozolin/BPA and ‘high’-dose vinclozolin/genistein mixtures (figure 2b).
Figure 2.
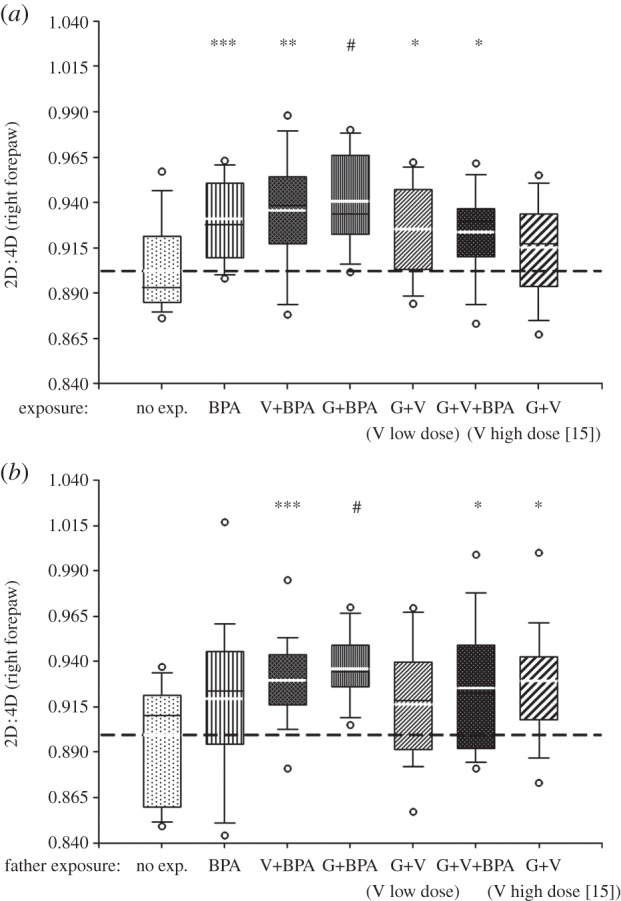
Box plot displaying the 10th, 25th, 50th, 75th and 90th percentile values, the extreme 5th and 95th percentiles (circles) and the mean value (thick white line) for right forepaw 2D : 4D in (a) the F1 males exposed to the compounds and (b) in their unexposed male offspring (F2) carried by unexposed dams. Mann–Whitney non-parametric comparisons (#p < 0.001, ***p < 0.005, **p < 0.01, *p < 0.05) versus controls (abbreviations for both the F1 and F2 and doses for the F1: BPA, bisphenol A, 5 µg kg−1 d−1; V, vinclozolin, 10 µg kg−1 d−1, ‘low’ or 1000 µg kg−1 d−1 ‘high’ and G, genistein, 1000 µg kg−1 d−1, see [15]).
Relative AGD did not differ significantly between the seven groups tested, in the male F1 or F2 (F = 1.18, p = 0.32 and F = 0.82, p = 0.55, respectively) despite mean values being lower in all exposure groups than in controls (data not shown). Overall, relative AGDs were not associated with digit ratios in the F1 or F2 (data not shown) in all studied animals or in the controls only (for the 2D : 4D digit ratio, see the electronic supplementary material, table S5).
4. Discussion
To our knowledge, our study is the first showing sex differences of digit length ratios in the Wistar rat. Sexual differences of digit ratios have been inconstantly found in rats or various mouse strains [9,26,27], and generally it remains to be determined how the accuracy and precision of measurement as well as the number of animals studied (owing to notable interindividual variations in digit length) may impact the conclusions. We believe that the method we used here (X-ray combined with image analysis) on a relatively important number of rats was a valuable asset to highlight subtle differences in size. We show here that prenatal exposure to environmental levels of BPA, alone or with low doses of genistein or vinclozolin, significantly modifies male offspring digit lengths, resulting in a feminized 2D : 4D. Similar effects were found in the next, unexposed male generation sired by exposed fathers with unexposed mothers.
