Abstract
The marine tropics contain five major biogeographic regions (East Pacific, Atlantic, Indian Ocean, Indo-Australian Archipelago (IAA) and Central Pacific). These regions are separated by both hard and soft barriers. Reconstructing ancestral vicariance, we evaluate the extent of temporal concordance in vicariance events across three major barriers (Terminal Tethyan Event (TTE), Isthmus of Panama (IOP), East Pacific Barrier, EPB) and two incomplete barriers (either side of the IAA) for the Labridae, Pomacentridae and Chaetodontidae. We found a marked lack of temporal congruence within and among the three fish families in vicariance events associated with the EPB, TTE and IOP. Vicariance across hard barriers separating the Atlantic and Indo-Pacific (TTE, IOP) is temporally diffuse, with many vicariance events preceding barrier formation. In marked contrast, soft barriers either side of the IAA hotspot support tightly concordant vicariance events (2.5 Myr on Indian Ocean side; 6 Myr on Central Pacific side). Temporal concordance in vicariance points to large-scale temporally restricted gene flow during the Late Miocene and Pliocene. Despite different and often complex histories, both hard and soft barriers have comparably strong effects on the evolution of coral reef taxa.
Keywords: coral reef fishes, vicariance, barriers, Lagrange, DEC model, ancestral biogeography
1. Introduction
The tropical world has been largely restricted to the low latitudes since the formation of the Circum-Antarctic Current at approximately 37 Myr ago [1]. Since that time, the tropics have been divided into three major realms by a series of barriers. These barriers can be ‘hard’ or ‘soft’ depending on their defining isolating mechanism. Hard barriers are caused by the formation of land bridges that physically split marine populations. Soft barriers often invoke hydrological processes (water currents, large distance) that disrupt the movement of adults and/or dispersal of pelagic larvae and are viewed as permeable in nature [2]. Although many studies have described sister species/lineages that are divided by marine barriers [3–5], few have examined patterns at a global biogeographic scale or at the family level. To understand the effect that marine barriers have had on present-day biodiversity and the relative role played by both hard and soft barriers, a temporal perspective is required in taxa containing a large number of species that occupy these major ocean basins.
Several phylogenetic studies of reef-associated fishes have explored the role that biogeography and barriers to dispersal have played in the divergence of lineages [5–8]. Recent phylogeographic studies have revealed cryptic diversity within species [9] and the influence of porous hydrological barriers [10]. Each study has provided valuable insights, yet an overview of the role of barriers is lacking.
Historically, three barriers have divided the circumtropical belt into three major realms: the Indo-Pacific, Atlantic and East Pacific. These three barriers are
(1) The final closure of the Tethys seaway (Terminal Tethyan Event, TTE) dated to approximately 12 Myr (12–18 Myr; [11]). This ‘hard’ land barrier at the northern tip of the Red Sea, cut off low-latitude gene flow from the Indian Ocean to the Atlantic. Vicariance associated with the TTE has been identified in numerous dated phylogenies of marine taxa, including coral reef fishes [3,12–14] and gastropods [15–17]. Although dispersal around the Horn of Africa [18] and Lessepsian migration are possible [19], the TTE represents the largest hard barrier in tropical marine biogeography and has been important in the early provinciality of the marine tropics and in several reef-associated percomorph lineages [20–22]. However, the timing and frequency of the many vicariance events across the TTE have not been examined across multiple groups.
(2) The closure of the Isthmus of Panama (IOP) dated to 3.1 Myr [23]. The IOP marked the final separation of the Atlantic/Caribbean region from the East Pacific and is another ‘hard’ barrier. As per the TTE, the effects of this closure are seen in sister taxa from several different faunal groups [4,24]. This relatively young hard barrier has been well studied by both geologists and molecular biologist [24–26], and its effects on phylogenies haven been comprehensively reviewed [4]. Recent geochemical and geological study has shown that the IOP may have had an extended temporal history, with an unbroken chain of volcanic islands in this region as far back as the Eocene [26,27]. On a large scale, the IOP and TTE effectively isolated the Indo-Pacific and the Atlantic realms. It is between these two regions that most differences are seen today in terms of the taxonomic composition of reef taxa [21].
(3) The EPB separates the Indo-Pacific from the East Pacific by a 5000 km expanse of open ocean [21]. It is a ‘soft’ barrier, as it does not represent a direct physical barrier between marine populations. It has had a large impact on the long-term separation of assemblages in the Indo-Pacific and East Pacific, but it has not been a permanent barrier to dispersal. While this barrier is believed to have been in effect throughout the past 65 Myr [28], there are examples of both fish and invertebrate lineages that have crossed the barrier [29,30], with most successful dispersal of taxa from west to east [30].
The lack of hard barriers in the Indo-Pacific has allowed many taxa to maintain widespread ranges spanning from the east coast of Africa to islands in the central Pacific, or in some cases to the Pacific coast of the Americas [31]. Nevertheless, regional faunas are readily identified. The Indo-Pacific can be separated into three broad regions: the Indian Ocean, the Indo-Australian Archipelago (IAA) hotspot and the Central West Pacific Islands [13,32–34]. These three regions are characterized by both provincial endemics and widespread species [21,35–37], and are presumably created and maintained by soft barriers between the regions (lying either side of the IAA). However, the permeable nature of barriers within the Indo-Pacific [38–40] and rapid dispersal potential of marine fishes [41] means that the present-day distribution of taxa may have blurred the history or role of vicariance between the three regions. The effects of these porous barriers in the Indo-Pacific have been seen in several population genetic studies, resulting in both temporal and geographical structuring of haplotypes [42,43]. However, the influence of these barriers on the speciation of taxa that are widespread today requires further investigation. Given the uncertainty surrounding the historical effectiveness of such barriers to dispersal, there is an expectation that the soft barriers separating the three regions in the Indo-Pacific will have a more temporally diffuse pattern of vicariance, unlike the clear ‘hard’ barriers of the IOP and the TTE. However, by examining family-level chronologies on a large geographical scale, vicariant cladogenesis may be identified in deeper lineages [44], and the relative timing of vicariance in these soft barriers can be determined and compared with hard barriers.
To address these issues, we implemented ancestral range reconstruction methods [45] to examine patterns of vicariance in three reef fish families: Labridae, Pomacentridae and Chaetodontidae. These families are among the most widespread, diverse and abundant on coral reefs globally [21]. Recently published chronologies of the three families [46] contain species restricted to each of the five major biogeographic regions, as well as species with widespread ranges [5,35,47,48]. Previous studies have explored the biogeographic evolution of taxa within each of the families [3,13,49–51]. However, there has been no explicit examination of patterns of vicariance within these groups and how they are related to known hard and soft barriers (but see Blum [32]). Using recently developed software for biogeographic reconstruction [45], hypothetical biogeographic scenarios along the molecular lineage can be modelled from extant ranges. Within this framework, implied vicariance events can now be examined and temporal patterns evaluated.
The aim of this study, therefore, was to identify congruence in patterns of vicariance in the biogeographic histories of the Labridae, Pomacentridae and Chaetodontidae. In a global context, this temporal perspective will allow the role of barriers and vicariance between regions to be quantified and compared with the palaeogeographical history of the regions. The specific questions to be answered are
(1) Do families of coral reef fishes display congruent patterns of inferred vicariance across major biogeographic barriers?
(2) What is the temporal pattern of vicariance events associated with biogeographic barriers and how well does this reflect known geological events? and
(3) How do hard and soft barriers differ in the intensity (spread) of vicariance events through time (are hard barriers temporally ‘tighter’ than soft ones)?
2. Material and methods
Recently reconstructed chronograms for the families Labridae, Pomacentridae and Chaetodontidae were used in the ancestral range inheritance analysis [46]. The geographical ranges of each species in each of the chronograms were assessed using published sources [48,52–54] and FishBase [55]. Geographical ranges were divided into five separate regions: (i) Indian Ocean; (ii) IAA; (iii) Central Pacific; (iv) East Pacific; and (v) Atlantic (see electronic supplementary material, table S1). Presence within a geographical region required a record of one location within the region; there was no limit to the number or order of regions occupied (see electronic supplementary material, table S2). The presence or absence of a species in each region was coded as a character state to be used in the ancestral range reconstruction.
(a). Ancestral range reconstruction
Reconstruction of ancestral ranges based on the time-calibrated phylogenies was implemented in the program Lagrange v. 2.01 [45]. We concentrate on the cladogenetic history of nodes on the time-calibrated phylogenies, specifically vicariant inheritance between regions. A vicariance event was defined as the splitting of an ancestral widespread lineage into two daughter lineages that were divided between two adjacent regions. A dispersal, extinction, cladogenesis (DEC) model was used to reconstruct ancestral patterns of vicariance among the five designated regions. For each node, Lagrange ranks the range inheritance scenarios based on the fractional likelihood they received by the DEC model. A vicariance event was recorded only when it was the most likely range inheritance scenario for a particular node.
Constraints were placed on the DEC model to accurately reflect the past formation of known barriers. The constrained model reduced the probability of dispersal from the Central Pacific to the East Pacific to 0.05 for the entire duration of the chronogram for each family (i.e. from root to tip) reflecting the EPB [30]. The probability of dispersal from the Indian Ocean to the Atlantic Ocean was reduced to 0.05 from 18 Myr onwards, reflecting the closure of the Tethys seaway [11], but allowing the possibility of dispersal around the Horn of Africa [18]. Dispersal from the Atlantic to the East Pacific was not allowed from 3.1 Myr to present, reflecting the closure of the IOP [23]. This model reflects the formation of barriers a priori so as to reduce the possibility of erroneously implying dispersal across a known hard barrier during the analysis (e.g. IOP). Ultimately, the cladogenetic history of each family will determine the timing of associated vicariance events.
Two types of analyses were undertaken: the first examines the relative rates of vicariance through time; the second examines ages of vicariance events through time. The first analysis, examined the relative frequency of vicariance events, i.e. the number of vicariance events in each time period relative to the number of possible events in that time period (= the number of nodes in the trees in each period). In test 1a, the null expectation is of a constant rate of vicariance through time, i.e. the frequency of vicariance events to possible events will be constant in each time period. The number of vicariance events, as a fraction of the potential events (nodes), was compared among time periods for each of the three families separately, using a Chi-squared goodness-of-fit test. To satisfy the assumption of the Chi-squared test, time periods were chosen (Pliocene/Pleistocene, Miocene to Eocene) to ensure that expected numbers of vicariance events were not below five [56]. Furthermore, if vicariance was constant through time, then the frequency of events (i.e. events relative to potential events) associated with each of the individual barriers should also be similar among time periods. In test 1b, we examine whether there was any significant difference in the frequencies of vicariance events associated with the five individual barriers through time using a Fisher's exact test. This test was used to compare the frequencies of vicariance events among barriers across time periods (Pliocene/Pleistocene, Miocene, Oligocene, Eocene). Given the low number of events for some barriers and time periods, the Fisher exact test was chosen as it remains valid for small sample sizes. The observed number of inferred vicariance events in each time period for each barrier were compared with a null expectation based on the total number of vicariance events recorded in each time period. The expected number of vicariance events is the product of the proportion of total vicariance associated with each barrier (vicariance events at that barrier/all vicariance events) and the proportion of the total number of vicariance events associated with each time period (vicariance in each epoch/all vicariance events). Thus, the expected number of vicariance events in the Miocene at the IOP barrier = the total number of events in the IOP × the fraction of all events in the Miocene (across all barriers). The second analysis examined the means and variance of estimated vicariance ages. Given that some barriers are geologically much younger than others, the expectation is that these barriers will have much younger mean ages of inferred vicariance. We therefore compare the mean ages of inferred vicariance events among barriers and among families using a two-way ANOVA, with families and barriers as fixed factors.
3. Results
The inferred vicariance events based on the DEC model are revealed when mapped onto specific nodes in the family chronologies (see electronic supplementary material, figures S1–S3). The distribution of vicariance events across all barriers, in all three families, revealed that the majority of vicariance occurred in the Late Miocene to Early Pliocene (figure 1a). For each of the three families, the number of vicariance events as a proportion of possible vicariance events (nodes) was not found to be significantly different among time periods (Labridae, χ2 = 0.018, p = 0.89, d.f. = 1; Pomacentridae, χ2 = 0.11, p = 0.73, d.f. = 1; Chaetodontidae, χ2 = 0.001, p = 0.98, d.f. = 1). As the number of potential vicariance events (nodes) increased or decreased through time, so does the total number of inferred vicariance events. However, Fisher's exact test did identify a significant temporal effect on the frequency of vicariance associated with individual barriers (p = 0.0007), with the frequency of events attributed to individual barriers varying across four time periods (Eocene, Oligocene, Miocene, Pliocene/Pleistocene). Examination of observed frequencies, in comparison with the expected values, suggests that both the TTE and IOP had a higher than expected frequency of vicariance events in the Miocene and lower than expected in the Pliocene/Pleistocene (figure 1b). The IAA/Central Pacific barrier displayed a similar pattern. However, the Indian Ocean/IAA barrier displayed the opposite pattern, with the plots suggesting a lower than expected frequency of events in the Miocene and higher than expected frequency in the Pliocene/Pleistocene epochs (figure 1b). The pattern of events associated with the EPB was inverse to what would be expected in each epoch (figure 1b).