Interestingly, the magnitude of digit ratio values in male rats exposed to xenohormones was similar to the values measured in control females. Our results, particularly for 2D : 4D are consistent with those of Zheng & Cohn [11], because the EASs studied are known to be oestrogenic or antiandrogenic compounds and may counteract physiological steroid action in males. Zheng and Cohn showed that 4D length modulation by sex hormones in mice may be limited to specific phalanxes according to the ligands used, with decreases in androgen or increases in oestrogen activity decreasing phalanx length. We measured the entire length of the digit and could not determine whether a particular phalanx was responsible for the differences in 2D : 4D and other ratios. Nevertheless, our results suggest indirectly that the developmental basis of 2D : 4D should be similar in mice, rats and humans [11,28]. Only two studies on digit length ratios in rats have been published, both indicating that testosterone supply or prenatal exposures modifying testosterone levels modulate forepaw digit length and digit ratios in prepubertal rats [26,27]. In humans and mice, various conditions are more frequently associated with right rather than left 2D : 4D [29,30]. Our results are consistent with these findings because we found a left–right asymmetry in both digit lengths and ratios for our EAS exposures, with a greater effect on the right forepaw than on the left. Our results showing differences in 2D : 4D and in several of the other five possible digit ratios are also consistent with previous studies, in which digit ratios other than 2D : 4D were found to differ as a function of sex or exposure conditions [27,31–33]. Further studies are required to determine whether the other digit ratios are mediated by mechanisms similar to those for 2D : 4D [11].
The 2D : 4D values obtained for each exposure group, including the controls, were markedly scattered (figure 2a,b), as reported for other endpoints in male Wistar rats exposed to a low-dose EAS alone or an EASs mixture [15]. Thus, factors other than EAS exposure presumably contribute to overall variability. It has been suggested that the considerably less pronounced sexual dimorphism of 2D : 4D in mice than in humans may reflect hormone transfer between male and female fetuses in rodent uterine horns [34], because the degree of exposure to sex steroids during sexual differentiation varies naturally in rodents, owing to the proximity within the uterus of fetuses of both sexes [35]. We have no reason to think that this confounding effect affects any one group more than another.
As digit lengths were measured in young adults, postnatal factors/exposures may play a role in determining final digit length. However, 2D : 4D are fixed by the age of 2 years in humans [36], and Zheng & Cohn [11] showed that postnatal modulation of androgen or oestrogen signalling does not affect digit ratio. Moreover, the finding of digit ratio differences between both the F1 (continuously exposed from conception to adulthood) and F2 (unexposed) young adults and controls suggests that the digit ratio differences observed in adults largely reflect prenatal rather than ‘postnatal’ exposure. We cannot rule out a role for other unknown factors (for example, a ‘contamination’ factor related to the husbandry conditions, which however should affect all exposed or unexposed animals), but the ‘EAS exposure factor’ appears to be the major factor determining 2D : 4D here, even in the unexposed F2 rats sired by exposed males.
Zheng & Cohn [11] were the first to show that the action of an AR antagonist, flutamide, in males, or an antioestrogen, fulvestrant, in females, displaced 2D : 4D towards a feminized ratio in males and a masculinized ratio in females. We used a similar approach, but with realistic, low ‘environmental’ doses of EAS alone or in combination. Genistein and BPA mostly bind ER alpha, whereas vinclozolin is an AR antagonist. As digit formation is partly controlled by oestrogen, ER alpha, testosterone and AR, our findings indicate that developing digits are a key target of oestrogen-like and antiandrogenic chemicals at low ‘environmental’ doses. Such properties appear to be sufficient to explain the effect on digit length ratios reported here. We observed no additive or synergistic effect of the mixtures including BPA over BPA alone, but digit lengths and ratios were generally modified slightly (generally an increase in 2D : 4D) in the presence of vinclozolin or genistein. These slight changes may result from complex modes of actions at low doses. They may also reflect the fact that the compounds selected do not have the single property of acting at the ER or AR. For example, genistein has been reported to exhibit antiandrogenic activity in vitro, in addition to its well-established oestrogenic activity [37] and has been identified as an alternative ligand of AR in vivo [38]. In a reporter gene assay, BPA was shown to act as an AR antagonist, with detectable activity [39] and BPA has been shown to affect multiple steps in AR activation and function [40]. Vinclozolin and its principal metabolites are generally considered to be potent antiandrogens but have also been shown to act as agonists of ER alpha and beta [41]. The EASs studied have a lower potency than natural ligands, but their ability to act via more than one mechanism must be taken into account when considering their combined effect, together with the absence of additive or synergistic effects for the low doses selected.
It has recently been reported that a mixture of BPA and genistein in vitro may disrupt the development of chondrocytes in micromass cultures of rat embryonic limb bud cells, with additive effects at lower concentrations and synergism at higher concentrations [42]. Similarly, a recent study indicated that in utero exposure to a more potent xenoestrogen, diethylstilbestrol, affects lumbar and femoral bone, giving a feminized phenotype in males [43]. These studies indicate that xenoestrogens, alone or in combination, in vitro and in utero, may have a detrimental sex-linked effect on the developing skeleton.
We jointly assessed the AGD—a confirmed sexually dimorphic marker in rodents that is disrupted during the perinatal period by sex steroids as well as endocrine disruptor exposure (at non-environmental levels) [18]—because it represents the reference endpoint routinely measured in reproductive toxicology as well as in tests for regulatory purposes. Therefore, we considered comparatively assessing both methods, because this may have implications in various domains such as clinical studies (it is obviously easier to measure digit length ratios than AGD in humans) or reproductive toxicology, with the particular question of the effect of low doses and mixtures of EASs. It was recently claimed in a review on AGD and digit ratio that the former classical approach in reproductive toxicology (where most studies on prenatal exposure to EASs involve high non-environmental doses) is superior to the latter, in either animal or human studies [44]. Our results based on realistic low-dose exposures to EASs is in contrast with this appraisal because we found differences in digit ratios but not in AGD according to the various exposure conditions. Thus, we believe that adding digit ratio measurements could be useful for reproductive toxicology or regulatory purposes. It should be kept in mind that because this trait is permanently fixed, it can be measured at any postnatal developmental period.
Our results—obtained with different experimental conditions and for different mammalian species—are consistent with those of Zheng & Cohn [11], Hurd et al. [45] and Manno [30]. Digit ratios are thought to be exclusively dependent on the ratio of prenatal testosterone and oestrogen levels, whereas AGD is determined by these hormones both prenatally and postnatally [46]. Fetal position in rodent uterine horns, as discussed above, may affect both AGD [45] and digit ratio. Our study is also different from previous studies in the continuous exposure of the pups to BPA or mixtures during the lactation period. Digit ratios were found to be fixed exclusively by prenatal exposure, whereas AGD was modulated by the compounds both during gestation and postnatal exposure of the pups (the transmission to pups to the three compounds tested via milk has been demonstrated; ([47,48], J. P. Cravedi 2005, unpublished data). In summary, digit ratios and AGDs in experimental studies involving continuous exposure to endocrine disruptors are different markers that may be jointly assessed for the detection of associations with other phenotypic changes. Overall, according to the questions raised concerning the exposure period, both biomarkers may be complementary and equally useful.