Figure 1.
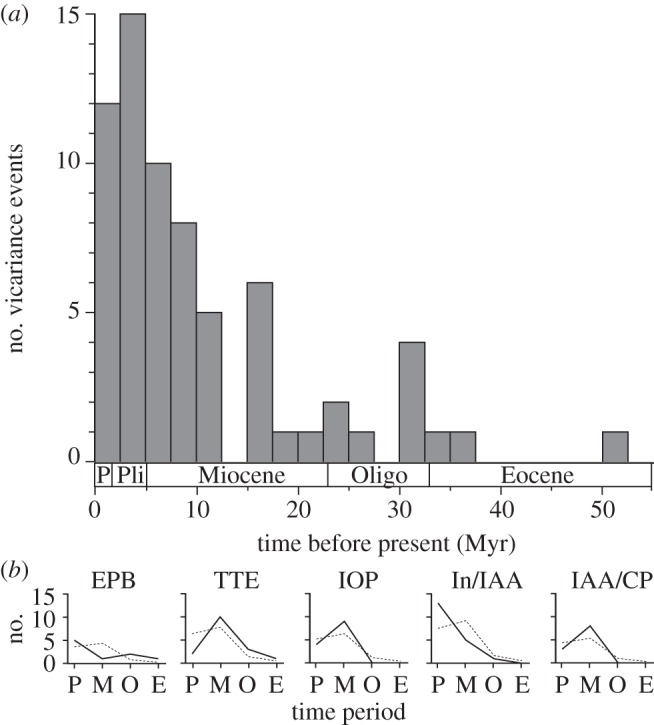
(a) Frequency histogram of vicariance events across all barriers in all three families. (b) Observed (solid line) versus expected (dashed line) frequency of vicariance events in four time periods (P, Plio/Pleistocene; M, Miocene; O, Oligocene; E, Eocene) for each of the five barriers. Expected frequencies are based on the product of the total vicariance associated with each barrier and the relative frequency of vicariance in each time period (based on the distribution among times in the entire dataset).
The mean age of vicariance across each of the barriers (figure 2b) exhibits marked among-family and within barrier variation. Possibly as a result of this variation, the mean ages of vicariance events associated with specified barriers (figure 2a) exhibited no significant difference (F4,53 = 1.89, p = 0.12). Likewise, no significant difference was detected among families (F2,53 = 2.78, p = 0.07), nor was there a significant interaction detected between barrier and family (F8,53 = 0.08, p = 0.58). Despite the lack of significance in the mean ages of vicariance events among barriers, the age distributions of vicariance events at the various barriers exhibit strongly contrasting patterns (as indicated by Fisher's exact test). These patterns are examined in the context of each associated barrier below.
Figure 2.
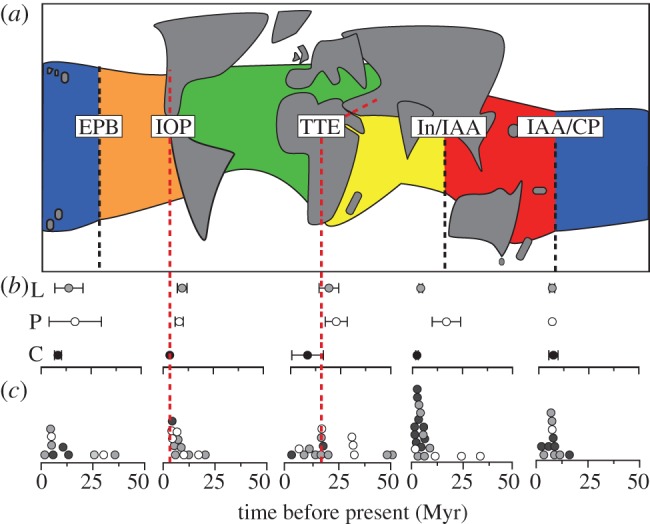
Distribution of reconstructed vicariance events associated with barriers between biogeographic regions. (a) Schematic diagram of world map identifying boundaries between regions (dashed lines) with known historical barriers: IOP and TTE (see text). Lines for IOP and TTE are extended down indicating the timing of known final barrier formation; (b) mean age (±s.e.) of vicariance events associated barriers (L, Labridae; P, Pomacentridae; C, Chaetodontidae) and (c) distribution of vicariance events across each barrier. Each circle represents a vicariance event across the associated barrier as implied from the Lagrange reconstruction. Family is indicated by circle colour as in (b) (Labridae, grey; Pomacentridae, white; Chaetodontidae, black). (Online version in colour.)
(a). Terminal Tethyan Event (18 Myr)
Reconstructed vicariance events between the Indian Ocean and the Atlantic for the Labridae and Pomacentridae have similar mean ages (approx. 19 and 22 Myr, respectively). However, both families contain outlier events: older in the Labridae (approx. 50 Myr, hypsigenyine) and younger in the Pomacentridae (4.2 Myr, Abudefdufinae (cf. ICZN designation Gliphysodon)). Vicariance events in the Labridae are more frequent closer to the final closure of the Tethys seaway (figure 2c), whereas the Pomacentridae has been affected by pre-TTE vicariance, with three events occurring at approximately 30 Myr. There were two vicariance events recorded in the Chaetodontidae: recently at 0.5 Myr (post-TTE) and close to the TTE at 16.3 Myr. Overall, there is a wide range of vicariance events among the three families, with a marginally higher density occurring around the TTE (approx. 15–16 Myr; figure 2c).
(b). Isthmus of Panama (3.1 Myr)
The IOP resulted in several vicariance events in the Labridae and Pomacentridae, and once in the Chaetodontidae, separating lineages between the Atlantic and East Pacific regions (figure 2). There was one event implied from the reconstruction in the chaetodontid tree, which occurred very close (at 3.3 Myr) to the final closure of the IOP (at 3.1 Myr). Both the Labridae and the Pomacentridae had a wider distribution of vicariance ages (mean ages of 9.4 and 7.9 Myr, respectively), with the highest density of events occurring just before the closure of the IOP (figure 2c).
(c). East Pacific Barrier (65 Myr)
Vicariance related to the EPB appears to have occurred in all three families (figure 2), separating lineages in the Central Pacific and East Pacific regions. Vicariance appears in two discrete time periods: from the Late Eocene/Oligocene (approx. 35–25 Myr) in the Labridae and Pomacentridae, and from the Late Miocene/Pliocene (approx. 9–1 Myr) in all three families (figure 2c). The majority of inferred vicariance events are in the latter period.
(d). Indian Ocean/Indo-Australian Archipelago
Both the Labridae and Chaetodontidae show marked congruence in the mean age of vicariance between the regions (4.1 and 2.2 Myr, respectively; figure 2b), with the distribution of vicariance events clustering tightly within the last 10 Myr (figure 2c). In particular, vicariance in the past 5 Myr is higher than expected (figure 1b). The Pomacentridae have a wider range of events with two older events occurring in the Oligocene/Miocene, but otherwise this family also shows congruence with the Labridae and Chaetodontidae, with two events occurring during the End Miocene and Early Pliocene (figure 2c).
(e). Indo-Australian Archipelago/Central Pacific
Vicariance between the IAA and Central Pacific regions was also remarkably concentrated in the Late Miocene for all three families. The Labridae and Chaetodontidae do have slightly older events as outliers, but the mean ages, are very similar (6.7 and 7.3 Myr, respectively; figure 2b). Only one vicariance event was observed in the Pomacentridae at approximately 6.7 Myr. There is marked congruence among the three families with an overall majority of events occurring between 6 and 7 Myr (figure 2c).
4. Discussion
(a). Barriers and vicariance through time
Vicariance between marine regions during the evolutionary history of the Labridae, Pomacentridae and Chaetodontidae has been associated with several well-known barriers to gene flow: the TTE at 12–18 Myr; and the IOP at 3.1 Myr; the EPB from 65 Myr to present. However, the reconstruction highlights a complicated evolutionary history in which barriers assumed to be temporally distinct were not found to be (EPB, TTE and IOP), and others regarded as historically permeable (IAA/Indian Ocean, IAA/Central Pacific) show strong temporal concordance among these three reef fish families. Overall, the rates of vicariance (relative to potential vicariance events) appear to be constant throughout the cladogenic history of each family. Furthermore, there is no difference in the mean ages of vicariance events among barriers. However, the frequency of vicariance associated with individual barriers was found to vary significantly among epochs. It is the distribution of vicariance events through time that appears to mark the greatest difference among the five key barriers. To examine the nature of vicariance through time, each barrier will be considered separately below.
(i). Terminal Tethyan Event
The final closure of the Tethys seaway estimated at 12 Myr [11], in conjunction with the EPB, effectively cut the marine tropics in two. It has been invoked as a major vicariance event in the early evolution of many reef-associated fish lineages [8,12,57,58]. While this may be true, the reconstruction identifies no temporally concordant pattern of vicariance between the Indian Ocean and Atlantic lineages among the three families. Nor was the mean age of TTE vicariance between the Indian Ocean and Atlantic distinct from other geological barriers. However, the distribution of events through time suggests that TTE vicariance is characterized by more older events and fewer younger events than expected (figure 1b). Few of the implied vicariance events are closely associated with the period surrounding the TTE (12–18 Myr), although four of the vicariance events are close to the 15 Myr mid-TTE cut-off. The reconstruction reveals a staggered pattern of pre-TTE (Pomacentridae, Labridae), mid-TTE (Pomacentridae, Labridae, Chaetodontidae) and post-TTE (Labridae, Chaetodontidae and an Abudefduf lineage) vicariance between the Indian Ocean and the Atlantic. The temporal accumulation of vicariance events mid-TTE and the fewer younger events than expected may be evidence of the effectiveness of the land barrier, however, the diffuse temporal pattern of vicariance is unexpected given the definitive ‘hard’ nature of this land bridge. The pattern of pre-TTE vicariance is consistent with that found in marine gastropods [17,59]. The pre-TTE events associated with the Labridae and the Pomacentridae may be linked to the formation of the Paratethys during the Oligocene [20]. However, Reid et al. [17] suggest that a similar TTE division in mangrove snails may be related to climatic changes in the Early Miocene. The post-TTE events occur in lineages with circum-African distributions and subtropical to temperate ranges (Scarus, Thalassoma, Chaetodon, Abudefduf) and are most likely associated with recent periods of dispersal connecting the two regions around the Cape of Good Hope [5,18,57].
(ii). Isthmus of Panama
The IOP has been a hard barrier, separating several lineages either side of the Americas for at least 3.1 Myr [4], but it is also the end product of a 12 Myr process of gradual separation [23]. From the ancestral reconstruction, vicariance events appear throughout this preceding 12 Myr period (and possibly earlier) and reach a peak just before the IOP closure (figure 2c). This evidence is consistent with previous work showing vicariance of geminate pairs predating the IOP [24,58,60] (reviewed by Lessios [4]). Even older vicariance across the IOP (more than 18 Myr) in the Labridae and Pomacentridae (figure 2c) may be evidence of disruption to gene flow in the Early Miocene [26]. The extended temporal influence of the IOP highlights the disruption in gene flow between the East Pacific and the Atlantic long before the final formation of the ‘hard’ isthmus.
(iii). East Pacific Barrier
The EPB is the oldest barrier that separates the Indo-Pacific from the East Pacific and Atlantic realms. In place since the Late Cretaceous, it has been a constant feature of the Tertiary, where it acts as a soft barrier to dispersal [21,61]. However, vicariance following dispersal from the Central Pacific to the East Pacific has been reported for some 80 fish species [62]. By contrast, there has been little dispersal in the other direction [30,63]. In this way, the EPB has acted as a unidirectional filter permitting limited movement from west to east and even less from east to west [30]. The pattern of vicariance associated with the EPB appears inverse to the pattern expected among epochs (figure 1b). The timing of vicariance events among the three families (approx. 35–25 and approx. 9–1 Myr) are not temporally concordant, spanning most of the Cenozoic, and suggest that the periodic breaches of this barrier may have more than one cause. However, despite the unidirectional dispersal across the barrier, there are several lineages present in the chronograms of the Labridae (Calotomus carolinus, Scarus rubroviolaceus, Scarus ghobban, Novaculichthys taeniourus, Stethojulis bandanensis) and one from the Chaetodontidae (Forcipiger flavissimus) that have been able to maintain gene flow across the EPB, possibly in both directions [30]. Breaching of the barrier and subsequent vicariance may therefore be a regular occurrence with no specific temporal focus, whereas barriers to establishment (more unoccupied niches, less chance of introgression) facilitate west–east movement, contrary to the prevailing currents.