We found that digit ratios were also feminized in the second generation sired by fathers exposed to BPA or some mixtures. When mothers are exposed to a toxicant during pregnancy (F0), the developing embryo (F1) and the developing germline that will give rise to the F2 generation are also directly exposed to the compound [49]. This is particularly true if the F1 is continuously exposed, as in this study. Our findings for digit ratios in the F2 thus indicate that BPA, alone or in mixture with other EASs, may modify the epigenome of the F2 generation. Owing to the design of this study, which was limited to F1 exposed and F2 unexposed generations, we cannot confirm the existence of a true transgenerational effect [50,51]. However, abnormal spermatogenesis, reduced fertility, changes to the expression of ER alpha and Steroid Receptor Coactivator-1 in the brain have been reported to persist in male F2 and in completely unexposed male F3 animals after perinatal exposure to a low environmental dose of BPA [13,52]. Changes were observed even after exposure to the parent chemical had ceased. Such findings imply that the effects of perinatal BPA exposure may be vertically transmitted in the germline, affecting the reproductive function not only of the F1, but also of subsequent (F2 and F3) generations. Other abnormalities have been found in subsequent generations of unexposed animals resulting from a parental F1 generation perinatally exposed to low-dose BPA [53]. Thus, the proposed epigenetic mechanism may also apply to transgenerational abnormalities of digit development after initial perinatal exposure to low-dose BPA or the mixtures tested. Further investigations are required to determine the underlying molecular basis of the permanent effects.
Our findings and the results of other studies on digit lengths and ratios in laboratory rodents [9,11,26,27,45] show this endpoint to be a potentially useful indicator that may be associated with gestational testosterone levels, the testosterone to oestrogen ratio, or prenatal hormonal disruption of the uterine microenvironment. It may indicate hormonal disruptions in response to various types of exposure, e.g. prenatal exposure to high doses of alcohol [27]. Thanks to the similarity of the mechanisms underlying digit development during gestation in rodents and humans, this endpoint may constitute an interesting index for assessing various human exposure risks (particularly in the context of the developmental origins of health and disease, and the endocrine disruptors hypothesis). X-ray measurements are clearly more accurate and precise than direct or photographic methods. Owing to a number of confounding factors (discussed above) and the low doses used, it remains possible that direct or photographic methods would not have detected the differences reported here. Photographic measurements of 2D : 4D in field voles have been shown to have a higher measurement error and give consistently higher estimates of 2D : 4D than X-rays. These findings suggest that X-rays, owing to their greater accuracy, should be preferred to photographs for digit ratio measurements in rodents [54]. However, significant associations between digit ratios and various traits have been found with methods other than X-rays: measurements from photographs of sectioned paws stored in formalin [55], measurements from photographs of live animals [26] and direct measurements [9,28]. Thus, such non-invasive methods may be preferred over the invasive, labour-intensive and costly method based on X-rays of sectioned paws. Further studies are required to determine whether simpler methods of digit measurement can clearly demonstrate changes owing to low-dose endocrine disruption, as in this study, and detect associations between digit ratios and a number of other hormonally disrupted endpoints.
In summary, our results for the first generation provide further indirect evidence of the prenatal influence of oestrogens and testosterone on the digit formation, because oestrogenic and antiandrogenic compounds are found to modify digit lengths and digit length ratios. Digit ratio appears to be a relevant new indicator for studying the in utero hormonal factors affecting specific behaviours and disease predispositions in humans, in the context of real-life environmental exposure. Our data also suggest that measurements of digit ratio may be an interesting supplementary endpoint for the assessment of epigenetically modulated effects.
Acknowledgements
We thank the INRA (Institut National de la Recherche Agronomique) animal facility at Dijon, A. Vernon from the EA 2496 animal facility who carried out all the X-rays, A. Finet for the English revision of the manuscript and D. Vaiman for his statistical advice and constructive comments on the manuscript.
The experiments described in this work were approved by the Ethics Committee of the University of Burgundy (ref.: CIME 16-Dec-2009, no. A1909).
Data accessibility
F1 and F2 digit length and ratio input files are deposited in the Dryad Repository: http://dx.doi.org/10.5061/dryad.s2np7.
Funding statement
This project was supported by the French Programme on Endocrine Disruption (PNRPE; contract MEDD CV 05147).