(iv). Indian Ocean/Indo-Australian Archipelago
Despite being the youngest barrier, the mean age of the vicariance events associated with this barrier was not found to be significantly different from other barriers. However, the pattern of vicariance across the Indian Ocean/IAA boundary is unusual, with the distribution of vicariance events suggesting that vicariance associated with this barrier is higher than expected in the Pliocene/Pleistocene (figure 1b). Of all deviations from expectation across time or boundaries, the greatest deviation is in the excessively large number of Plio/Pleistocene vicariance events across the Indian/IAA barrier (figure 1b). The majority of vicariance events occurred between 2 and 6 Myr (figure 2c), especially within the Labridae and Chaetodontidae. There are several barriers that have been reported between the Indian Ocean and the IAA, but their position and temporal history are still unclear [21,32,64,65]. While vicariance appears to have occurred from the Late Miocene (and possibly as far back as the Oligocene for the Pomacentridae), the majority have occurred in a narrow time interval at approximately 2.5 Myr. This temporal concordance for such a complex region is remarkable. The reconstruction points to a barrier, or series of barriers that have historically affected lineages from the Late Miocene, with an increasing impact towards the end of the Pliocene. The ongoing nature of this barrier may be evident in population studies across the two regions, with temporal clades containing haplotypes from both regions [43,66]. However, the nature and location of the barrier is hard to identify. Past vicariance about the 40° line was noted by Winterbottom [64], whereas numerous barriers exist within the IAA [21,37]. Given that the dates of the vicariance in the reconstruction pre-date the Pleistocene, sea-level changes do not appear to have been a major driver of vicariance; however, changing ocean currents present a possible mechanism for changing levels of connectivity between the Indian Ocean and IAA regions. Hopefully, more detailed tectonic, eustatic, climatic, oceanographic and geomorphological studies of the region will help elucidate the underlying patterns [67–69].
(v). Indo-Australian Archipelago/Central Pacific
Vicariance between the IAA and the Central Pacific is very similar to the Indian Ocean/IAA vicariance in that although the mean age of vicariance events was not found to be different from the other barriers, the distribution of vicariance events is striking, with most events being restricted to a relatively short time period. Although events extend back to the Miocene (approx. 15 Myr; figure 2c) most events between the IAA/Central Pacific occur in the Late Miocene (5–7.5 Myr) with a distinct peak at about approximately 6 Myr. This concordance among taxa in a geographically indistinct soft barrier is, again, remarkable. Previous work has highlighted the importance of sea-level changes during the Pleistocene and Holocene in structuring species populations from the IAA and Central Pacific [70,71]; however, the ages of vicariance reported here are much older, again, making sea-level changes unlikely as a mechanism for vicariance. The congruence between vicariance on both sides of the IAA points to a global effect, possibly climate change or changing ocean currents, in separating lineages either side of the IAA.
(b). Consequences of ‘hard’ and ‘soft’ barriers
The reconstruction presented herein for vicariance across major regional barriers highlights the complex history and relative effects of ‘hard’ and ‘soft’ barriers. Based on their physical separation of ocean basins, there may be an expectation that both the IOP and the TTE would have a definitive timing of vicariance close to, or shortly before the formation of the associated land bridges, making them appear temporally distinct. This is not the case. In both localities, an extended period of vicariance pre-dated the final closure of the barrier. This extended period of vicariance has previously been reported in numerous taxa for the IOP [4], and for pomacanthids [57] and marine snails [17] across the TTE. This similarity to previous, independent findings provides some confidence in the patterns inferred from the current reconstruction (problems of extinction and taxon sampling are also likely to be limited; electronic supplementary material, section Discussion). These ‘hard’ barriers do not have a strong temporal signal and lineages have responded on different timescales. It is these extended periods of vicariance that result in the lack of a statistical difference in the mean age of vicariance events associated with hard barriers. This is not an unexpected result given the gradual formation of these barriers. The patterns of vicariance across soft barriers between the IAA and the Indian and Pacific Oceans, however, are harder to explain.
For the ‘soft’ barriers between the Indian Ocean/IAA and the IAA/Central Pacific, one might expect that the permeable and complex nature of these barriers would result in a wider distribution of vicariance events, especially in the Central Pacific, where numerous islands form ‘stepping stones’. This also does not appear to be the case. Although the mean age of vicariance associated with these barriers are, again, not statistically different from other barriers, there is clear evidence that observed frequencies of vicariance do vary among barriers (figure 1b), with soft barriers having temporally restricted vicariance. Vicariance either side of the IAA was largely restricted to two narrow times: 2.5 Myr on the Indian Ocean side; and 6 Myr on the Pacific side (figure 2). Such tight temporally restricted vicariance in an extensive area of connectivity is surprising. This pattern also appears to be relatively consistent across the three families. As in previous studies from a wider range of taxa [69,72], the ages of the vicariance events either side of the IAA are much older than expected if sea-level changes were the primary cause. Given the ages, changes in climatic conditions and oceanic currents may be key factors, rather than tectonics [68,72]. However, it needs to be considered that the biogeographic event may considerably pre-date vicariance, as in the Caribbean where the closing of the isthmus at 3.1 Myr, did not trigger an extinction event until a million years later [25].
Our results suggest there is a need to be careful in interpreting the intensity or impact of ‘hard’ barriers, as ‘soft’ barriers seem to have a more intense or temporally concordant impact. The key question remains regarding the underlying process driving vicariance between the IAA and the Indian Ocean, or the IAA and the Central Pacific. Further analysis is required to answer this exciting question.
(c). Ecology and vicariance
The variation in patterns of vicariance among the three families (figure 2) may highlight an ecological component in the effect of marine barriers. With respect to the three families examined here, the Labridae and the Pomacentridae appear to share more older hard barrier events, whereas the Labridae and the Chaetodontidae share more younger, soft barrier events. The common older patterns in the Labridae and Pomacentridae may be related to their shared older evolutionary history [14]. The younger vicariance seen in the Indo-Pacific for the Labridae and Chaetodontidae may reflect similar reproductive modes. Both labrids and chaetodontids spawn in the water column, whereas pomacentrids lay demersal eggs. In addition, labrid and chaetodontid taxa have, on average, longer pelagic larval durations (PLDs; approx. 39 and approx. 36 days, respectively) when compared with that of pomacentrids (approx. 22 days) [73]. A longer PLD may allow labrids and chaetodontids to disperse further and hence increased the opportunity for vicariance. However, this is unlikely to be a major influence in the Indo-Pacific where lineages in all three families can have large Indo-Pacific ranges. Luiz et al. [2] showed that when it came to crossing hydrological barriers in the Atlantic, PLD was far less important than other traits such as the ability to raft with flotsam and broad environmental tolerance. These two characteristics may be important for post-TTE vicariance in labrids, chaetodontids and the Abudefduf lineage.
5. Conclusion
Temporally congruent patterns of implied vicariance of molecular lineages highlight the complex history of barrier formation in the marine tropics. All five barriers separating the five biogeographic regions have a long history of associated vicariance events. Hard barriers separating the Atlantic from the Indo-Pacific are temporally diffuse, whereas soft barriers either side of the IAA hotspot support tightly concordant vicariance events between 2.5 and 6 Myr. Although the location of soft barriers may be geographically indistinct, they are biogeographically important and part of a period of exceptional biogeographic change for reef-associated taxa in the Late Miocene/Pliocene.
Acknowledgements
We thank L. van Herwerden, C. Goatley, A. Hoey, J. Tanner and two anonymous reviewers for valuable comments and/or helpful discussions.
Funding statement
This work was supported by The Australian Research Council (DRB).
References
- 1.Kamp P, Waghorn D, Nelson C. 1990. Late Eocene–Early Oligocene integrated isotope stratigraphy and biostratigraphy for paleoshelf sequences in southern Australia: paleoceanographic implications. Palaeogeogr. Palaeoclim. Palaeoecol. 80, 311–323 (doi:10.1016/0031-0182(90)90140-3) [Google Scholar]
- 2.Luiz OJ, Madin JS, Robertson DR, Rocha LA, Wirtz P, Floeter SR. 2012. Ecological traits influencing range expansion across large oceanic dispersal barriers: insights from tropical Atlantic reef fishes. Proc. R. Soc. B 279, 1033–1040 (doi:10.1098/rspb.2011.1525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber PH, Bellwood DR. 2005. Biodiversity hotspots: evolutionary origins of biodiversity in wrasses (Halichoeres: Labridae) in the Indo-Pacific and new world tropics . Mol. Phylogenet. Evol. 35, 235–253 (doi:10.1016/j.ympev.2004.10.004) [DOI] [PubMed] [Google Scholar]
- 4.Lessios HA. 2008. The great American schism: divergence of marine organisms after the rise of the Central American Isthmus. Annu. Rev. Ecol. Evol. Syst. 39, 63–91 (doi:10.1146/annurev.ecolsys.38.091206.095815) [Google Scholar]
- 5.Floeter SR, et al. 2007. Atlantic reef fish biogeography and evolution. J. Biogeogr. 35, 22–47 (10.1111/j.1365-2699.2007.01790.x) [Google Scholar]
- 6.Hanel R, Westneat MW, Sturmbauer C. 2002. Phylogenetic relationships, evolution of broodcare behavior, and geographic speciation in the wrasse tribe Labrini. J. Mol. Evol. 55, 776–789 (doi:10.1007/s00239-002-2373-6) [DOI] [PubMed] [Google Scholar]
- 7.Westneat MW, Alfaro ME. 2005. Phylogenetic relationships and evolutionary history of the reef fish family Labridae. Mol. Phylogenet. Evol. 36, 370–390 (doi:10.1016/j.ympev.2005.02.001) [DOI] [PubMed] [Google Scholar]
- 8.Fessler JL, Westneat MW. 2007. Molecular phylogenetics of the butterflyfishes (Chaetodontidae): taxonomy and biogeography of a global coral reef fish family. Mol. Phylogenet. Evol. 45, 50–68 (doi:10.1016/j.ympev.2007.05.018) [DOI] [PubMed] [Google Scholar]
- 9.Rocha LA. 2004. Mitochondrial DNA and color pattern variation in three western Atlantic Halichoeres (Labridae), with the revalidation of two species. Copeia 2004, 770–782 (doi:10.1643/CG-04-106) [Google Scholar]
- 10.Drew JA, Barber PH. 2012. Comparative phylogeography in Fijian coral reef fishes: a multi-taxa approach towards marine reserve design. PLoS ONE 7, e47710 (doi:10.1371/journal.pone.0047710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steininger FF, Rögl F. 1979. The paratethys history. A contribution towards the Neogene geodynamics of the alpine orogene. Ann. Geol. Pays Hell 3, 1153–1165 [Google Scholar]
- 12.Klanten S, van Herwerden L, Choat JH, Blair D. 2004. Patterns of lineage diversification in the genus Naso (Acanthuridae). Mol. Phylogenet. Evol. 32, 221–235 (doi:10.1016/j.ympev.2003.11.008) [DOI] [PubMed] [Google Scholar]
- 13.Read CI, Bellwood DR, van Herwerden L. 2006. Ancient origins of Indo-Pacific coral reef fish biodiversity: a case study of the leopard wrasses (Labridae: Macropharyngodon). Mol. Phylogenet. Evol. 38, 808–819 (doi:10.1016/j.ympev.2005.08.001) [DOI] [PubMed] [Google Scholar]
- 14.Cowman PF, Bellwood DR, van Herwerden L. 2009. Dating the evolutionary origins of wrasse lineages and the rise of trophic novelty on coral reefs. Mol. Phylogenet. Evol. 52, 621–631 (doi:10.1016/j.ympev.2009.05.015) [DOI] [PubMed] [Google Scholar]
- 15.Williams ST, Reid DG. 2004. Speciation and diversity on tropical rocky shores: a global phylogeny of snails of the genus Echinolittorina. Evolution 58, 2227–2251 (doi:10.1554/03-565) [DOI] [PubMed] [Google Scholar]
- 16.Williams ST, Duda TF. 2008. Did tectonic activity stimulate Oligomiocene speciation in the Indo-West Pacific? Evolution 62, 1618–1634 (doi:10.1111/j.1558-5646.2008.00399.x) [DOI] [PubMed] [Google Scholar]
- 17.Reid DG, Dyal P, Williams ST. 2010. Global diversification of mangrove fauna: a molecular phylogeny of Littoraria (Gastropoda: Littorinidae). Mol. Phylogenet. Evol. 55, 185–201 (doi:10.1016/j.ympev.2009.09.036) [DOI] [PubMed] [Google Scholar]
- 18.Bowen BW, Muss A, Rocha LA, Grant WS. 2006. Shallow mtDNA coalescence in Atlantic pygmy angelfishes (genus Centropyge) indicates a recent invasion from the Indian Ocean. J. Hered. 97, 1–12 (doi:10.1093/jhered/esj006) [DOI] [PubMed] [Google Scholar]
- 19.Goren M, Aronov A. 2002. First record of the Indo-Pacific parrotfish Scarus ghobban in the eastern Mediterranean. Cybium 26, 239–240 [Google Scholar]
- 20.Rögl VF. 1998. Palaeogeographic considerations for Mediterranean and Paratethys seaways (Oligocene to Miocene). Ann. Nat. Hist. Mus. Wien. 99(A), 279–310 [Google Scholar]
- 21.Bellwood DR, Wainwright PW. 2002. The history and biogeography of fishes on coral reefs. In Coral reef fishes: dynamics and diversity in a complex ecosystem (ed. Sale PF.), pp. 5–32 London, UK: Academic Press [Google Scholar]
- 22.Cowman PF, Bellwood DR. 2012. The historical biogeography of coral reef fishes: global patterns of origination and dispersal. J. Biogeogr. 40, 209–224 (doi:10.1111/jbi.12003) [Google Scholar]
- 23.Coates AG, Obando JA. 1996. The geologic evolution of the Central American Isthmus. In Evolution and environment in tropical America (eds Jackson JBC, Budd AF, Coates AG.), pp. 21–56 Chicago, IL: University of Chicago Press [Google Scholar]
- 24.Knowlton N, Weigt LA. 1998. New dates and new rates for divergence across the Isthmus of Panama. Proc. R. Soc. Lond. B 265, 2257–2263 (doi:10.1098/rspb.1998.0568) [Google Scholar]
- 25.O'Dea A, Jackson JBC, Fortunato H, Smith JT, D'Croz L, Johnson KG, Todd JA. 2007. Environmental change preceded Caribbean extinction by 2 million years. Proc. Natl Acad. Sci. USA 104, 5501–5506 (doi:10.1073/pnas.0610947104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farris DW, et al. 2011. Fracturing of the Panamanian Isthmus during initial collision with South America. Geology 39, 1007 (doi:10.1130/G32237.1) [Google Scholar]