References
- 1.Galis F, Ten Broek CM, Van Dongen S, Wijnaendts LC. 2010. Sexual dimorphism in the prenatal digit ratio (2D : 4D). Arch. Sex. Behav. 39, 57–62 (doi:10.1007/s10508-009-9485-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutchmaya S, Baron-Cohen S, Raggatt P, Knickmeyer R, Manning JT. 2004. 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Hum. Dev. 77, 23–28 (doi:10.1016/j.earlhumdev.2003.12.002) [DOI] [PubMed] [Google Scholar]
- 3.Manning JT, Bundred PE. 2000. The ratio of 2nd to 4th digit length: a new predictor of disease predisposition? Med. Hypotheses 54, 855–857 (doi:10.1054/mehy.1999.0000) [DOI] [PubMed] [Google Scholar]
- 4.Manning JT, Baron-Cohen S, Wheelwright S, Sanders G. 2001. The 2nd to 4th digit ratio and autism. Dev. Med. Child Neurol. 43, 160–164 (doi:10.1111/j.1469-8749.2001.tb00181.x) [PubMed] [Google Scholar]
- 5.Rahman AA, et al. 2011. Hand pattern indicates prostate cancer risk. Br. J. Cancer 104, 175–177 (doi:10.1038/sj.bjc.6605986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manning JT, Scutt D, Wilson J, Lewis-Jones DI. 1998. The ratio of 2nd to 4th digit length: a predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum. Reprod. 13, 3000–3004 (doi:10.1093/humrep/13.11.3000) [DOI] [PubMed] [Google Scholar]
- 7.Auger J, Eustache F. 2011. Second to fourth digit ratios, male genital development and reproductive health: a clinical study among fertile men and testis cancer patients. Int. J. Androl. 34, e49–e58 (doi:10.1111/j.1365-2605.2010.01124.x) [DOI] [PubMed] [Google Scholar]
- 8.Breedlove SM. 2010. Minireview: organizational hypothesis: instances of the fingerpost. Endocrinology 151, 4116–4122 (doi:10.1210/en.2010-0041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown WM, Finn CJ, Breedlove SM. 2002. Sexual dimorphism in digit-length ratios of laboratory mice. Anat. Rec. 267, 231–234 (doi:10.1002/ar.10108) [DOI] [PubMed] [Google Scholar]
- 10.Roney JR, Whitham JC, Leoni M, Bellem A, Wielebnowski N, Maestripieri D. 2004. Relative digit lengths and testosterone levels in Guinea baboons. Horm. Behav. 45, 285–290 (doi:10.1016/j.yhbeh.2003.12.008) [DOI] [PubMed] [Google Scholar]
- 11.Zheng Z, Cohn MJ. 2011. Developmental basis of sexually dimorphic digit ratios. Proc. Natl Acad. Sci. USA 108, 16 289–16 294 (doi:10.1073/pnas.1108312108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farabollini F, Porrini S, Dessi-Fulgherit F. 1999. Perinatal exposure to the estrogenic pollutant bisphenol A affects behavior in male and female rats. Pharmacol. Biochem. Behav. 64, 687–694 (doi:10.1016/S0091-3057(99)00136-7) [DOI] [PubMed] [Google Scholar]
- 13.Salian S, Doshi T, Vanage G. 2009. Perinatal exposure of rats to bisphenol A affects the fertility of male offspring. Life Sci. 85, 742–752 (doi:10.1016/j.lfs.2009.10.004) [DOI] [PubMed] [Google Scholar]
- 14.Hsieh MH, Breyer BN, Eisenberg ML, Baskin LS. 2008. Associations among hypospadias, cryptorchidism, anogenital distance, and endocrine disruption. Curr. Urol. Rep. 9, 137–142 (doi:10.1007/s11934-008-0025-0) [DOI] [PubMed] [Google Scholar]
- 15.Eustache F, Mondon F, Canivenc-Lavier MC, Lesaffre C, Fulla Y, Berges R, Cravedi JP, Vaiman D, Auger J. 2009. Chronic dietary exposure to a low-dose mixture of genistein and vinclozolin modifies the reproductive axis, testis transcriptome, and fertility. Environ. Health Perspect. 117, 1272–1279 (doi:10.1289/ehp.0800158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. 2008. Exposure of the US population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 116, 39–44 (doi:10.1289/ehp.10753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. 2007. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 24, 139–177 (doi:10.1016/j.reprotox.2007.07.010) [DOI] [PubMed] [Google Scholar]
- 18.Hotchkiss AK, Lambright CS, Ostby JS, Parks-Saldutti L, Vandenbergh JG, Gray LE., Jr 2007. Prenatal testosterone exposure permanently masculinizes anogenital distance, nipple development, and reproductive tract morphology in female Sprague-Dawley rats. Toxicol. Sci. 96, 335–345 (doi:10.1093/toxsci/kfm002) [DOI] [PubMed] [Google Scholar]
- 19.Lakind JS, Naiman DQ. 2011. Daily intake of bisphenol A and potential sources of exposure: 2005–2006 National Health and Nutrition Examination Survey. J. Expo. Sci. Environ. Epidemiol. 21, 272–279 (doi:10.1038/jes.2010.9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka K, Sasaki S, Murakami K, Okubo H, Takahashi Y, Miyake Y. 2008. Relationship between soy and isoflavone intake and periodontal disease: the Freshmen in Dietetic Courses Study II . BMC Pub. Health 8, 39 (doi:10.1186/1471-2458-8-39) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US EPA 2003. Fed reg. vinclozolin; notice of filing a pesticide petition to establish a tolerance for a certain pesticide chemical in or on food, pp. 14628–14635 See https://www.federalregister.gov/articles/2003/03/26/03-7246/
- 22.Leblanc JC, Malmauret L, Guérin T, Bordet F, Boursier B, Verger P. 2000. Estimation of the dietary intake of pesticide residues, lead, cadmium, arsenic and radionuclides in France. Food Addit. Contam. 17, 925–932 (doi:10.1080/026520300750038108) [DOI] [PubMed] [Google Scholar]
- 23.European Food Safety Authority 2008. Reasoned opinion of EFSA prepared by PRAPeR on MRLs of concern for the active substance vinclozolin. EFSA Sci. Rep. 166, 1–36 (doi:10.2903/j.efsa.2008.166r) [Google Scholar]
- 24.Hass U, Scholze M, Christiansen S, Dalgaard M, Vinggaard AM, Axelstad M, Metzdorff SB, Kortenkamp A. 2007. Combined exposure to anti-androgens exacerbates disruption of sexual differentiation in the rat. Environ. Health Perspect. 115(Suppl. 1), 122–128 (doi:10.1289/ehp.9360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marois G. 1968. Action of progesterone, testosterone and estradiol on the anogenital distance and somatic sexual differentiation in rats. Biol. Med. 57, 44–90 [PubMed] [Google Scholar]
- 26.Talarovicová A, Krsková L, Blazeková J. 2009. Testosterone enhancement during pregnancy influences the 2D : 4D ratio and open field motor activity of rat siblings in adulthood. Horm. Behav. 55, 235–239 (doi:10.1016/j.yhbeh.2008.10.010) [DOI] [PubMed] [Google Scholar]
- 27.McMechan AP, O'Leary-Moore SK, Morrison SD, Hannigan JH. 2004. Effects of prenatal alcohol exposure on forepaw digit length and digit ratios in rats. Dev. Psychobiol. 45, 251–258 (doi:10.1002/dev.20035) [DOI] [PubMed] [Google Scholar]