- 27.Montes C, et al. 2012. Evidence for middle Eocene and younger land emergence in central Panama: implications for isthmus closure. GSA Bull. 124, 780–799 (doi:10.1130/B30528.1) [Google Scholar]
- 28.Rosen BR, Smith AB. 1988. Tectonics from fossils? Analysis of reef coral and sea urchin distributions from Late-Cretaceous to recent, using a new method. In Gondwana and Tethys (eds Audley-Charles MG, Hallam A.), pp. 275–306 Oxford, NY: Oxford University Press [Google Scholar]
- 29.Lessios HA, Kessing BD, Robertson DR. 1998. Massive gene flow across the world's most potent marine biogeographic barrier. Proc. R. Soc. Lond. B 265, 583–588 (doi:10.1098/rspb.1998.0334) [Google Scholar]
- 30.Lessios HA, Robertson DR. 2006. Crossing the impassable: genetic connections in 20 reef fishes across the eastern Pacific barrier. Proc. R. Soc. B 273, 2201–2208 (doi:10.1098/rspb.2006.3543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes TP, Bellwood DR, Connolly SR. 2002. Biodiversity hotspots, centres of endemicity, and the conservation of coral reefs. Ecol. Lett. 5, 775–784 (doi:10.1046/j.1461-0248.2002.00383.x) [Google Scholar]
- 32.Blum SD. 1989. Biogeography of the Chaetodontidae: an analysis of allopatry among closely related species. Environ. Biol. Fish. 25, 9–31 (doi:10.1007/BF00002198) [Google Scholar]
- 33.Bernardi G, Bucciarelli G, Costagliola D, Robertson DR, Heiser JB. 2004. Evolution of coral reef fish Thalassoma spp. (Labridae). 1. Molecular phylogeny and biogeography. Mar. Biol. 144, 369–375 (doi:10.1007/s00227-003-1199-0) [Google Scholar]
- 34.Briggs JC, Bowen BW. 2012. A realignment of marine biogeographic provinces with particular reference to fish distributions. J. Biogeogr. 39, 12–30 (doi:10.1111/j.1365-2699.2011.02613.x) [Google Scholar]
- 35.Jones GP, Caley MJ, Munday PL. 2002. Rarity in coral reef fish communities. In Coral reef fishes, dynamics and diversity in a complex ecosystem (ed. Sale PF.), pp. 81–101 London, UK: Academic Press [Google Scholar]
- 36.Connolly S, Bellwood DR, Hughes TP. 2003. Indo-Pacific biodiversity of coral reefs: deviations from a mid-domain model. Ecology 84, 2178–2190 (doi:10.1890/02-0254) [Google Scholar]
- 37.Hoeksema B. 2007. Delineation of the Indo-Malayan centre of maximum marine biodiversity: the coral triangle. In Biogeography, time, and place: distributions, barriers, and islands (ed. Renema W.), pp. 117–178 Dordrecht, The Netherlands: Springer [Google Scholar]
- 38.Barber PH, Palumbi SR, Erdmann MV, Moosa MK. 2000. A marine Wallace's line? Nature 406, 692–693 (doi:10.1038/35021135) [DOI] [PubMed] [Google Scholar]
- 39.Barber PH, Palumbi SR, Erdmann MV, Moosa MK. 2002. Sharp genetic breaks among populations of a benthic marine crustacean indicate limited oceanic larval transport: patterns, causes, and consequences. Mol. Ecol. 11, 659–674 (doi:10.1046/j.1365-294X.2002.01468.x) [DOI] [PubMed] [Google Scholar]
- 40.Santini F, Winterbottom R. 2002. Historical biogeography of Indo-western Pacific coral reef biota: is the Indonesian region a centre of origin? J. Biogeogr. 29, 189–205 (doi:10.1046/j.1365-2699.2002.00669.x) [Google Scholar]
- 41.Quenouille B, Hubert N, Bermingham E, Planes S. 2011. Speciation in tropical seas: allopatry followed by range change. Mol. Phylogenet. Evol. 58, 546–552 (doi:10.1016/j.ympev.2010.12.009) [DOI] [PubMed] [Google Scholar]
- 42.Gaither MR, Toonen RJ, Robertson DR, Planes S, Bowen BW. 2009. Genetic evaluation of marine biogeographical barriers: perspectives from two widespread Indo-Pacific snappers (Lutjanus kasmira and Lutjanus fulvus). J. Biogeogr. 37, 133–147 (doi:10.1111/j.1365-2699.2009.02188.x) [Google Scholar]
- 43.Horne JB, van Herwerden L, Choat JH, Robertson DR. 2008. High population connectivity across the Indo-Pacific: congruent lack of phylogeographic structure in three reef fish congeners. Mol. Phylogenet. Evol. 49, 629–638 (doi:10.1016/j.ympev.2008.08.023) [DOI] [PubMed] [Google Scholar]
- 44.Losos JB, Glor RE. 2003. Phylogenetic comparative methods and the geography of speciation. Trends Ecol. Evol. 18, 220–227 (doi:10.1016/S0169-5347(03)00037-5) [Google Scholar]
- 45.Ree RH, Smith SA. 2008. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Syst. Biol. 57, 4–14 (doi:10.1080/10635150701883881) [DOI] [PubMed] [Google Scholar]
- 46.Cowman PF, Bellwood DR. 2011. Coral reefs as drivers of cladogenesis: expanding coral reefs, cryptic extinction events, and the development of biodiversity hotspots. J. Evol. Biol. 24, 2543–2562 (doi:10.1111/j.1420-9101.2011.02391.x) [DOI] [PubMed] [Google Scholar]
- 47.de Moura R, Sazima I. 2000. Species richness and endemism levels of the Southwestern Atlantic reef fish fauna. In Proc. 9th Int. Coral Reef Symp. 1, 1–6 [Google Scholar]
- 48.Kuiter RH. 2002. Butterflyfishes, bannerfishes and their relatives: a comprehensive guide to Chaetodontidae and Microcanthidae. Chorleywood, UK: TMC Publishing [Google Scholar]
- 49.McCafferty S, Bermingham E, Quenouille B, Planes S, Hoelzer G, Asoh K. 2002. Historical biogeography and molecular systematics of the Indo-Pacific genus Dascyllus (Teleostei: Pomacentridae). Mol. Ecol. 11, 1377–1392 (doi:10.1046/j.1365-294X.2002.01533.x) [DOI] [PubMed] [Google Scholar]
- 50.Beldade R, Heiser JB, Robertson DR, Gasparini JL, Floeter SR, Bernardi G. 2009. Historical biogeography and speciation in the Creole wrasses (Labridae, Clepticus). Mar. Biol. 156, 679–687 (doi:10.1007/s00227-008-1118-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Craig MT, Eble JA, Bowen BW. 2010. Origins, ages and population histories: comparative phylogeography of endemic Hawaiian butterflyfishes (genus Chaetodon). J. Biogeogr. 37, 2125–2136 (doi:10.1111/j.1365-2699.2010.02358.x) [Google Scholar]
- 52.Allen GR. 1991. Damselfishes of the world. Melle, Germany: Aquarium Systems [Google Scholar]
- 53.Randall JE, Allen GR, Steene RC. 1996. Fishes of the great barrier reef and coral sea. London, UK: Crawford House Publishing [Google Scholar]
- 54.Randall JE. 2005. Reef and shore fishes of the South Pacific. Honolulu, HI: University of Hawaii Press [Google Scholar]
- 55.Froese F, Pauly D.2011. Fishbase. Available from www.fishbase.org .