- 28.Manning JT. 2011. Resolving the role of prenatal sex steroids in the development of digit ratio. Proc. Natl Acad. Sci. USA 108, 16 143–16 144 (doi:10.1073/pnas.1108312108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manning JT, Fink B. 2008. Digit ratio (2D : 4D), dominance, reproductive success, asymmetry, and sociosexuality in the BBC Internet Study. Am. J. Hum. Biol. 20, 451–461 (doi:10.1002/ajhb.20767) [DOI] [PubMed] [Google Scholar]
- 30.Manno FA. 2008. Measurement of the digit lengths and the anogenital distance in mice. Physiol. Behav. 93, 364–368 (doi:10.1016/j.physbeh.2007.09.011) [DOI] [PubMed] [Google Scholar]
- 31.McFadden D, Shubel E. 2002. Relative lengths of fingers and toes in human males and females. Horm. Behav. 42, 492–500 (doi:10.1006/hbeh.2002.1833) [DOI] [PubMed] [Google Scholar]
- 32.Kyriakidis I, Papaioannidou P. 2008. Epidemiologic study of the sexually dimorphic second to fourth digit ratio (2D : 4D) and other finger ratios in Greek population. Coll. Antropol. 32, 1093–1098 [PubMed] [Google Scholar]
- 33.Voracek M. 2009. Comparative study of digit ratios (2D : 4D and other) and novel measures of relative finger length: testing magnitude and consistency of sex differences across samples. Percept. Mot. Skills 108, 83–93 (doi:10.2466/pms.108.1.83-93) [DOI] [PubMed] [Google Scholar]
- 34.Hauser H, Gandelman R. 1983. Contiguity to males in utero affects avoidance responding in adult female mice. Science 220, 437–438 (doi:10.1126/science.6836288) [DOI] [PubMed] [Google Scholar]
- 35.Morley-Fletcher S, Palanza P, Parolaro D, Viganò D, Laviola G. 2003. Intrauterine position has long-term influence on brain mu-opioid receptor density and behaviour in mice. Psychoneuroendocrinology 28, 386–400 (doi:10.1016/S0306-4530(02)00030-6) [DOI] [PubMed] [Google Scholar]
- 36.Knickmeyer RC, Woolson S, Hamer RM, Konneker T, Gilmore JH. 2011. 2D : 4D ratios in the first 2 years of life: stability and relation to testosterone exposure and sensitivity. Horm. Behav. 60, 256–263 (doi:10.1016/j.yhbeh.2011.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg Zand RS, Jenkins DJ, Diamandis EP. 2000. Genistein: a potent natural antiandrogen. Clin. Chem. 46, 887–888 [PubMed] [Google Scholar]
- 38.Wang H, Li J, Gao Y, Xu Y, Pan Y, Tsuji I, Sun ZJ, Li XM. 2010. Xeno-oestrogens and phyto-oestrogens are alternative ligands for the androgen receptor. Asian J. Androl. 12, 535–547 (doi:10.1038/aja.2010.14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu LC, Sun H, Chen JF, Bian Q, Qian J, Song L, Wang XR. 2005. Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicology 216, 197–203 (doi:10.1016/j.tox.2005.08.006) [DOI] [PubMed] [Google Scholar]
- 40.Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K. 2003. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol. Sci. 75, 40–46 (doi:10.1093/toxsci/kfg150) [DOI] [PubMed] [Google Scholar]
- 41.Molina-Molina JM, et al. 2006. Steroid receptor profiling of vinclozolin and its primary metabolites. Toxicol. Appl. Pharmacol. 216, 44–54 (doi:10.1016/j.taap.2006.04.005) [DOI] [PubMed] [Google Scholar]
- 42.Xiao Y, Liu R, Xing L, Xu Y, Shang L, Hao W. 2011. Combined developmental toxicity of bisphenol A and genistein in micromass cultures of rat embryonic limb bud and midbrain cells. Toxicol. In Vitro 25, 153–159 (doi:10.1016/j.tiv.2010.10.010) [DOI] [PubMed] [Google Scholar]