- 56.Motulsky H. 2010. Intuitive biostatistics a nonmathematical guide to statistical thinking, pp. 447 Oxford, UK: Oxford University Press [Google Scholar]
- 57.Bellwood DR, van Herwerden L, Konow N. 2004. Evolution and biogeography of marine angelfishes (Pisces: Pomacanthidae). Mol. Phylogenet. Evol. 33, 140–155 (doi:10.1016/j.ympev.2004.04.015) [DOI] [PubMed] [Google Scholar]
- 58.Bellwood DR, Klanten S, Cowman PF, Pratchett MS, Konow N, van Herwerden L. 2010. Evolutionary history of the butterflyfishes (f: Chaetodontidae) and the rise of coral feeding fishes. J. Evol. Biol. 23, 335–349 (doi:10.1111/j.1420-9101.2009.01904.x) [DOI] [PubMed] [Google Scholar]
- 59.Malaquias MAE, Reid DG. 2009. Tethyan vicariance, relictualism and speciation: evidence from a global molecular phylogeny of the opisthobranch genus Bulla. J. Biogeogr. 36, 1760–1777 (doi:10.1111/j.1365-2699.2009.02118.x) [Google Scholar]
- 60.Lessios HA. 1998. The first stage of speciation as seen in organisms separated by the Isthmus of Panama. In Endless forms: species and speciation (eds Howard DJ, Berlocher S.), pp. 186–201 Oxford, UK: Oxford University Press [Google Scholar]
- 61.Ekman S. Leipzig, Germany:: Akademische Verlagsgesellschaft; 1935. Tiergeographie des Meeres. [Google Scholar]
- 62.Robertson DR, Grove JS, McCosker JE. 2004. Tropical transpacific shore fishes. Pac. Sci. 58, 507–565 (doi:10.1353/psc.2004.0041) [Google Scholar]
- 63.Vermeij GJ. 2004. Nature: an economic history. Princeton, NJ: Princeton University Press [Google Scholar]
- 64.Winterbottom R. 1986. Revision and vicariance biogeography of the subfamily Congrogadinae (Pisces: Perciformes: Pseudochromidae). Indo-Pac. Fish. 9, 1–34 [Google Scholar]
- 65.Springer VG, Williams JT. 1994. The Indo-West Pacific blenniid fish genus Istiblennius reappraised: a revision of Istiblennius, Blenniella, and Paralticus, new genus. Smithson Contrib. Zool. 565, 1–193 (doi:10.5479/si.00810282.565) [Google Scholar]
- 66.Gaither MR, Bowen BW, Bordenave TR, Rocha LA, Newman SJ, Gomez JA, van Herwerden L, Craig MT. 2011. Phylogeography of the reef fish Cephalopholis argus (Epinephelidae) indicates Pleistocene isolation across the Indo-Pacific Barrier with contemporary overlap in the Coral Triangle. BMC Evol. Biol. 11, 189 (doi:10.1186/1471-2148-11-189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hall R. 1998. The plate tectonics of the Cenozoic SE Asia and the distribution of land and sea. In Biogeography and geological evolution of SE Asia (eds Hall R, Holloway JD.), pp. 99–131 Leiden, The Netherlands: Backhuys Publishers [Google Scholar]
- 68.Hall R. 2002. Cenozoic geological and plate tectonic evolution of SE Asia and the SW Pacific: computer-based reconstructions, model and animations. J. Asian Earth Sci. 20, 353–431 (doi:10.1016/S1367-9120(01)00069-4) [Google Scholar]
- 69.Gower DJ, Johnson KG, Richardson JE, Rosen BR, Rüber L, Williams ST. 2012. Biotic evolution and environmental change in Southeast Asia. Cambridge, UK: Cambridge University Press [Google Scholar]
- 70.Bernardi G, Holbrook SJ, Schmitt RJ, Crane NL, DeMartini E. 2002. Species boundaries, populations and colour morphs in the coral reef three-spot damselfish (Dascyllus trimaculatus) species complex. Proc. R. Soc. Lond. B 269, 599–605 (doi:10.1098/rspb.2001.1922). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fauvelot C, Bernardi G, Planes S. 2003. Reductions in the mitochondrial DNA diversity of coral reef fish provide evidence of population bottlenecks resulting from Holocene sea-level change. Evolution 57, 1571–1583 (doi:10.1554/02-173) [DOI] [PubMed] [Google Scholar]
- 72.Renema W, et al. 2008. Hopping hotspots: global shifts in marine biodiversity. Science 321, 654–657 (doi:10.1126/science.1155674) [DOI] [PubMed] [Google Scholar]
- 73.Depczynski M, Bellwood DR. 2006. Extremes, plasticity, and invariance in vertebrate life history traits: insights from coral reef fishes. Ecology 87, 3119–3127 (doi:10.2307/20069341) [DOI] [PubMed] [Google Scholar]