- 43.Rowas SA, Haddad R, Gawri R, Al Ma'awi AA, Chalifour LE, Antoniou J, Mwale F. 2012. Effect of in utero exposure to diethylstilbestrol on lumbar and femoral bone, articular cartilage, and the intervertebral disc in male and female adult mice progeny with and without swimming exercise. Arthritis Res. Ther. 14, R17 (doi:10.1186/ar3696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dean A, Sharpe RM. 2013. Anogenital distance or digit length ratio as measures of fetal androgen exposure: relationship to male reproductive development and its disorders. J. Clin. Endocrinol. Metab. 98, 2230–2238 (doi:10.1210/jc.2012-4057) [DOI] [PubMed] [Google Scholar]
- 45.Hurd PL, Bailey AA, Gongal PA, Yan RH, Greer JJ, Pagliardini S. 2008. Intrauterine position effects on anogenital distance and digit ratio in male and female mice. Arch. Sex. Behav. 37, 9–18 (doi:10.1007/s10508-007-9259-z) [DOI] [PubMed] [Google Scholar]
- 46.van den Driesche S, Scott HM, MacLeod DJ, Fisken M, Walker M, Sharpe RM. 2011. Relative importance of prenatal and postnatal androgen action in determining growth of the penis and anogenital distance in the rat before, during and after puberty. Int. J. Androl. 34, e578–e586 (doi:10.1111/j.1365-2605.2011.01175.x) [DOI] [PubMed] [Google Scholar]
- 47.Kurebayashi H, Nagatsuka S, Nemoto H, Noguchi H, Ohno Y. 2005. Disposition of low doses of 14C-bisphenol A in male, female, pregnant, fetal, and neonatal rats. Arch. Toxicol. 79, 243–252 (doi:10.1007/s00204-004-0628-2) [DOI] [PubMed] [Google Scholar]
- 48.Doerge DR, Vanlandingham M, Twaddle NC, Delclos KB. 2010. Lactational transfer of bisphenol A in Sprague-Dawley rats. Toxicol. Lett. 199, 372–376 (doi:10.1016/j.toxlet.2010.09.022) [DOI] [PubMed] [Google Scholar]
- 49.Jirtle RL, Skinner MK. 2007. Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 8, 253–262 (doi:10.1038/nrg2045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lange UC, Schneider R. 2010. What an epigenome remembers. Bioessays 32, 659–668 (doi:10.1002/bies.201000030) [DOI] [PubMed] [Google Scholar]
- 51.Reik W. 2007. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 447, 425–432 (doi:10.1038/nature05918) [DOI] [PubMed] [Google Scholar]
- 52.Salian S, Doshi T, Vanage G. 2009. Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to bisphenol A. Life Sci. 85, 11–18 (doi:10.1016/j.lfs.2009.04.005) [DOI] [PubMed] [Google Scholar]
- 53.Salian S, Doshi T, Vanage G. 2011. Perinatal exposure of rats to bisphenol A affects fertility of male offspring: an overview. Reprod. Toxicol. 31, 359–362 (doi:10.1016/j.reprotox.2010.10.008) [DOI] [PubMed] [Google Scholar]
- 54.Lilley T, Laaksonen T, Huitu O, Helle S. 2009. Digit length ratio (2D/4D): comparing measurements from X-rays and photographs in field voles (Microtus agrestis). Behav. Ecol. Sociobiol. 63, 1539–1547 (doi:10.1016/j.physbeh.2009.11.015) [Google Scholar]
- 55.Yan RH, Malisch JL, Hannon RM, Hurd PL, Garland T., Jr 2008. Selective breeding for a behavioural trait changes digit ratio. PLoS ONE 3, e3216 (doi:10.1371/journal.pone.0003216) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
F1 and F2 digit length and ratio input files are deposited in the Dryad Repository: http://dx.doi.org/10.5061/dryad.s2np7.


